Abstract
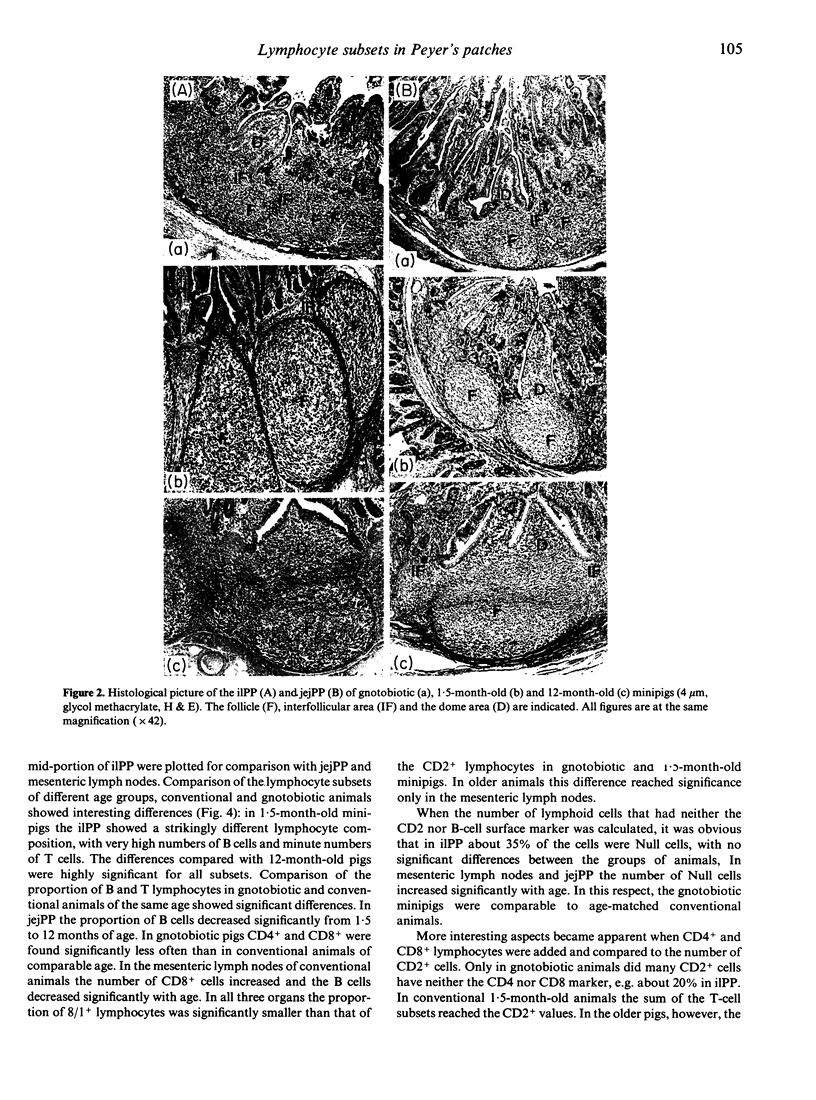
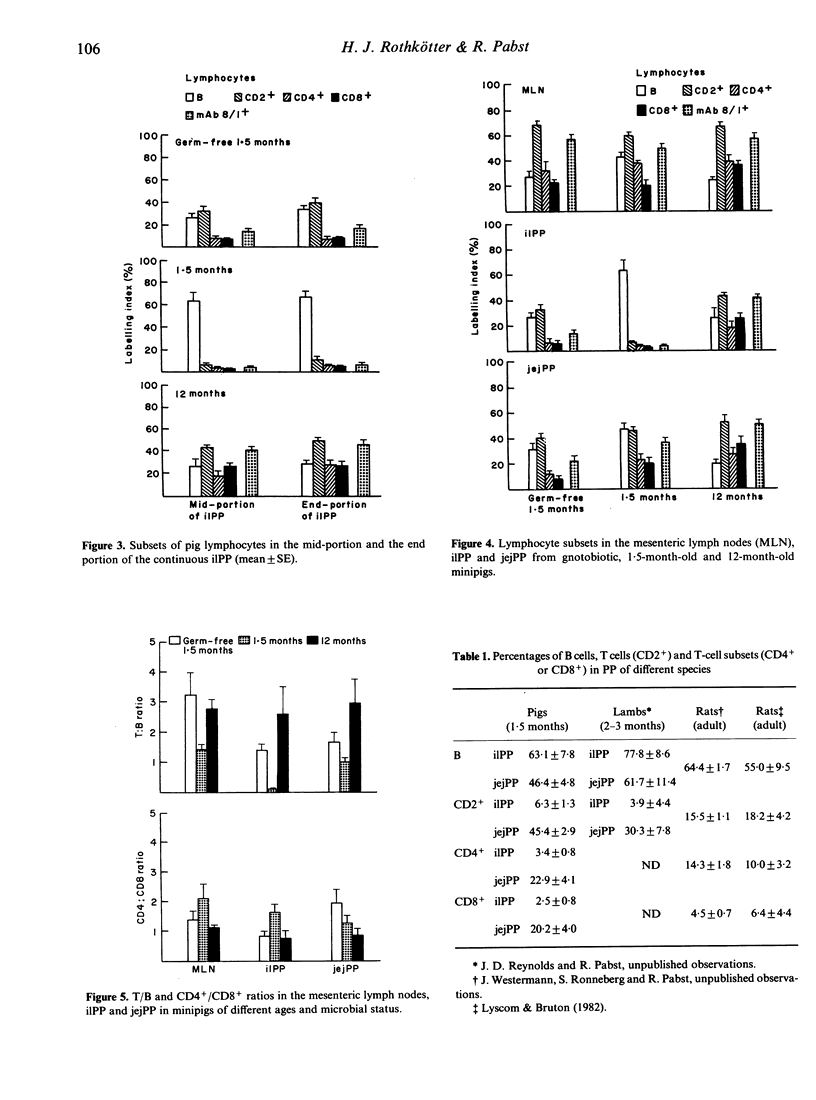
The size and location of Peyer's patches (PP) in the jejunum and ileum and their composition of lymphocyte subsets (B, CD2+, CD4+, CD8+) have been studied in conventional and gnotobiotic Göttingen minipigs. Each PP in the small intestine remained at the same site and was of comparable length between 2 and 12 months of age. In 1.5-month-old conventional minipigs the histology of the compartments differed between the continuous PP in the terminal ileum (ilPP) and the discrete PP in the jejunum (jejPP). No such difference was seen in gnotobiotic or in 12-month-old animals. The composition of lymphocyte subsets showed striking differences with significantly more B and less T, CD4+ and CD8+ cells in ilPP in 1.5-month-old minipigs in comparison with 12-month-olds. Mesenteric lymph nodes and jejPP displayed a typical pattern of lymphocyte subsets. The size of the lymphocyte compartments in PP and their cellular composition depends largely on age and microbial influences from the gut lumen, which might be of major importance for studies on the function of the gut-associated immune system in the pig.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen W. D., Porter P. The relative frequencies and distribution of immunoglobulin-bearing cells in the intestinal mucosa of neonatal and weaned pigs and their significance in the development of secretory immunity. Immunology. 1977 May;32(5):819–824. [PMC free article] [PubMed] [Google Scholar]
- Binns R. M., Licence S. T. Patterns of migration of labelled blood lymphocyte subpopulations: evidence for two types of Peyer's patch in the young pig. Adv Exp Med Biol. 1985;186:661–668. doi: 10.1007/978-1-4613-2463-8_81. [DOI] [PubMed] [Google Scholar]
- Brown P. J., Bourne F. J. Development of immunoglobulin-containing cell populations in intestine, spleen, and mesenteric lymph node of the young pig, as demonstrated by peroxidase-conjugated antiserums. Am J Vet Res. 1976 Nov;37(11):1309–1314. [PubMed] [Google Scholar]
- Butler J. E., Klobasa F., Werhahn E. The differential localization of IgA, IgM and IgG in the gut of suckled neonatal piglets. Vet Immunol Immunopathol. 1981 Feb;2(1):53–65. doi: 10.1016/0165-2427(81)90038-6. [DOI] [PubMed] [Google Scholar]
- Chu R. M., Glock R. D., Ross R. F. Changes in gut-associated lymphoid tissues of the small intestine of eight-week-old pigs infected with transmissible gastroenteritis virus. Am J Vet Res. 1982 Jan;43(1):67–76. [PubMed] [Google Scholar]
- Dziaba K. A., Lambrecht G., Petzoldt K. Intestinal and serum antibody response in gnotobiotic piglets to oral immunization with Escherichia coli. Comp Immunol Microbiol Infect Dis. 1985;8(3-4):267–272. doi: 10.1016/0147-9571(85)90005-0. [DOI] [PubMed] [Google Scholar]
- Gerber H. A., Morris B., Trevella W. The role of gut-associated lymphoid tissues in the generation of immunoglobulin-bearing lymphocytes in sheep. Aust J Exp Biol Med Sci. 1986 Jun;64(Pt 3):201–213. doi: 10.1038/icb.1986.22. [DOI] [PubMed] [Google Scholar]
- Jonjić N., Jonjić S., Saalmüller A., Rukavina D., Koszinowski U. H. Distribution of T-lymphocyte subsets in porcine lymphoid tissues. Immunology. 1987 Mar;60(3):395–401. [PMC free article] [PubMed] [Google Scholar]
- Jonjić S., Koszinowski U. H. Monoclonal antibodies reactive with swine lymphocytes. I. Antibodies to membrane structures that define the cytolytic T lymphocyte subset in the swine. J Immunol. 1984 Aug;133(2):647–652. [PubMed] [Google Scholar]
- Lyscom N., Brueton M. J. Intraepithelial, lamina propria and Peyer's patch lymphocytes of the rat small intestine: isolation and characterization in terms of immunoglobulin markers and receptors for monoclonal antibodies. Immunology. 1982 Apr;45(4):775–783. [PMC free article] [PubMed] [Google Scholar]
- Opstelten D., Deenen G. J., Rozing J., Hunt S. V. B lymphocyte-associated antigens on terminal deoxynucleotidyl transferase-positive cells and pre-B cells in bone marrow of the rat. J Immunol. 1986 Jul 1;137(1):76–84. [PubMed] [Google Scholar]
- Pabst R., Geist M., Rothkötter H. J., Fritz F. J. Postnatal development and lymphocyte production of jejunal and ileal Peyer's patches in normal and gnotobiotic pigs. Immunology. 1988 Jul;64(3):539–544. [PMC free article] [PubMed] [Google Scholar]
- Pabst R. The anatomical basis for the immune function of the gut. Anat Embryol (Berl) 1987;176(2):135–144. doi: 10.1007/BF00310046. [DOI] [PubMed] [Google Scholar]
- Pescovitz M. D., Lunney J. K., Sachs D. H. Murine anti-swine T4 and T8 monoclonal antibodies: distribution and effects on proliferative and cytotoxic T cells. J Immunol. 1985 Jan;134(1):37–44. [PubMed] [Google Scholar]
- Pescovitz M. D., Lunney J. K., Sachs D. H. Preparation and characterization of monoclonal antibodies reactive with porcine PBL. J Immunol. 1984 Jul;133(1):368–375. [PubMed] [Google Scholar]
- Reynolds J. D. Peyer's patches and the early development of B lymphocytes. Curr Top Microbiol Immunol. 1987;135:43–56. doi: 10.1007/978-3-642-71851-9_3. [DOI] [PubMed] [Google Scholar]
- Reynolds J., Pabst R., Bordmann G. Evidence for the existence of two distinct types of Peyer's patches in sheep. Adv Exp Med Biol. 1985;186:101–109. doi: 10.1007/978-1-4613-2463-8_12. [DOI] [PubMed] [Google Scholar]
- Saalmüller A., Jonjic S., Bühring H. J., Reddehase M. J., Koszinowski U. H. Monoclonal antibodies reactive with swine lymphocytes. II. Detection of an antigen on resting T cells down-regulated after activation. J Immunol. 1987 Mar 15;138(6):1852–1857. [PubMed] [Google Scholar]
- Tykkä H. T., Vaittinen E. J., Mahlberg K. L., Railo J. E., Pantzar P. J., Sarna S., Tallberg T. A randomized double-blind study using CaNa2EDTA, a phospholipase A2 inhibitor, in the management of human acute pancreatitis. Scand J Gastroenterol. 1985 Jan;20(1):5–12. doi: 10.3109/00365528509089625. [DOI] [PubMed] [Google Scholar]
- Tzipori S., Robins-Browne R. M., Gonis G., Hayes J., Withers M., McCartney E. Enteropathogenic Escherichia coli enteritis: evaluation of the gnotobiotic piglet as a model of human infection. Gut. 1985 Jun;26(6):570–578. doi: 10.1136/gut.26.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. D., Stokes C. R., Bourne F. J. Morphology and functional characteristics of isolated porcine intraepithelial lymphocytes. Immunology. 1986 Sep;59(1):109–113. [PMC free article] [PubMed] [Google Scholar]