Abstract
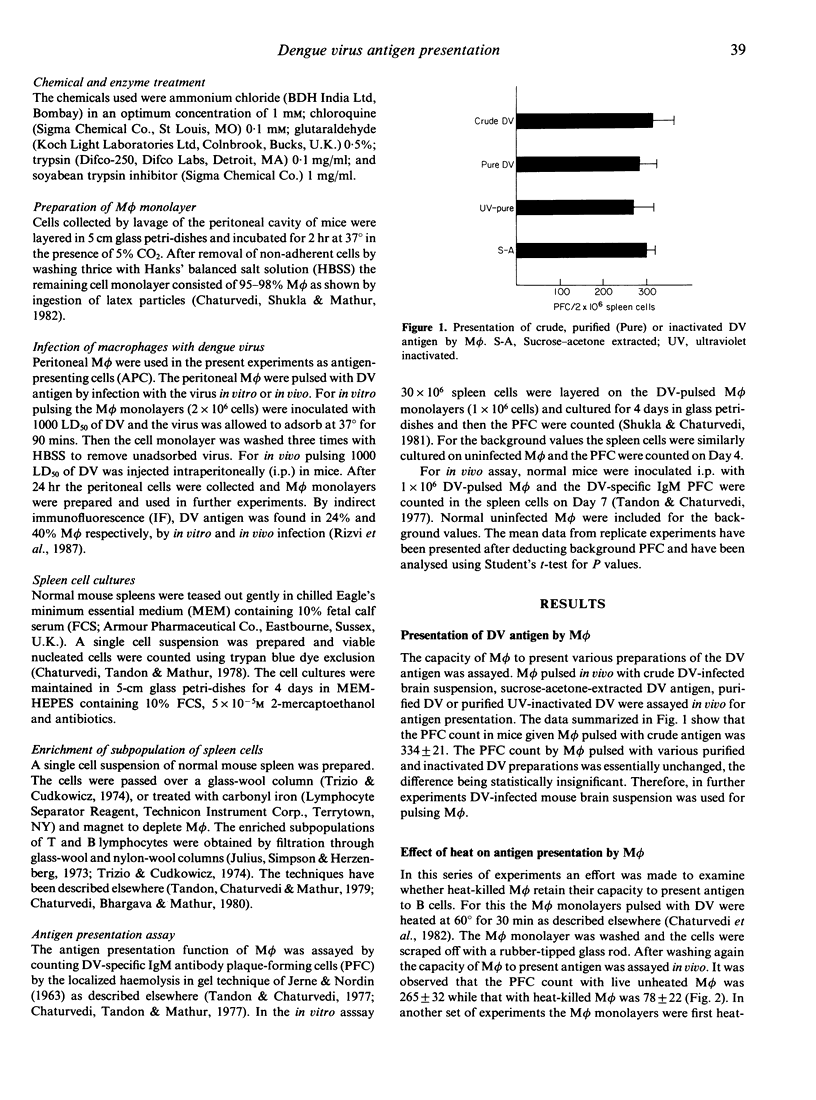
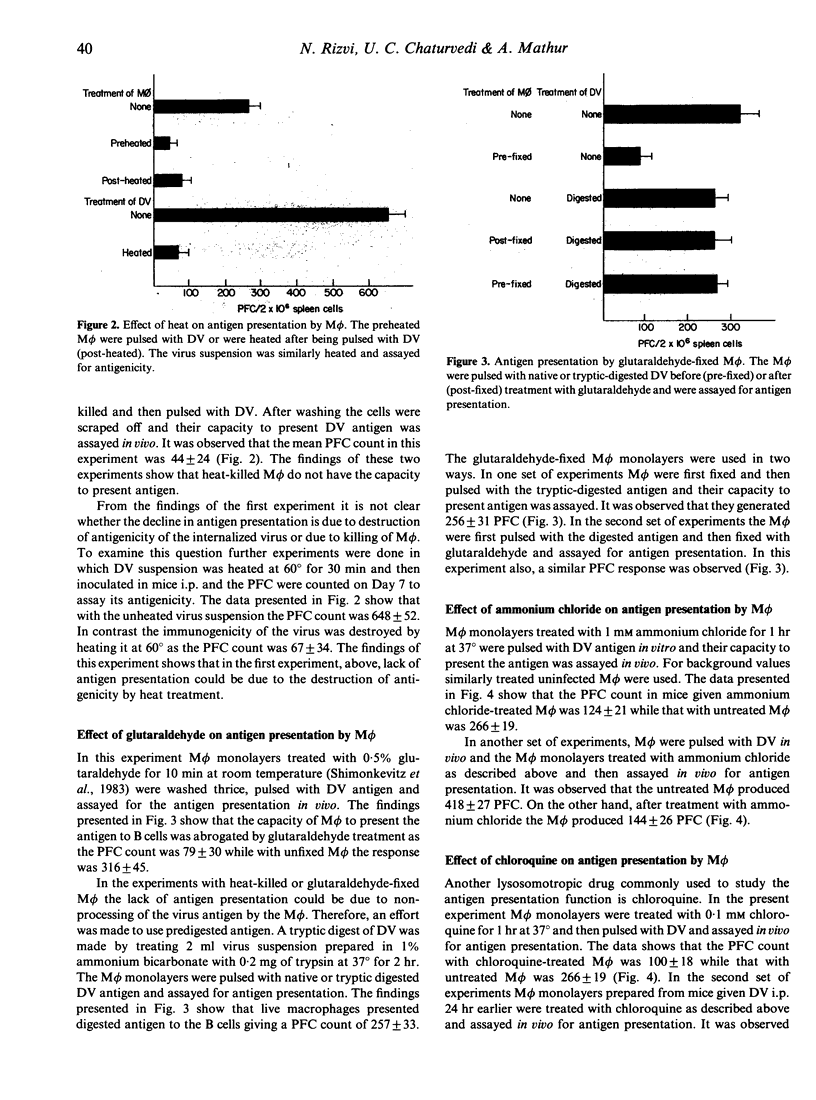
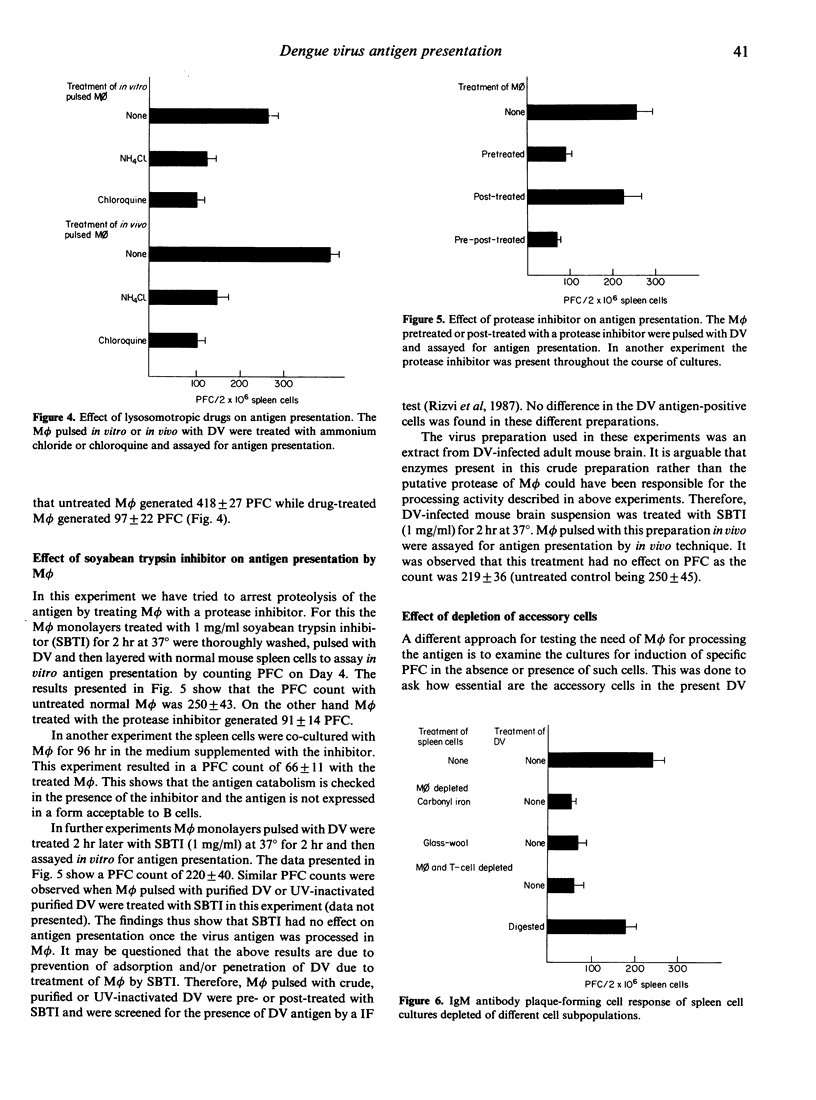
The study was undertaken to investigate the role of dengue type 2 virus (DV)-infected mouse peritoneal macrophages (M phi) in presentation of the DV antigen to B lymphocytes as shown by counting virus-specific IgM antibody plaque-forming cells (PFC). It was observed that heat-killed or glutaraldehyde-fixed M phi did not present the antigen. Pretreatment of M phi with the lysosomotropic compounds ammonium chloride and chloroquine inhibited the antigen presentation. Depletion of M phi from the spleen cell cultures abrogated the immune response to DV. The tryptic-digested DV antigen could stimulate immune responses in B-lymphocyte enriched (depleted of M phi and T cells) spleen cell cultures, and the digested antigen could be presented by glutaraldehyde-fixed M phi. Pretreatment of M phi with a trypsin inhibitor abrogated antigen presentation. The findings thus show that even for presentation to B cells the DV antigen must be processed by M phi by a trypsin-like protease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahuguna L. M., Chaturvedi U. C. Histochemical and biochemical studies in ultraviolet irradiated tissue culture. Br J Exp Pathol. 1974 Aug;55(4):314–319. [PMC free article] [PubMed] [Google Scholar]
- Buus S., Werdelin O. A group-specific inhibitor of lysosomal cysteine proteinases selectively inhibits both proteolytic degradation and presentation of the antigen dinitrophenyl-poly-L-lysine by guinea pig accessory cells to T cells. J Immunol. 1986 Jan;136(2):452–458. [PubMed] [Google Scholar]
- Chaturvedi U. C., Bhargava A., Mathur A. Production of cytotoxic factor in the spleen of dengue virus-infected mice. Immunology. 1980 Aug;40(4):665–671. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Nagar R., Mathur A. Effect of dengue virus infection on Fc-receptor functions of mouse macrophages. J Gen Virol. 1983 Nov;64(Pt 11):2399–2407. doi: 10.1099/0022-1317-64-11-2399. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Tandon H. O., Mathur A. Control of in vitro and in vivo spread of coxsackievirus B4 infection by sensitized spleen cells and antibody. J Infect Dis. 1978 Aug;138(2):181–190. doi: 10.1093/infdis/138.2.181. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Tandon P., Mathur A. Effect of immunosuppression on dengue virus infection in mice. J Gen Virol. 1977 Sep;36(3):449–458. doi: 10.1099/0022-1317-36-3-449. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Rizvi N., Chaturvedi U. C., Nagar R., Mathur A. Macrophage functions during dengue virus infection: antigenic stimulation of B cells. Immunology. 1987 Nov;62(3):493–498. [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser L. J., Rosenthal A. S. Adherent cell function in murine T lymphocyte antigen recognition. I. A. macrophage-dependent T cell proliferation assay in the mouse. J Immunol. 1978 Jun;120(6):1991–1995. [PubMed] [Google Scholar]
- Rosenwasser L. J., Rosenthal A. S. Adherent cell function in murine T lymphocyte antigen recognition. II. Definition of genetically restricted and nonrestricted macrophage functions in T cell proliferation. J Immunol. 1978 Dec;121(6):2497–2501. [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla M. I., Chaturvedi U. C. Dengue virus-induced suppressor factor stimulates production of prostaglandin to mediate suppression. J Gen Virol. 1981 Oct;56(Pt 2):241–249. doi: 10.1099/0022-1317-56-2-241. [DOI] [PubMed] [Google Scholar]
- Stollar V. Studies on the nature of dengue viruses. IV. The structural proteins of type 2 dengue virus. Virology. 1969 Nov;39(3):426–438. doi: 10.1016/0042-6822(69)90091-9. [DOI] [PubMed] [Google Scholar]
- Tandon P., Chaturvedi U. C., Mathur A. Dengue virus-induced thymus-derived suppressor cells in the spleen of mice. Immunology. 1979 Dec;38(4):653–658. [PMC free article] [PubMed] [Google Scholar]
- Trizio D., Cudkowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974 Oct;113(4):1093–1097. [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Lu C. Y., Allen P. M. Antigen presentation: comments on its regulation and mechanism. J Immunol. 1984 Jan;132(1):1–5. [PubMed] [Google Scholar]
- Walden P., Nagy Z. A., Klein J. Antigen presentation by liposomes: inhibition with antibodies. Eur J Immunol. 1986 Jun;16(6):717–720. doi: 10.1002/eji.1830160622. [DOI] [PubMed] [Google Scholar]
- Walden P., Nagy Z. A., Klein J. Induction of regulatory T-lymphocyte responses by liposomes carrying major histocompatibility complex molecules and foreign antigen. Nature. 1985 May 23;315(6017):327–329. doi: 10.1038/315327a0. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]



