Abstract
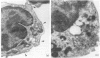
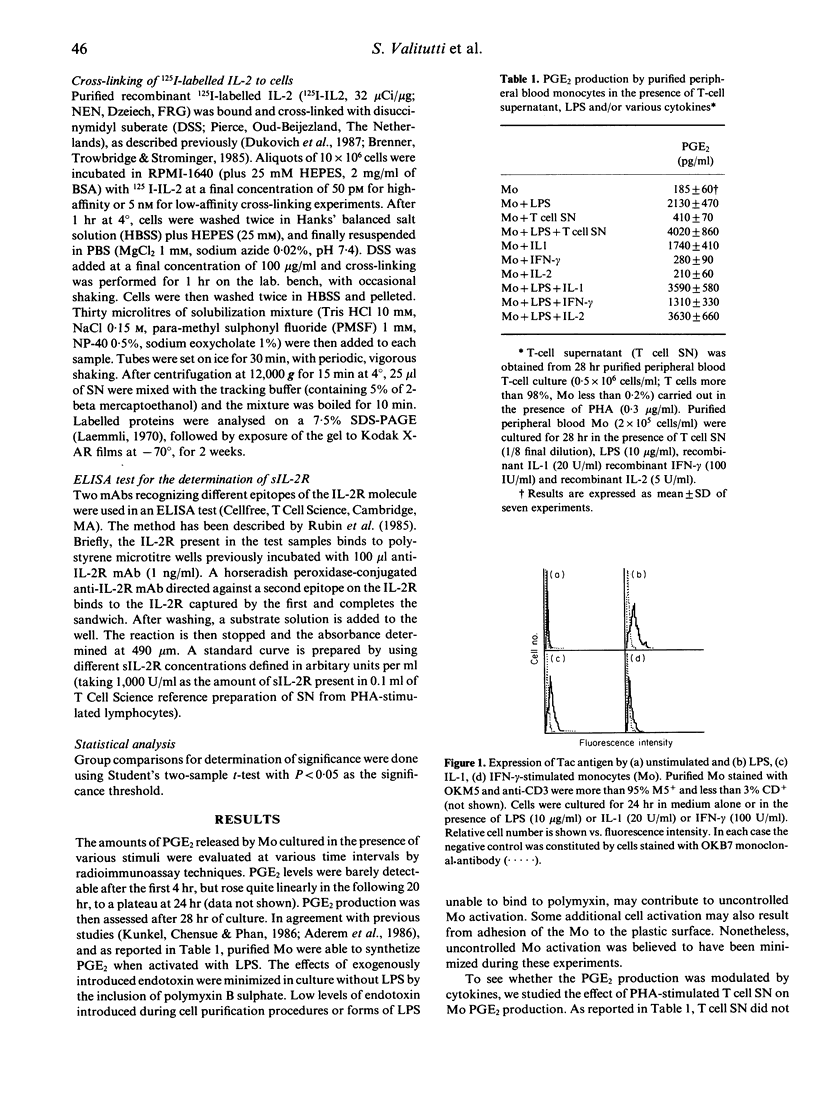
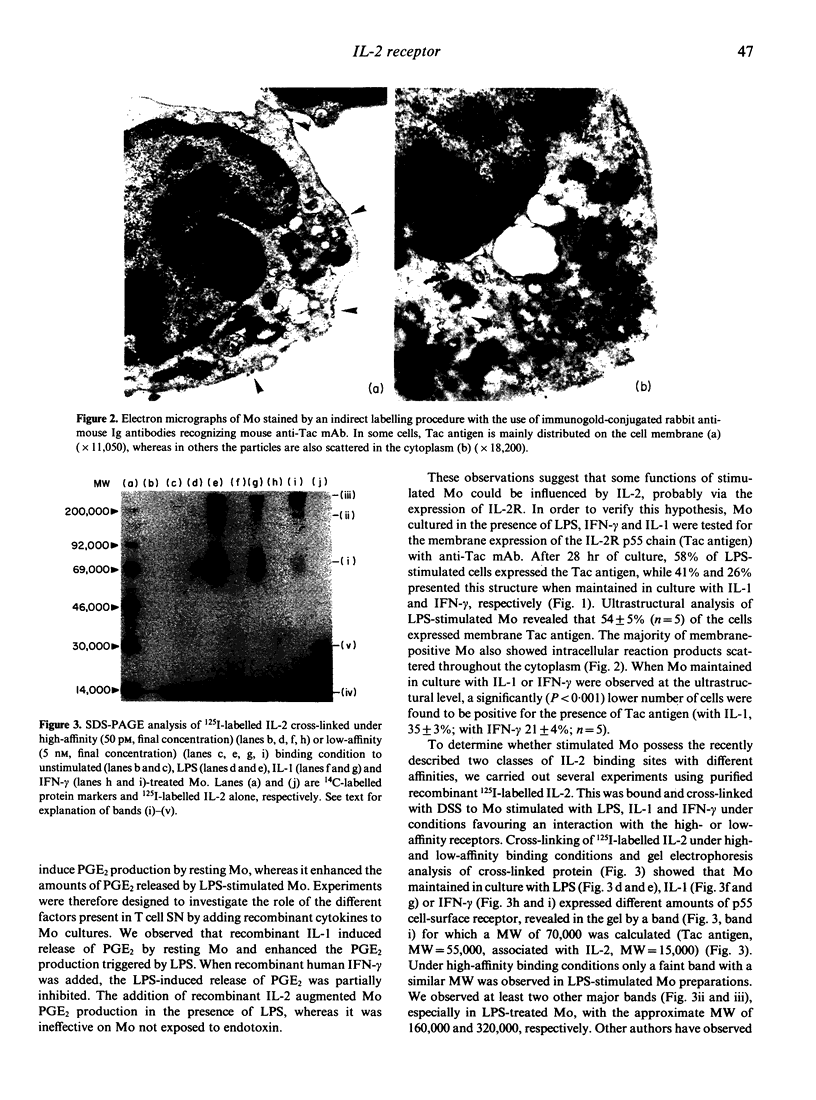
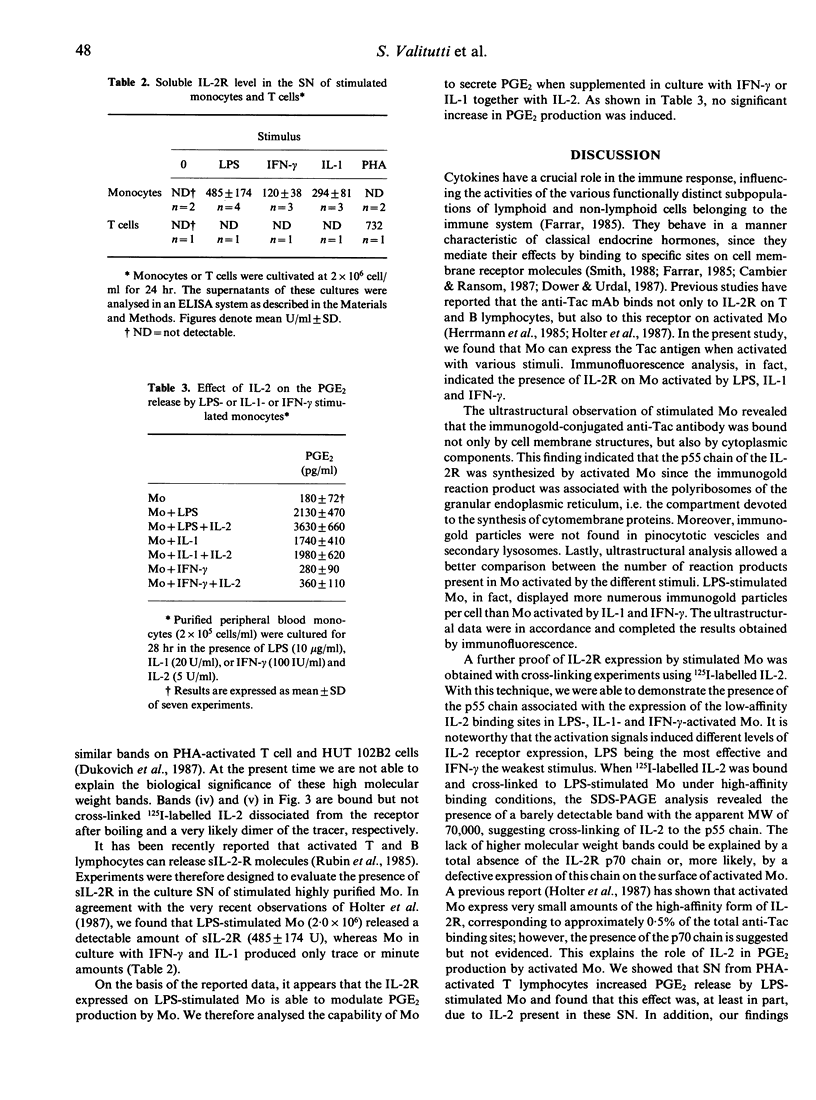
Human peripheral blood monocytes (Mo) synthesize prostaglandin E2 (PGE2) when activated with lipopolysaccharide (LPS). This production is strongly enhanced by the addition of supernatant from phytohaemagglutinin (PHA)-activated T cells. To evaluate the factor(s) responsible for this enhancement we studied the effect of several cytokines on the PGE2 metabolism. Recombinant interleukin-1 (IL-1) or recombinant IL-2 strongly enhanced PGE2 synthesis in LPS-stimulated Mo cultures, whereas recombinant interferon-gamma (IFN-gamma) partially inhibited its production. To see whether the effect of IL-2 on Mo was due to the presence of IL-2 receptor (IL-2R) on the cell surface, flow cytometric analysis and electron microscopy were used to investigate IL-2R expression in unstimulated and stimulated Mo. Stimulated, but not resting, Mo displayed the p55 IL-2R chain on their cellular surface and associated with the polyribosomes of the rough endoplasmic reticulum in the cytoplasm. This finding strongly suggested that the p55 chain of the IL-2R was synthesized by activated Mo. To confirm this result, 125I-labelled IL-2 was bound under high- and low-affinity conditions and cross-linked to Mo cultured in the presence of LPS, IFN-gamma or IL-1. The cross-linked 125I-IL-2/IL-2R complexes were analysed by SDS-PAGE. Mo cultured with LPS, IFN-gamma and IL-1 expressed the p55 protein detected by low-affinity cross-linking, whereas only LPS-stimulated Mo displayed a barely detectable band with an apparent MW of 70,000 under high-affinity binding conditions. In addition, stimulated Mo were found capable of producing the soluble form of IL-2R. Finally, LPS-activated Mo only responded to the addition of IL-2 by an increase in PGE2 production, suggesting that the function of IL-2R on activated Mo is linked to the stimulus applied.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Cohen D. S., Wright S. D., Cohn Z. A. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J Exp Med. 1986 Jul 1;164(1):165–179. doi: 10.1084/jem.164.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney R. J., Naruns P., Davies P., Humes J. L. Antigen-antibody complexes stimulate the synthesis and release of prostaglandins by mouse peritoneal macrophages. Prostaglandins. 1979 Oct;18(4):605–616. doi: 10.1016/0090-6980(79)90027-3. [DOI] [PubMed] [Google Scholar]
- Bonney R. J., Wightman P. D., Davies P., Sadowski S. J., Kuehl F. A., Jr, Humes J. L. Regulation of prostaglandin synthesis and of the selective release of lysosomal hydrolases by mouse peritoneal macrophages. Biochem J. 1978 Nov 15;176(2):433–442. doi: 10.1042/bj1760433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M. B., Trowbridge I. S., Strominger J. L. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985 Jan;40(1):183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Cambier J. C., Ransom J. T. Molecular mechanisms of transmembrane signaling in B lymphocytes. Annu Rev Immunol. 1987;5:175–199. doi: 10.1146/annurev.iy.05.040187.001135. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Welte K., Mertelsmann R., Dupont B. Prostaglandin E2 acts at two distinct pathways of T lymphocyte activation: inhibition of interleukin 2 production and down-regulation of transferrin receptor expression. J Immunol. 1985 Aug;135(2):1172–1179. [PubMed] [Google Scholar]
- Ciabattoni G., Pugliese F., Spaldi M., Cinotti G. A., Patrono C. Radioimmunoassay measurement of prostaglandins E2 and F2alpha in human urine. J Endocrinol Invest. 1979 Apr-Jun;2(2):173–182. doi: 10.1007/BF03349310. [DOI] [PubMed] [Google Scholar]
- Dukovich M., Wano Y., Le thi Bich Thuy, Katz P., Cullen B. R., Kehrl J. H., Greene W. C. A second human interleukin-2 binding protein that may be a component of high-affinity interleukin-2 receptors. Nature. 1987 Jun 11;327(6122):518–522. doi: 10.1038/327518a0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Herrmann F., Cannistra S. A., Levine H., Griffin J. D. Expression of interleukin 2 receptors and binding of interleukin 2 by gamma interferon-induced human leukemic and normal monocytic cells. J Exp Med. 1985 Sep 1;162(3):1111–1116. doi: 10.1084/jem.162.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter W., Goldman C. K., Casabo L., Nelson D. L., Greene W. C., Waldmann T. A. Expression of functional IL 2 receptors by lipopolysaccharide and interferon-gamma stimulated human monocytes. J Immunol. 1987 May 1;138(9):2917–2922. [PubMed] [Google Scholar]
- Holter W., Grunow R., Stockinger H., Knapp W. Recombinant interferon-gamma induces interleukin 2 receptors on human peripheral blood monocytes. J Immunol. 1986 Mar 15;136(6):2171–2175. [PubMed] [Google Scholar]
- Jerrells T. R., Dean J. H., Richardson G. L., Herberman R. B. Depletion of monocytes from human peripheral blood mononuclear leukocytes: comparison of the sephadex G-10 column method with other commonly used techniques. J Immunol Methods. 1980;32(1):11–29. doi: 10.1016/0022-1759(80)90113-1. [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Racine L., Griffiths G. W. Attempts to quantitate immunocytochemistry at the electron microscope level. Histochem J. 1980 May;12(3):317–332. doi: 10.1007/BF01006953. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Phan S. H. Prostaglandins as endogenous mediators of interleukin 1 production. J Immunol. 1986 Jan;136(1):186–192. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maggiano N., Larocca L. M., Piantelli M., Acuto O., Musiani P. Expression of T cell receptor-alpha and -beta subunits in human thymocytes. An immunocytologic study. J Immunol. 1987 Sep 1;139(5):1438–1445. [PubMed] [Google Scholar]
- Malkovský M., Loveland B., North M., Asherson G. L., Gao L., Ward P., Fiers W. Recombinant interleukin-2 directly augments the cytotoxicity of human monocytes. Nature. 1987 Jan 15;325(6101):262–265. doi: 10.1038/325262a0. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Nabholz M., Conzelmann A., Acuto O., North M., Haas W., Pohlit H., von Boehmer H., Hengartner H., Mach J. P., Engers H. Established murine cytolytic T-cell lines as tools for a somatic cell genetic analysis of T-cell functions. Immunol Rev. 1980;51:125–156. doi: 10.1111/j.1600-065x.1980.tb00319.x. [DOI] [PubMed] [Google Scholar]
- Piantelli M., Lauriola L., Maggiano N., Ranelletti F. O., Musiani P. Role of interleukins 1 and 2 on human thymocyte mitogen activation. Cell Immunol. 1981 Nov 1;64(2):337–349. doi: 10.1016/0008-8749(81)90485-8. [DOI] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Samuelsson B., Goldyne M., Granström E., Hamberg M., Hammarström S., Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Zrike J. M., Hamill A. L., Kempe J., Cohn Z. A. Regulation of arachidonic acid metabolites in macrophages. J Exp Med. 1980 Aug 1;152(2):324–335. doi: 10.1084/jem.152.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Adorini L., Marubini E., Doria G. A microcomputer program for probit analysis of interleukin-2 (IL-2) titration data. J Immunol Methods. 1986 Feb 12;86(2):265–277. doi: 10.1016/0022-1759(86)90463-1. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. Interleukin 2. Annu Rev Immunol. 1984;2:319–333. doi: 10.1146/annurev.iy.02.040184.001535. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]