Abstract
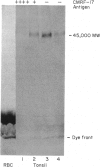
The identification of membrane molecules expressed on subpopulations of B lymphocytes is of potential significance because these molecules may be candidates for regulating the activation, proliferation and differentiation of B cells. A new monoclonal antibody, CMRF-17, which reacts with a subpopulation of tonsil B lymphocytes has been produced. The antibody did not react with T lymphocytes in tonsil or peripheral blood nor most peripheral blood B lymphocytes but did label erythrocytes and some platelets. In tonsil, the germinal centre cells, cells in the interfollicular region and endothelial cells were positive, but mantle zone B cells were negative. Double labelling experiments showed that CMRF-17 reacted with activated tonsillar lymphocytes. The antigen recognized by CMRF-17 was heat stable, resistant to treatment with proteolytic enzymes and neuraminidase and was shown to be a carbohydrate determinant on one or more glycolipids. These characteristics of the antigen recognized by CMRF-17 and its pattern of reactivity distinguish this antibody from other monoclonal antibodies recognizing B-cell activation markers. It was notable that of the B-lymphoid malignancies tested to date, including those of probable follicular origin, few stained with CMRF-17.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. E., McKenzie J. L., McLoughlin K., Beard M. E., Hart D. N. The inheritance of abnormal sialoglycoproteins found in a Gerbich negative individual. Pathology. 1986 Oct;18(4):407–412. doi: 10.3109/00313028609087560. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T., Childs R. A. Carbohydrates as antigenic determinants of glycoproteins. Biochem J. 1987 Jul 1;245(1):1–11. doi: 10.1042/bj2450001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman A. S., Freeman G., Horowitz J. C., Daley J., Nadler L. M. B7, a B-cell-restricted antigen that identifies preactivated B cells. J Immunol. 1987 Nov 15;139(10):3260–3267. [PubMed] [Google Scholar]
- Fyfe G., Cebra-Thomas J. A., Mustain E., Davie J. M., Alley C. D., Nahm M. H. Subpopulations of B lymphocytes in germinal centers. J Immunol. 1987 Oct 1;139(7):2187–2194. [PubMed] [Google Scholar]
- Golay J. T., Crawford D. H. Pathways of human B-lymphocyte activation blocked by B-cell specific monoclonal antibodies. Immunology. 1987 Oct;62(2):279–284. [PMC free article] [PubMed] [Google Scholar]
- Gruner K. R., van Eijk R. V., Mühlradt P. F. Structure elucidation of marker glycolipids of alloantigen-activated murine T lymphocytes. Biochemistry. 1981 Aug 4;20(16):4518–4522. doi: 10.1021/bi00519a002. [DOI] [PubMed] [Google Scholar]
- Hart D. N., Beard M. E., Hamer J. W., Heaton D. C., Neville M. A., Southern M. A cytological analysis of FMC-7 positive leukaemias. Hematol Oncol. 1986 Jul-Sep;4(3):205–212. doi: 10.1002/hon.2900040304. [DOI] [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Major histocompatibility complex antigens in rat kidney, ureter, and bladder. Localization with monoclonal antibodies and demonstration of Ia-positive dendritic cells. Transplantation. 1981 May;31(5):318–325. doi: 10.1097/00007890-198105010-00003. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Hjelmeland L. M. A nondenaturing zwitterionic detergent for membrane biochemistry: design and synthesis. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6368–6370. doi: 10.1073/pnas.77.11.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman F. M., Yanagihara E., Byrne B., Billing R., Baird S., Frisman D., Taylor C. R. Analysis of B-cell antigens in normal reactive lymphoid tissue using four B-cell monoclonal antibodies. Blood. 1983 Oct;62(4):775–783. [PubMed] [Google Scholar]
- Jelinek D. F., Lipsky P. E. Regulation of human B lymphocyte activation, proliferation, and differentiation. Adv Immunol. 1987;40:1–59. doi: 10.1016/s0065-2776(08)60237-0. [DOI] [PubMed] [Google Scholar]
- Jung L. K., Fu S. M. Selective inhibition of growth factor-dependent human B cell proliferation by monoclonal antibody AB1 to an antigen expressed by activated B cells. J Exp Med. 1984 Dec 1;160(6):1919–1924. doi: 10.1084/jem.160.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R., Levine P., Watanabe K., Hakomori S. Recent studies of glycolipid and glycoprotein profiles and characterization of the major glycolipid antigen in gastric cancer of a patient of blood group genotype pp (Tja-) first studied in 1951. Cancer Res. 1982 Dec;42(12):5249–5254. [PubMed] [Google Scholar]
- Kikutani H., Kimura R., Nakamura H., Sato R., Muraguchi A., Kawamura N., Hardy R. R., Kishimoto T. Expression and function of an early activation marker restricted to human B cells. J Immunol. 1986 Jun 1;136(11):4019–4026. [PubMed] [Google Scholar]
- Kokai Y., Ishii Y., Kikuchi K. Characterization of two distinct antigens expressed on either resting or activated human B cells as defined by monoclonal antibodies. Clin Exp Immunol. 1986 May;64(2):382–391. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murray L. J., Habeshaw J. A., Wiels J., Greaves M. F. Expression of Burkitt lymphoma-associated antigen (defined by the monoclonal antibody 38.13) on both normal and malignant germinal-centre B cells. Int J Cancer. 1985 Nov 15;36(5):561–565. doi: 10.1002/ijc.2910360508. [DOI] [PubMed] [Google Scholar]
- Schwarting G. A. Quantitative analysis of neutral glycosphingolipids from human lymphocyte subpopulations. Biochem J. 1980 Sep 1;189(3):407–412. doi: 10.1042/bj1890407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Sanders S. K., Butler J. L., Gartland G. L., Komiyama K., Cooper M. D. Identification of an early activation antigen (Bac-1) on human B cells. J Immunol. 1986 Aug 15;137(4):1208–1213. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Schooley R. T., Bhan A. K., Nadler L. M. Epstein-Barr virus superinduces a new human B cell differentiation antigen (B-LAST 1) expressed on transformed lymphoblasts. Cell. 1982 Sep;30(2):415–425. doi: 10.1016/0092-8674(82)90239-2. [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Azzo W., Al-Katib A., Chiorazzi N., Knowles D. M., 2nd Preparation and characterization of monoclonal antibodies recognizing three distinct differentiation antigens (BL1, BL2, BL3) on human B lymphocytes. J Immunol. 1984 Aug;133(2):684–691. [PubMed] [Google Scholar]
- Yokochi T., Holly R. D., Clark E. A. B lymphoblast antigen (BB-1) expressed on Epstein-Barr virus-activated B cell blasts, B lymphoblastoid cell lines, and Burkitt's lymphomas. J Immunol. 1982 Feb;128(2):823–827. [PubMed] [Google Scholar]
- Zola H., Moore H. A., Hohmann A., Hunter I. K., Nikoloutsopoulos A., Bradley J. The antigen of mature human B cells detected by the monoclonal antibody FMC7: studies on the nature of the antigen and modulation of its expression. J Immunol. 1984 Jul;133(1):321–326. [PubMed] [Google Scholar]
- von dem Borne A. E., Bos M. J., Joustra-Maas N., Tromp J. F., van't Veer M. B., van Wijngaarden-du Bois R., Tetteroo P. A. A murine monoclonal IgM antibody specific for blood group P antigen (globoside) Br J Haematol. 1986 May;63(1):35–46. doi: 10.1111/j.1365-2141.1986.tb07492.x. [DOI] [PubMed] [Google Scholar]