Abstract
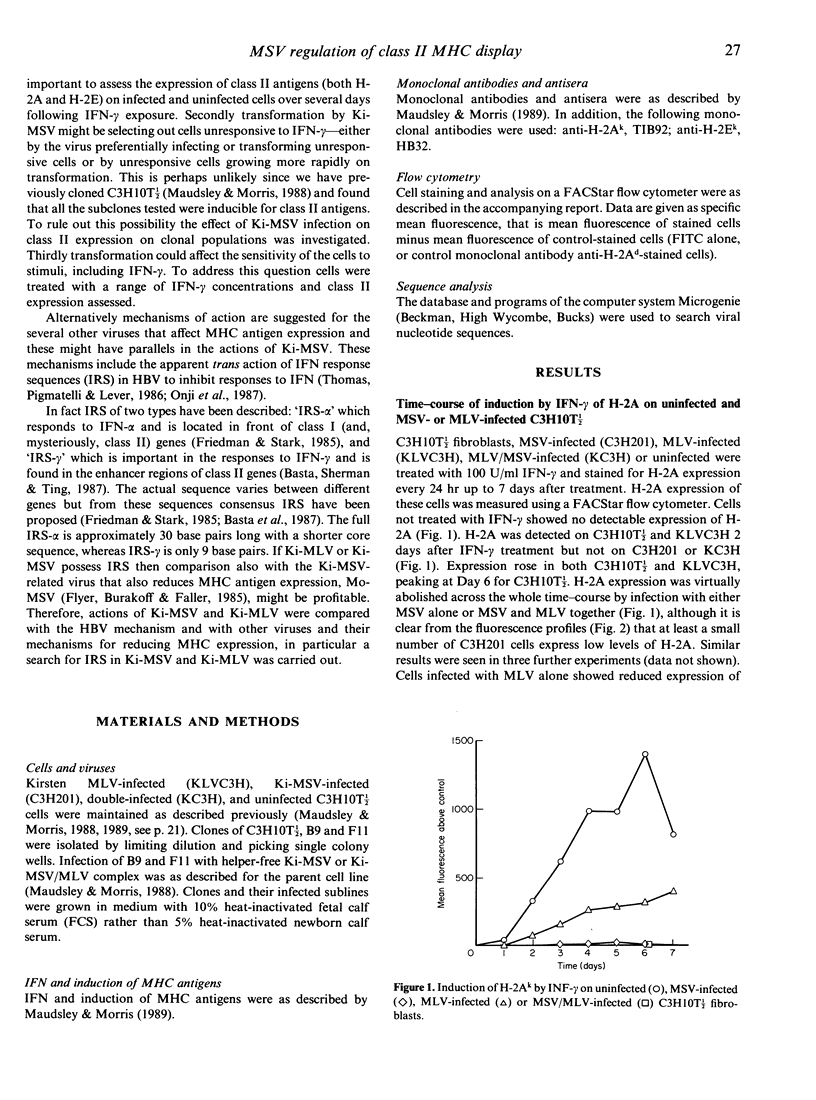
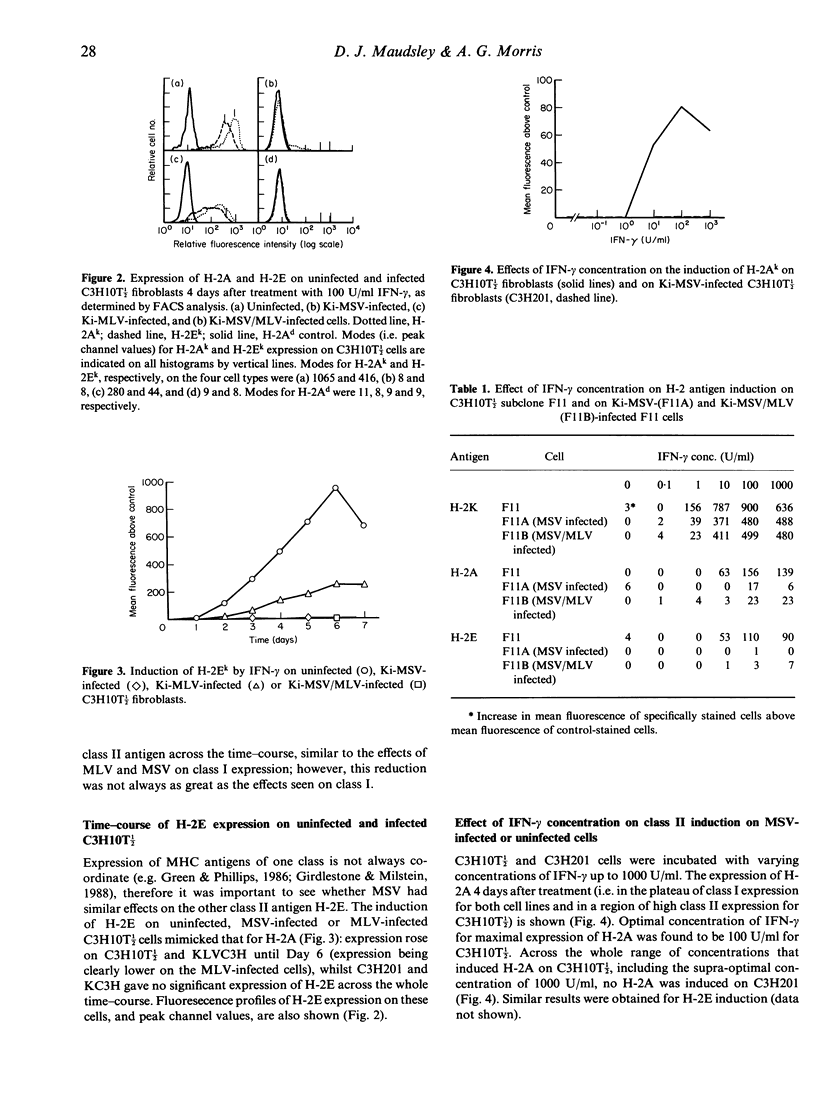
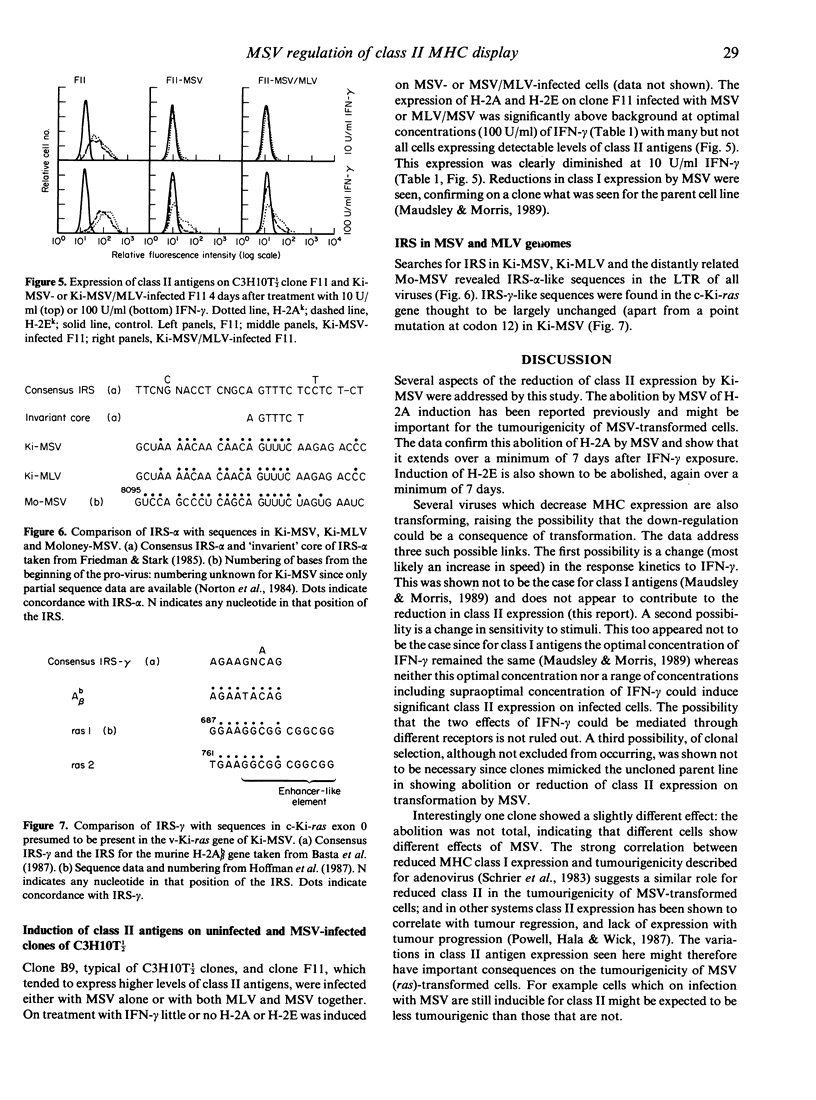
We have reported previously that the Kirsten murine sarcoma virus (Ki-MSV), which carries the v-Ki-ras oncogene, prevents the induction of the class II MHC antigen H-2A and reduces the induction of class I MHC antigens by interferon-gamma (IFN-gamma) on C3H10T 1/2 fibroblasts. It is here shown that the abolition by the virus of H-2A expression extends also to class II antigen H-2E and that this is maintained for at least 7 days after IFN treatment. In addition no concentration of IFN-gamma tested, including supra-optimal concentrations for class I antigen expression, induced class II antigens on MSV-infected cells. Thus MSV inhibits the induction by IFN-gamma of class II MHC antigens by a mechanism other than via a change in kinetics of response to, or in the sensitivity of the cells to, IFN. The possibility that transformation by MSV could result in the (selective) outgrowth of cells unresponsive to IFN was refuted by the observation that clones of C3H10T 1/2, when infected with Ki-MSV, expressed no or dramatically reduced levels of H-2A or H-2E. One C3H10T 1/2 clone chosen for high class II expression, when transformed with Ki-MSV, did express low levels of class II antigens at optimal concentrations of IFN-gamma, suggesting that the degree of the reduction of class II expression varies with the cells that are infected. Comparison with mechanisms whereby other viruses inhibit MHC antigen display revealed an interesting possibility: IFN response sequences (IRS) identified in the virus genomes might act in trans to (down) regulate MHC antigen expression. This could be an important mechanism determining the tumourigenicity of, and immune evasion by, Ki-MSV and other viruses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., McMichael A., Peterson P. A. Reduced allorecognition of adenovirus-2 infected cells. J Immunol. 1987 Jun 1;138(11):3960–3966. [PubMed] [Google Scholar]
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Basta P. V., Sherman P. A., Ting J. P. Identification of an interferon-gamma response region 5' of the human histocompatibility leukocyte antigen DR alpha chain gene which is active in human glioblastoma multiforme lines. J Immunol. 1987 Feb 15;138(4):1275–1280. [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Flyer D. C., Burakoff S. J., Faller D. V. Retrovirus-induced changes in major histocompatibility complex antigen expression influence susceptibility to lysis by cytotoxic T lymphocytes. J Immunol. 1985 Oct;135(4):2287–2292. [PubMed] [Google Scholar]
- Friedman R. L., Stark G. R. alpha-Interferon-induced transcription of HLA and metallothionein genes containing homologous upstream sequences. Nature. 1985 Apr 18;314(6012):637–639. doi: 10.1038/314637a0. [DOI] [PubMed] [Google Scholar]
- Girdlestone J., Milstein C. Differential expression and interferon response of HLA class I genes in thymocyte lines and response variants. Eur J Immunol. 1988 Jan;18(1):139–143. doi: 10.1002/eji.1830180121. [DOI] [PubMed] [Google Scholar]
- Green W. R., Phillips J. D. Differential induction of H-2K vs H-2D class I major histocompatibility complex antigen expression by murine recombinant interferon-gamma. J Immunol. 1986 Aug 1;137(3):814–818. [PubMed] [Google Scholar]
- Guy B., Kieny M. P., Riviere Y., Le Peuch C., Dott K., Girard M., Montagnier L., Lecocq J. P. HIV F/3' orf encodes a phosphorylated GTP-binding protein resembling an oncogene product. Nature. 1987 Nov 19;330(6145):266–269. doi: 10.1038/330266a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. K., Trusko S. P., Freeman N., George D. L. Structural and functional characterization of the promoter region of the mouse c-Ki-ras gene. Mol Cell Biol. 1987 Jul;7(7):2592–2596. doi: 10.1128/mcb.7.7.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler J. L., Schatz C., Wasylyk C., Chatton B., Wasylyk B. A Harvey-ras responsive transcription element is also responsive to a tumour-promoter and to serum. Nature. 1988 Mar 17;332(6161):275–278. doi: 10.1038/332275a0. [DOI] [PubMed] [Google Scholar]
- Jennings S. R., Rice P. L., Kloszewski E. D., Anderson R. W., Thompson D. L., Tevethia S. S. Effect of herpes simplex virus types 1 and 2 on surface expression of class I major histocompatibility complex antigens on infected cells. J Virol. 1985 Dec;56(3):757–766. doi: 10.1128/jvi.56.3.757-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. H., Ferguson B. Q., Rosenberg M. Pentapeptide nuclear localization signal in adenovirus E1a. Mol Cell Biol. 1987 Jul;7(7):2451–2456. doi: 10.1128/mcb.7.7.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley D. J., Morris A. G. Kirsten murine sarcoma virus abolishes interferon gamma-induced class II but not class I major histocompatibility antigen expression in a murine fibroblast line. J Exp Med. 1988 Feb 1;167(2):706–711. doi: 10.1084/jem.167.2.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell B., Kirk C. G-protein control of inositol phosphate hydrolysis. Nature. 1986 Sep 11;323(6084):112–113. doi: 10.1038/323112b0. [DOI] [PubMed] [Google Scholar]
- Norton J. D., Connor J., Avery R. J. Genesis of Kirsten murine sarcoma virus: sequence analysis reveals recombination points and potential leukaemogenic determinant on parental leukaemia virus genome. Nucleic Acids Res. 1984 Sep 11;12(17):6839–6852. doi: 10.1093/nar/12.17.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell P. C., Hála K., Wick G. Aberrant expression of Ia-like antigens on tumor cells of regressing but not of progressing Rous sarcomas. Eur J Immunol. 1987 May;17(5):723–726. doi: 10.1002/eji.1830170523. [DOI] [PubMed] [Google Scholar]
- Savard P., DesGroseillers L., Rassart E., Poirier Y., Jolicoeur P. Important role of the long terminal repeat of the helper Moloney murine leukemia virus in Abelson virus-induced lymphoma. J Virol. 1987 Oct;61(10):3266–3275. doi: 10.1128/jvi.61.10.3266-3275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Thomas H. C., Pignatelli M., Lever A. M. Homology between HBV-DNA and a sequence regulating the interferon-induced antiviral system: possible mechanism of persistent infection. J Med Virol. 1986 May;19(1):63–69. doi: 10.1002/jmv.1890190110. [DOI] [PubMed] [Google Scholar]
- Vaessen R. T., Houweling A., van der Eb A. J. Post-transcriptional control of class I MHC mRNA expression in adenovirus 12-transformed cells. Science. 1987 Mar 20;235(4795):1486–1488. doi: 10.1126/science.3823900. [DOI] [PubMed] [Google Scholar]


