Abstract
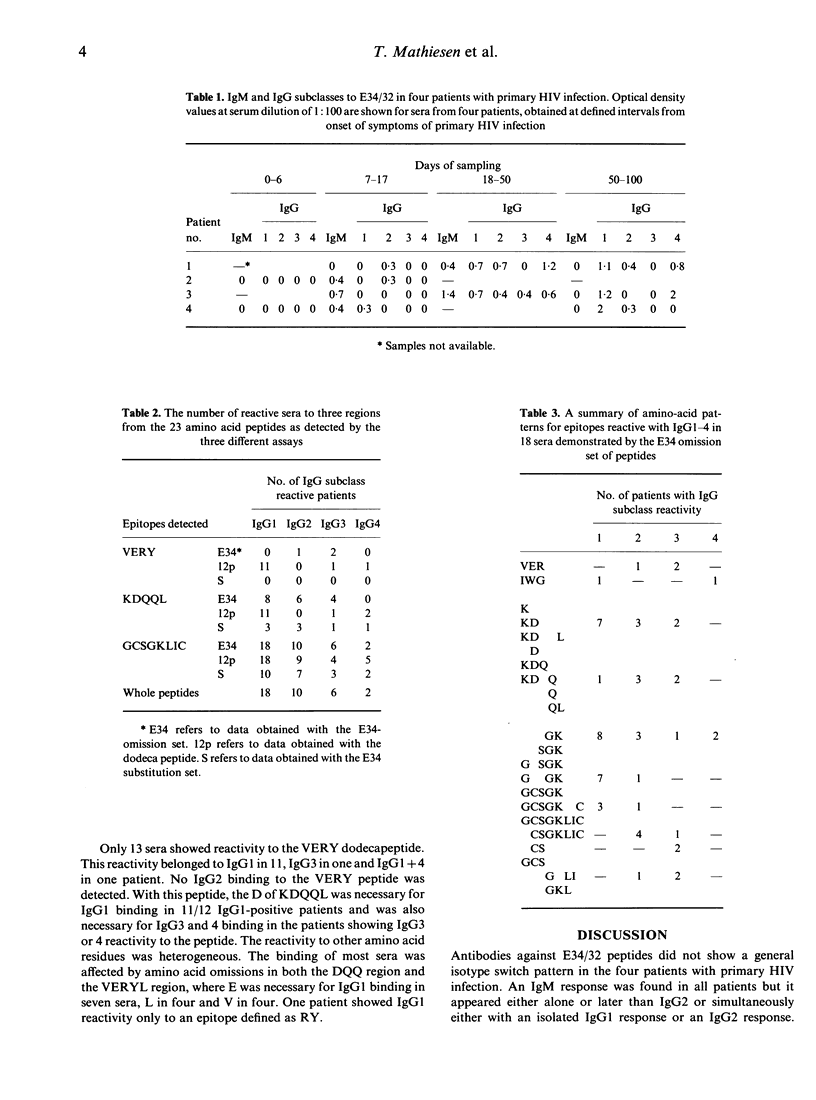
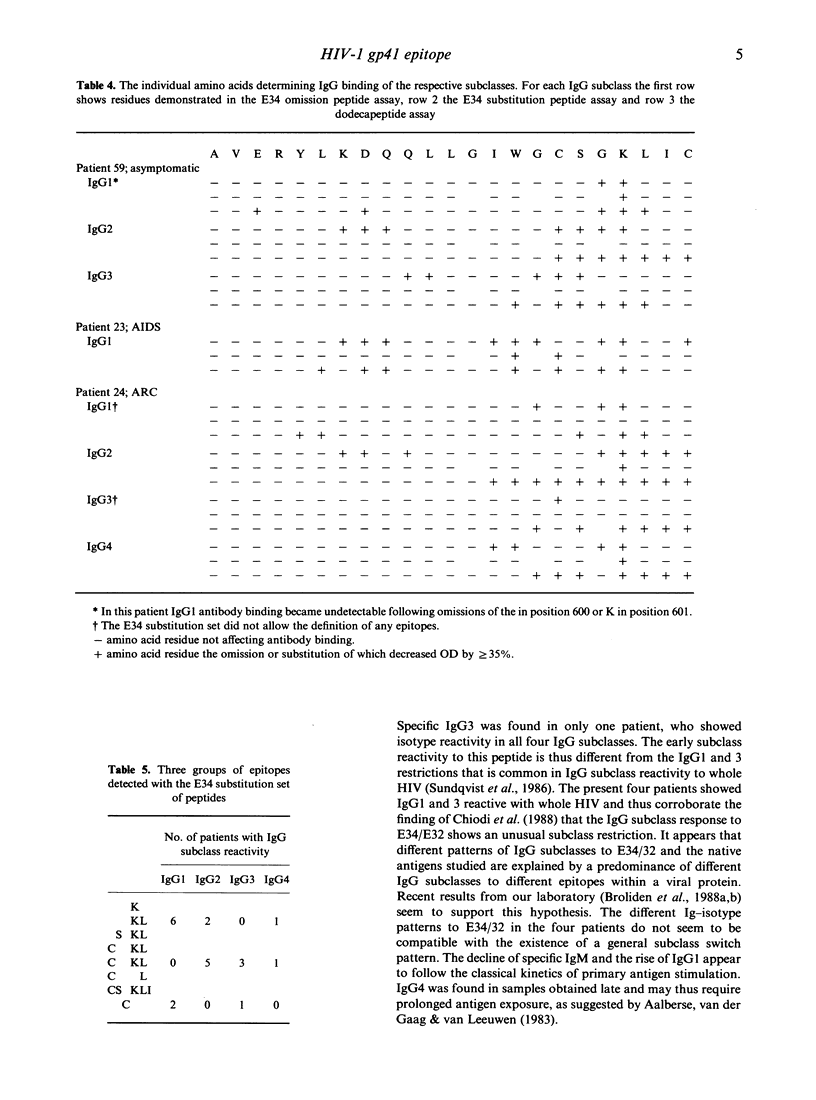
To define the amino acids involved in IgG subclass reactivity to two overlapping HIV-1 gp41 (E34/32; amino acid positions 582-613) peptides, sera from 18 HIV-infected individuals were studied. Peptides mimicking E34 but with single amino acid deletions or glycine substitutions were used to define the amino acid residues necessary for antibody binding. Two dominating immunogenic epitopes, containing highly hydrophilic amino acids, were found on the original peptide. Further analysis was undertaken with two corresponding omission sets of dodecapeptides representing halves of the complete E34 plus a terminal cystein peptide. The subclass reactivities usually differed between the patients with regard to the epitopes with which the different IgG subclasses reacted and also to the importance of different amino acids in antibody binding. The 600 glycine and the 601 lysine were involved in the binding of all IgG1, 2 and 4 and most IgG3. The development of E34/32-reactive IgM and IgG subclasses showed different patterns in four patients with primary HIV infections, contradicting the existence of a general pattern for the development of IgG subclasses to this peptide. The findings suggest that different progenitor clones are selected for synthesis of the different subclasses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalberse R. C., van der Gaag R., van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983 Feb;130(2):722–726. [PubMed] [Google Scholar]
- Alexander H., Johnson D. A., Rosen J., Jerabek L., Green N., Weissman I. L., Lerner R. A. Mimicking the alloantigenicity of proteins with chemically synthesized peptides differing in single amino acids. Nature. 1983 Dec 15;306(5944):697–699. doi: 10.1038/306697a0. [DOI] [PubMed] [Google Scholar]
- Chiodi F., von Gegerfeldt A., Albert J., Fenyö E. M., Gaines H., von Sydow M., Biberfeld G., Parks E., Norrby E. Site-directed ELISA with synthetic peptides representing the HIV transmembrane glycoprotein. J Med Virol. 1987 Sep;23(1):1–9. doi: 10.1002/jmv.1890230102. [DOI] [PubMed] [Google Scholar]
- Clark M. F., Barbara D. J. A method for the quantitative analysis of ELISA data. J Virol Methods. 1987 Feb;15(3):213–222. doi: 10.1016/0166-0934(87)90099-1. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J. Non-sequential expression of multiple immunoglobulin classes by isolated B-cell clones. Nature. 1977 Oct 27;269(5631):812–813. doi: 10.1038/269812a0. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J. A priori delineation of a peptide which mimics a discontinuous antigenic determinant. Mol Immunol. 1986 Jul;23(7):709–715. doi: 10.1016/0161-5890(86)90081-7. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Tainer J. A., Rodda S. J., Mason T. J., Alexander H., Getzoff E. D., Lerner R. A. Chemistry of antibody binding to a protein. Science. 1987 Mar 6;235(4793):1184–1190. doi: 10.1126/science.3823878. [DOI] [PubMed] [Google Scholar]
- Houghten R. A., Bray M. K., Degraw S. T., Kirby C. J. Simplified procedure for carrying out simultaneous multiple hydrogen fluoride cleavages of protected peptide resins. Int J Pept Protein Res. 1986 Jun;27(6):673–678. doi: 10.1111/j.1399-3011.1986.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Houghten R. A., Chang W. C., Li C. H. Human beta-endorphin: synthesis and characterization of analogs iodinated and tritiated at tyrosine residues 1 and 27. Int J Pept Protein Res. 1980 Oct;16(4):311–320. doi: 10.1111/j.1399-3011.1980.tb02592.x. [DOI] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A., Ostresh J. M., Klipstein F. A. Chemical synthesis of an octadecapeptide with the biological and immunological properties of human heat-stable Escherichia coli enterotoxin. Eur J Biochem. 1984 Nov 15;145(1):157–162. doi: 10.1111/j.1432-1033.1984.tb08535.x. [DOI] [PubMed] [Google Scholar]
- Khalife J., Guy B., Capron M., Kieny M. P., Ameisen J. C., Montagnier L., Lecocq J. P., Capron A. Isotypic restriction of the antibody response to human immunodeficiency virus. AIDS Res Hum Retroviruses. 1988 Feb;4(1):3–9. doi: 10.1089/aid.1988.4.3. [DOI] [PubMed] [Google Scholar]
- Mathiesen T., Persson M. A., Sundqvist V. A., Wahren B. Neutralization capacity and antibody dependent cell-mediated cytotoxicity of separated IgG subclasses 1, 3 and 4 against herpes simplex virus. Clin Exp Immunol. 1988 May;72(2):211–215. [PMC free article] [PubMed] [Google Scholar]
- Mathiesen T., Sönnerborg A., von Sydow M., Gaines H., Wahren B. IgG subclass reactivity against human immunodeficiency virus (HIV) and cytomegalovirus in cerebrospinal fluid and serum from HIV-infected patients. J Med Virol. 1988 May;25(1):17–26. doi: 10.1002/jmv.1890250104. [DOI] [PubMed] [Google Scholar]
- Modrow S., Hahn B. H., Shaw G. M., Gallo R. C., Wong-Staal F., Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987 Feb;61(2):570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. S., Naso R. B., Rosen J., Whalley A., Hom Y. L., Hoey K., Kennedy C. J., McCutchan J. A., Spector S. A., Richman D. D. Antibody to a synthetic oligopeptide in subjects at risk for human immunodeficiency virus infection. J Clin Microbiol. 1987 Aug;25(8):1498–1504. doi: 10.1128/jcm.25.8.1498-1504.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist V. A., Linde A., Kurth R., Werner A., Helm E. B., Popovic M., Gallo R. C., Wahren B. Restricted IgG subclass responses to HTLV-III/LAV and to cytomegalovirus in patients with AIDS and lymphadenopathy syndrome. J Infect Dis. 1986 May;153(5):970–973. doi: 10.1093/infdis/153.5.970. [DOI] [PubMed] [Google Scholar]
- Teale J. M. The potential of B lymphocytes for isotype expression. Fed Proc. 1982 Jul;41(9):2497–2501. [PubMed] [Google Scholar]


