Abstract
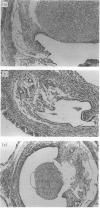
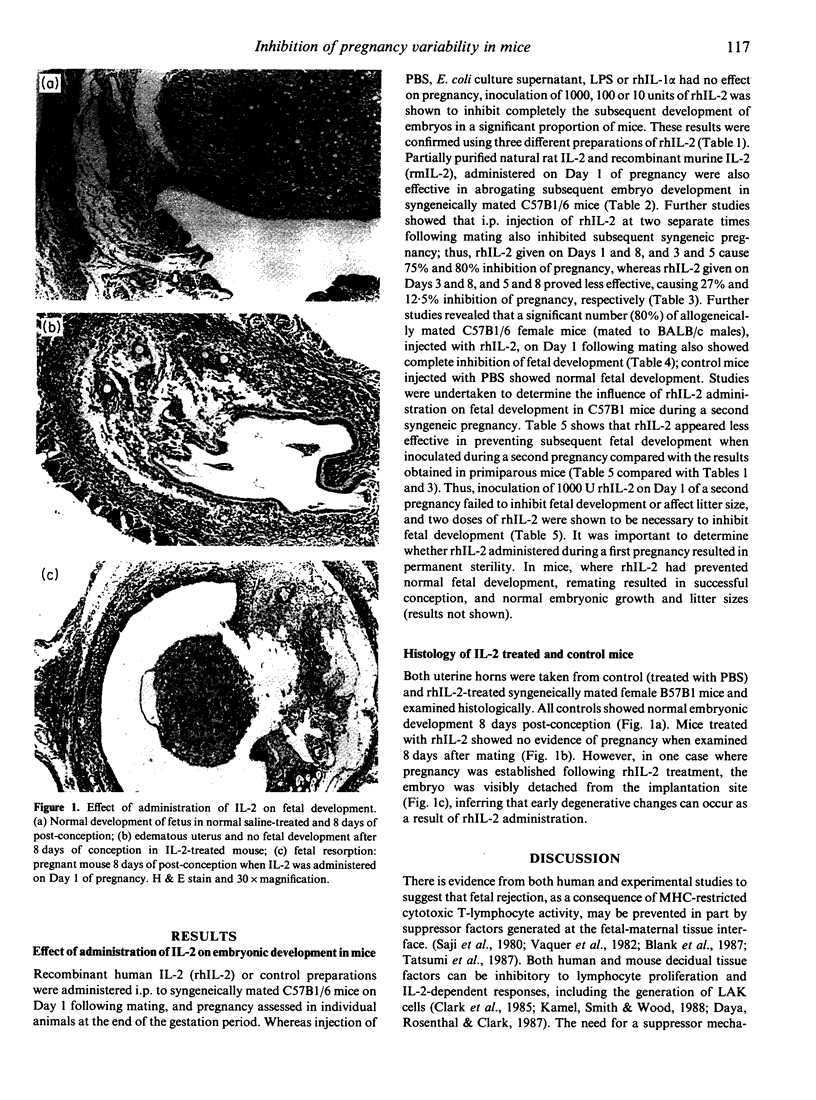
The influence of administration of interleukin-2 (IL-2) on syngeneic and allogeneic murine pregnancy has been investigated. Human or mouse recombinant IL-2 (rhIL-2 and rmIL-2), or partially purified rat IL-2, was inoculated i.p. into C57B1 mice following syngeneic mating but before embryo implantation. This inhibited subsequent fetal development in up to 100% of cases, compared with mice inoculated with control material, including recombinant human interleukin-1 alpha (IL-1 alpha), where no inhibition of pregnancy viability was observed. Similar data were obtained in both syngeneic and allogeneic matings when rhIL-2 was administered on Day 1 of pregnancy. Administration of rhIL-2 during the second pregnancy, rather than a first pregnancy, was less effective. Administration of rhIL-2 during the first pregnancy does not induce a permanent sterility. Histological examination of the endometrium further demonstrated that mice injected with rhIL-2 on Day 1 of their first pregnancy showed a complete absence of embryonic tissue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames I. H., Gates C. E., Garcia A. M., John P. A., Hennig A. K., Tomar R. H. Lysis of fresh murine mammary tumor cells by syngeneic natural killer cells and lymphokine-activated killer cells. Cancer Immunol Immunother. 1987;25(3):161–168. doi: 10.1007/BF00199142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M., Nebel L., Toder V. Trophoblast does not cause the cytotoxic T lymphocyte generation due to the lack of ability to stimulate interleukin-2 production. Am J Reprod Immunol Microbiol. 1987 Jun;14(2):49–53. doi: 10.1111/j.1600-0897.1987.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Bulmer J. N., Johnson P. M. The T-lymphocyte population in first-trimester human decidua does not express the interleukin-2 receptor. Immunology. 1986 Aug;58(4):685–687. [PMC free article] [PubMed] [Google Scholar]
- Campion P. D., Currey H. L. Cell-mediated immunity in pregnancy. Lancet. 1972 Oct 14;2(7781):830–830. doi: 10.1016/s0140-6736(72)92201-5. [DOI] [PubMed] [Google Scholar]
- Cheever M. A., Greenberg P. D., Irle C., Thompson J. A., Urdal D. L., Mochizuki D. Y., Henney C. S., Gillis S. Interleukin 2 administered in vivo induces the growth of cultured T cells in vivo. J Immunol. 1984 May;132(5):2259–2265. [PubMed] [Google Scholar]
- Clark D. A., Chaput A., Walker C., Rosenthal K. L. Active suppression of host-vs-graft reaction in pregnant mice. VI. Soluble suppressor activity obtained from decidua of allopregnant mice blocks the response to IL 2. J Immunol. 1985 Mar;134(3):1659–1664. [PubMed] [Google Scholar]
- Combe B., Pope R. M., Fischbach M., Darnell B., Baron S., Talal N. Interleukin-2 in rheumatoid arthritis: production of and response to interleukin-2 in rheumatoid synovial fluid, synovial tissue and peripheral blood. Clin Exp Immunol. 1985 Mar;59(3):520–528. [PMC free article] [PubMed] [Google Scholar]
- Daya S., Rosenthal K. L., Clark D. A. Immunosuppressor factor(s) produced by decidua-associated suppressor cells: a proposed mechanism for fetal allograft survival. Am J Obstet Gynecol. 1987 Feb;156(2):344–350. doi: 10.1016/0002-9378(87)90281-x. [DOI] [PubMed] [Google Scholar]
- Ettinghausen S. E., Rosenberg S. A. Immunotherapy of murine sarcomas using lymphokine activated killer cells: optimization of the schedule and route of administration of recombinant interleukin-2. Cancer Res. 1986 Jun;46(6):2784–2792. [PubMed] [Google Scholar]
- Farrar J. J., Benjamin W. R., Hilfiker M. L., Howard M., Farrar W. L., Fuller-Farrar J. The biochemistry, biology, and role of interleukin 2 in the induction of cytotoxic T cell and antibody-forming B cell responses. Immunol Rev. 1982;63:129–166. doi: 10.1111/j.1600-065x.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Gupta S., Gillis S., Thornton M., Goldberg M. Autologous mixed lymphocyte reaction in man. XIV. Deficiency of the autologous mixed lymphocyte reaction in acquired immune deficiency syndrome (AIDS) and AIDS related complex (ARC). In vitro effect of purified interleukin-1 and interleukin-2. Clin Exp Immunol. 1984 Nov;58(2):395–401. [PMC free article] [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Hill J. A., Haimovici F., Anderson D. J. Products of activated lymphocytes and macrophages inhibit mouse embryo development in vitro. J Immunol. 1987 Oct 1;139(7):2250–2254. [PubMed] [Google Scholar]
- Kasakura S. A factor in maternal plasma during pregnancy that suppresses the reactivity of mixed leukocyte cultures. J Immunol. 1971 Nov;107(5):1296–1301. [PubMed] [Google Scholar]
- Kim B., Rosenstein M., Weiland D., Eberlein T. J., Rosenberg S. A. Clonal analysis of the lymphoid cells mediating skin allograft rejection. Mediation of graft rejection in vivo by cloned Lyt-1+2- proliferative, noncytotoxic long-term cell lines. Transplantation. 1983 Nov;36(5):525–532. doi: 10.1097/00007890-198311000-00011. [DOI] [PubMed] [Google Scholar]
- Nakayama E., Asano S., Kodo H., Miwa S. Suppression of mixed lymphocyte reaction by cells of human first trimester pregnancy endometrium. J Reprod Immunol. 1985 Aug;8(1):25–31. doi: 10.1016/0165-0378(85)90075-0. [DOI] [PubMed] [Google Scholar]
- Nicholas N. S., Panayi G. S. Inhibition of interleukin-2 production by retroplacental sera: a possible mechanism for human fetal allograft survival. Am J Reprod Immunol Microbiol. 1985 Sep;9(1):6–11. doi: 10.1111/j.1600-0897.1985.tb00332.x. [DOI] [PubMed] [Google Scholar]
- Nicholas N. S., Panayi G. S., Nouri A. M. Human pregnancy serum inhibits interleukin-2 production. Clin Exp Immunol. 1984 Dec;58(3):587–595. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Mulé J. J., Spiess P. J., Reichert C. M., Schwarz S. L. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985 May 1;161(5):1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji F., Koyama M., Kameda T., Negoro T., Nakamuro K., Tanizawa O. Effect of a soluble factor secreted from cultured human trophoblast cells on in vitro lymphocyte reactions. Am J Reprod Immunol Microbiol. 1987 Apr;13(4):121–124. doi: 10.1111/j.1600-0897.1987.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Salup R. R., Mathieson B. J., Wiltrout R. H. Precursor phenotype of lymphokine-activated killer cells in the mouse. J Immunol. 1987 Jun 1;138(11):3635–3639. [PubMed] [Google Scholar]
- Stimson W. H., Blackstock J. C. Identification of an immunosuppressive factor in pregnancy serum. Obstet Gynecol. 1976 Sep;48(3):305–311. [PubMed] [Google Scholar]
- Tatsumi K., Mori T., Mori E., Kanzaki H., Mori T. Immunoregulatory factor released from a cell line derived from human decidual tissue. Am J Reprod Immunol Microbiol. 1987 Mar;13(3):87–92. doi: 10.1111/j.1600-0897.1987.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Vaquer S., de la Hera A., Jordá J., Martínez C., Escudero M., Alvarez-Mon M. Diminished natural killer activity in pregnancy: modulation by interleukin 2 and interferon gamma. Scand J Immunol. 1987 Dec;26(6):691–698. doi: 10.1111/j.1365-3083.1987.tb02305.x. [DOI] [PubMed] [Google Scholar]
- Wagner H., Hardt C., Heeg K., Röllinghoff M., Pfizenmaier K. T-cell-derived helper factor allows in vivo induction of cytotoxic T cells in nu/nu mice. Nature. 1980 Mar 20;284(5753):278–278. doi: 10.1038/284278a0. [DOI] [PubMed] [Google Scholar]