Abstract
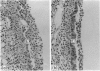
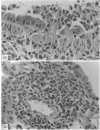
Previous studies have shown that influenza nucleoprotein (NP)-specific cytotoxic T-cell clones do not prevent influenza infection of mice but lead to a more rapid viral clearance and recovery of the host. Here we examine the histology of the lung to see if viral clearance by cytotoxic T cells (Tc) correlates with recovery of pulmonary pathology or if it is in any way deleterious. Intransasal (i.n.) A/X31 virus infection of BALB/c mice produces lung tissue changes lasting 8-10 days in BALB/c mice, with the most severe abnormalities appearing between Days 4 and 6 (e.g. loss of epithelium, airway obliteration, peribronchiolar and perivascular cell accumulation). The transfer of Tc clone T9/13 into i.n.-infected BALB/c mice induces a transient enhanced loss of epithelium on Day 4, while by Day 6 epithelial abnormalities are much reduced in the lung compared to control infected mice. This correlates with a significant reduction in lung virus titres by Day 6; by Day 8 virus is cleared in Tc recipients and lung histology is normal. Another Tc clone (T5/5) with greater cytolytic activity resulted in significant recovery of the lung tissues by Day 4. Tc clones also resulted in enhanced perivascular infiltration of cells and variation in the infiltrating cell type. Quantification in our system required careful attention to the level of the airway assessed. These histological findings showing an enhanced tissue recovery support the previous assessment of reduced lung viral levels following the transfer of Tc cells, and show that a transient increase in lung pathology can occur.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askonas B. A., Taylor P. M., Esquivel F. Cytotoxic T cells in influenza infection. Ann N Y Acad Sci. 1988;532:230–237. doi: 10.1111/j.1749-6632.1988.tb36342.x. [DOI] [PubMed] [Google Scholar]
- Baenziger J., Hengartner H., Zinkernagel R. M., Cole G. A. Induction or prevention of immunopathological disease by cloned cytotoxic T cell lines specific for lymphocytic choriomeningitis virus. Eur J Immunol. 1986 Apr;16(4):387–393. doi: 10.1002/eji.1830160413. [DOI] [PubMed] [Google Scholar]
- Bodmer H. C., Pemberton R. M., Rothbard J. B., Askonas B. A. Enhanced recognition of a modified peptide antigen by cytotoxic T cells specific for influenza nucleoprotein. Cell. 1988 Jan 29;52(2):253–258. doi: 10.1016/0092-8674(88)90514-4. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus. VI. Migration and activity in vivo in acute and persistent infection. J Immunol. 1986 Jan;136(2):698–704. [PubMed] [Google Scholar]
- CURTIS A. S. Area and volume measurements by random sampling methods. Med Biol Illus. 1960 Oct;10:261–266. [PubMed] [Google Scholar]
- Cannon M. J., Openshaw P. J., Askonas B. A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988 Sep 1;168(3):1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU C. Studies of influenza infection in ferrets by means of fluorescein-labelled antibody. I. The pathogenesis and diagnosis of the disease. J Exp Med. 1955 Jun 1;101(6):665–676. doi: 10.1084/jem.101.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacher A. E., Braciale V. L., Braciale T. J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984 Sep 1;160(3):814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M. R., Lukacher A. E., Braciale V. L., Braciale T. J., Bienenstock J. Characterization and in vivo distribution of influenza-virus-specific T-lymphocytes in the murine respiratory tract. Am Rev Respir Dis. 1987 Jan;135(1):245–249. doi: 10.1164/arrd.1987.135.1.245. [DOI] [PubMed] [Google Scholar]
- Taylor P. M., Askonas B. A. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986 Jul;58(3):417–420. [PMC free article] [PubMed] [Google Scholar]
- Taylor P. M., Davey J., Howland K., Rothbard J. B., Askonas B. A. Class I MHC molecules rather than other mouse genes dictate influenza epitope recognition by cytotoxic T cells. Immunogenetics. 1987;26(4-5):267–272. doi: 10.1007/BF00346521. [DOI] [PubMed] [Google Scholar]
- Wells M. A., Ennis F. A., Albrecht P. Recovery from a viral respiratory infection. II. Passive transfer of immune spleen cells to mice with influenza pneumonia. J Immunol. 1981 Mar;126(3):1042–1046. [PubMed] [Google Scholar]
- Yetter R. A., Lehrer S., Ramphal R., Small P. A., Jr Outcome of influenza infection: effect of site of initial infection and heterotypic immunity. Infect Immun. 1980 Aug;29(2):654–662. doi: 10.1128/iai.29.2.654-662.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Welsh R. M. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976 Nov;117(5 Pt 1):1495–1502. [PubMed] [Google Scholar]