Abstract
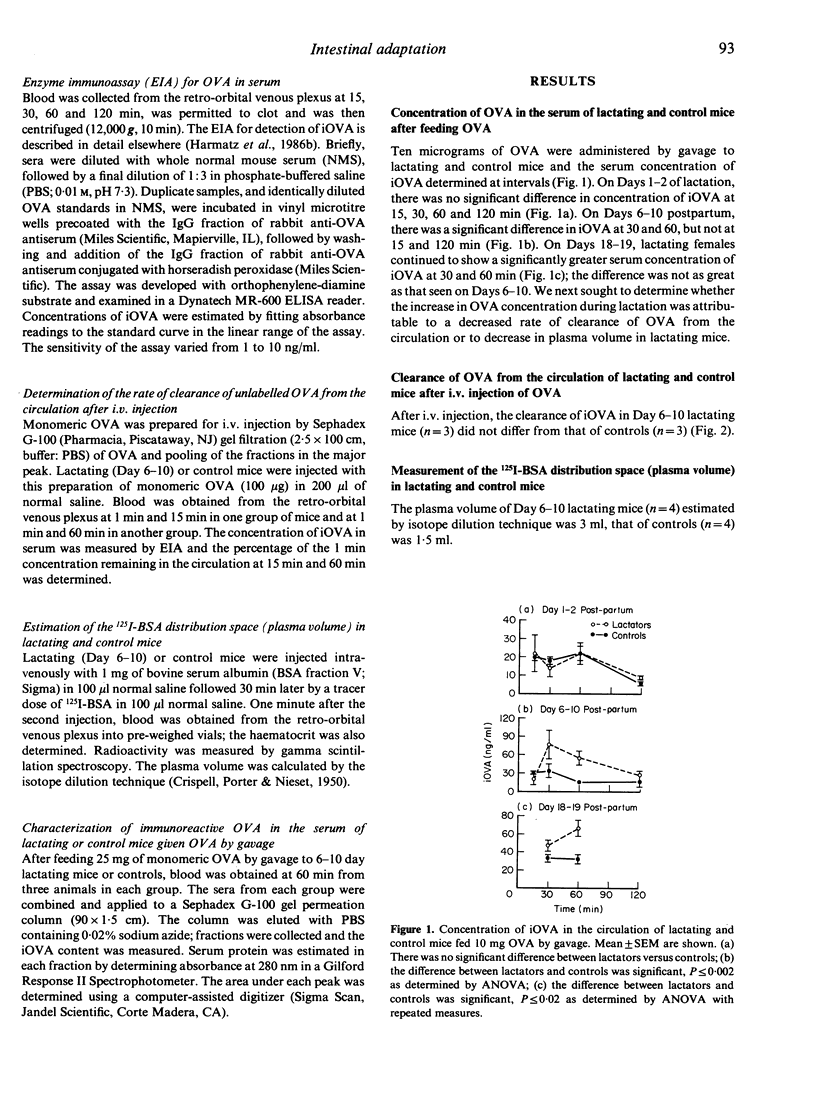
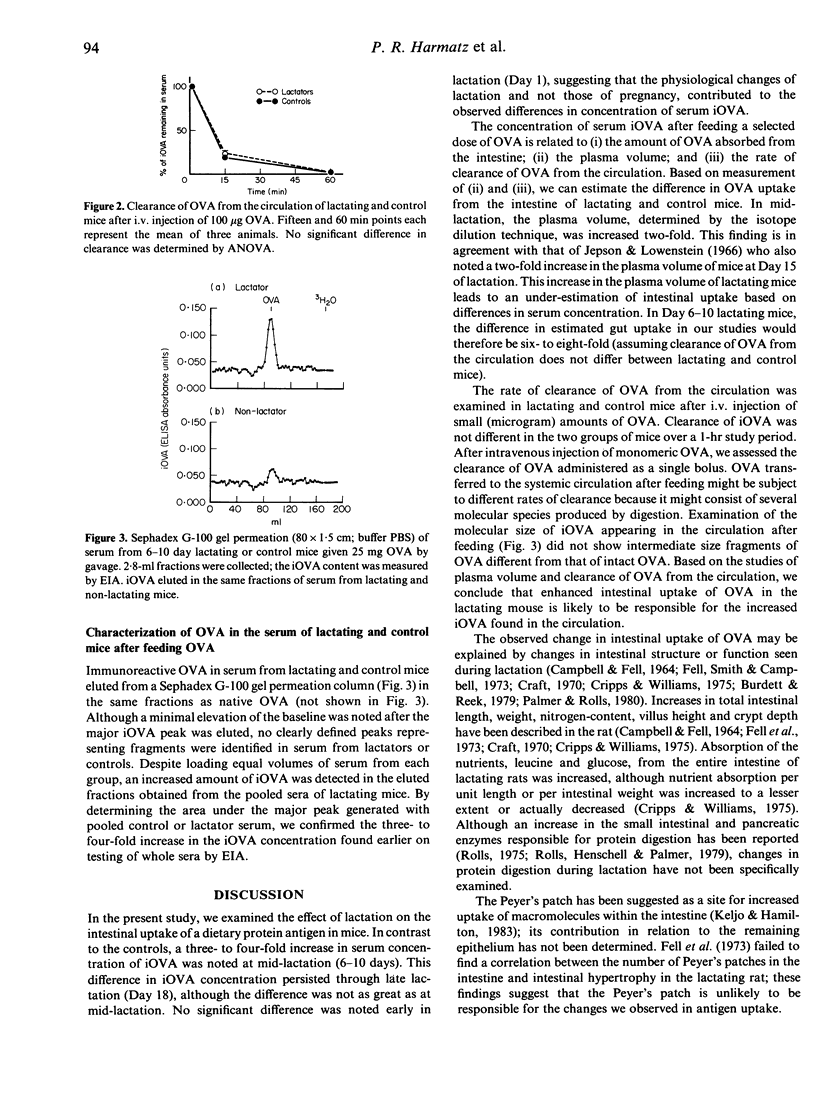
Small quantities of dietary protein antigens cross the intestinal epithelium of the lactating mouse, enter the circulation, are transferred across the mammary gland into the milk and reach the suckling neonate. In this study, we sought to determine whether intestinal uptake of ovalbumin (OVA) was enhanced in lactating compared to control mice. OVA was administered by gavage under ether anaesthesia. Blood was obtained at 15, 30, 60 and 120 min and immunoreactive OVA (iOVA) measured by enzyme immunoassay. At 30 and 60 min, a three- to four-fold higher concentration of iOVA was detected in lactating compared to control mice. Because this increase in concentration of iOVA might be explained by changes in plasma volume, rate of clearance of OVA from the circulation or altered uptake from the intestine, plasma volume was measured by isotope dilution after i.v. injection of 125I-bovine serum albumin (BSA) and clearance was assessed by measuring elimination of OVA from the circulation after i.v. injection of OVA. In comparison to controls, plasma volume of Day 7-10 lactating mice was increased two-fold and no difference in clearance rate was noted. Because the increase in concentration of iOVA in lactating mice is several-fold greater than in controls, we suggest that increased intestinal uptake of the protein occurs during lactation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burdett K., Reek C. Adaptation of the small intestine during pregnancy and lactation in the rat. Biochem J. 1979 Nov 15;184(2):245–251. doi: 10.1042/bj1840245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL R. M., FELL B. F. GASTRO-INTESTINAL HYPERTROPHY IN THE LACTATING RAT AND ITS RELATION TO FOOD INTAKE. J Physiol. 1964 May;171:90–97. doi: 10.1113/jphysiol.1964.sp007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRISPELL K. R., PORTER B., NIESET R. T. Studies of plasma volume using human serum albumin tagged with radioactive iodine. J Clin Invest. 1950 May;29(5):513–516. doi: 10.1172/JCI102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant A. J., Bailes J. A., Marsden R. A., Hewitt D. Effect of maternal dietary exclusion on breast fed infants with eczema: two controlled studies. Br Med J (Clin Res Ed) 1986 Jul 26;293(6541):231–233. doi: 10.1136/bmj.293.6541.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft I. L. The influence of pregnancy and lactation on the morphology and absorptive capacity of the rat small intestine. Clin Sci. 1970 Mar;38(3):287–295. doi: 10.1042/cs0380287. [DOI] [PubMed] [Google Scholar]
- Cripps A. W., Williams V. J. The effect of pregnancy and lactation on food intake, gastrointestinal anatomy and the absorptive capacity of the small intestine in the albino rat. Br J Nutr. 1975 Jan;33(1):17–32. doi: 10.1079/bjn19750005. [DOI] [PubMed] [Google Scholar]
- FELL B. F., SMITH K. A., CAMPBELL R. M. Hypertrophic and hyperplastic changes in the alimentary canal of the lactating rat. J Pathol Bacteriol. 1963 Jan;85:179–188. doi: 10.1002/path.1700850117. [DOI] [PubMed] [Google Scholar]
- Fälth-Magnusson K., Kjellman N. I., Sundqvist T., Magnusson K. E. Gastrointestinal permeability in atopic and non-atopic mothers, assessed with different-sized polyethyleneglycols (PEG 400 and PEG 1000). Clin Allergy. 1985 Nov;15(6):565–570. doi: 10.1111/j.1365-2222.1985.tb02310.x. [DOI] [PubMed] [Google Scholar]
- Harmatz P. R., Hanson D. G., Walsh M. K., Kleinman R. E., Bloch K. J., Walker W. A. Transfer of protein antigens into milk after intravenous injection into lactating mice. Am J Physiol. 1986 Aug;251(2 Pt 1):E227–E233. doi: 10.1152/ajpendo.1986.251.2.E227. [DOI] [PubMed] [Google Scholar]
- Jakobsson I., Lindberg T. Cow's milk proteins cause infantile colic in breast-fed infants: a double-blind crossover study. Pediatrics. 1983 Feb;71(2):268–271. [PubMed] [Google Scholar]
- Jepson J., Lowenstein L. Erythopoiesis during pregnancy and lactation in the mouse. II. Role of erythropoietin. Proc Soc Exp Biol Med. 1966 Apr;121(4):1077–1081. doi: 10.3181/00379727-121-30971. [DOI] [PubMed] [Google Scholar]
- Keljo D. J., Hamilton J. R. Quantitative determination of macromolecular transport rate across intestinal Peyer's patches. Am J Physiol. 1983 Jun;244(6):G637–G644. doi: 10.1152/ajpgi.1983.244.6.G637. [DOI] [PubMed] [Google Scholar]
- Lake A. M., Whitington P. F., Hamilton S. R. Dietary protein-induced colitis in breast-fed infants. J Pediatr. 1982 Dec;101(6):906–910. doi: 10.1016/s0022-3476(82)80008-5. [DOI] [PubMed] [Google Scholar]
- Nicklin S., Miller K. Naturally acquired tolerance to dietary antigen: effect of in utero and perinatal exposure on subsequent humoral immune competence in the rat. J Reprod Immunol. 1987 Feb;10(2):167–176. doi: 10.1016/0165-0378(87)90074-x. [DOI] [PubMed] [Google Scholar]
- Palmer M. F., Rolls B. A. Activities of some metabolic enzymes in the small intestinal mucosa during pregnancy and lactation in the rat. J Reprod Fertil. 1980 Sep;60(1):231–236. doi: 10.1530/jrf.0.0600231. [DOI] [PubMed] [Google Scholar]
- Peri B. A., Rothberg R. M. Specific suppression of antibody production in young rabbit kits after maternal ingestion of bovine serum albumin. J Immunol. 1981 Dec;127(6):2520–2525. [PubMed] [Google Scholar]
- Rolls B. A. Dipeptidase activity in the small intestinal mucosa during pregnancy and lactation in the rat. Br J Nutr. 1975 Jan;33(1):1–9. doi: 10.1079/bjn19750003. [DOI] [PubMed] [Google Scholar]
- Rolls B. A., Henschel M. J., Palmer M. F. The effects of pregnancy and lactation on the activities of trypsin and alpha-chymotrypsin in the rat pancreas. Br J Nutr. 1979 May;41(3):573–578. doi: 10.1079/bjn19790072. [DOI] [PubMed] [Google Scholar]
- Troncone R., Ferguson A. In mice, gluten in maternal diet primes systemic immune responses to gliadin in offspring. Immunology. 1988 Jul;64(3):533–537. [PMC free article] [PubMed] [Google Scholar]
- Turner M. W., Boulton P., Shields J. G., Strobel S., Gibson S., Miller H. R., Levinsky R. J. Intestinal hypersensitivity reactions in the rat. I. Uptake of intact protein, permeability to sugars and their correlation with mucosal mast-cell activation. Immunology. 1988 Jan;63(1):119–124. [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Weaver L. T., Coombs R. R. Does 'sugar' permeability reflect macromolecular absorption? A comparison of the gastro-intestinal uptake of lactulose and beta-lactoglobulin in the neonatal guinea pig. Int Arch Allergy Appl Immunol. 1988;85(1):133–135. doi: 10.1159/000234490. [DOI] [PubMed] [Google Scholar]
- Wold A. E., Dahlgren U. I., Ahlstedt S., Hanson L. A. Lack of IgA antibody response in secretions of rat dams during long-term ovalbumin feeding. Induction of systemic tolerance in pups but not in adult rats. Int Arch Allergy Appl Immunol. 1987;84(4):332–338. doi: 10.1159/000234446. [DOI] [PubMed] [Google Scholar]



