Abstract
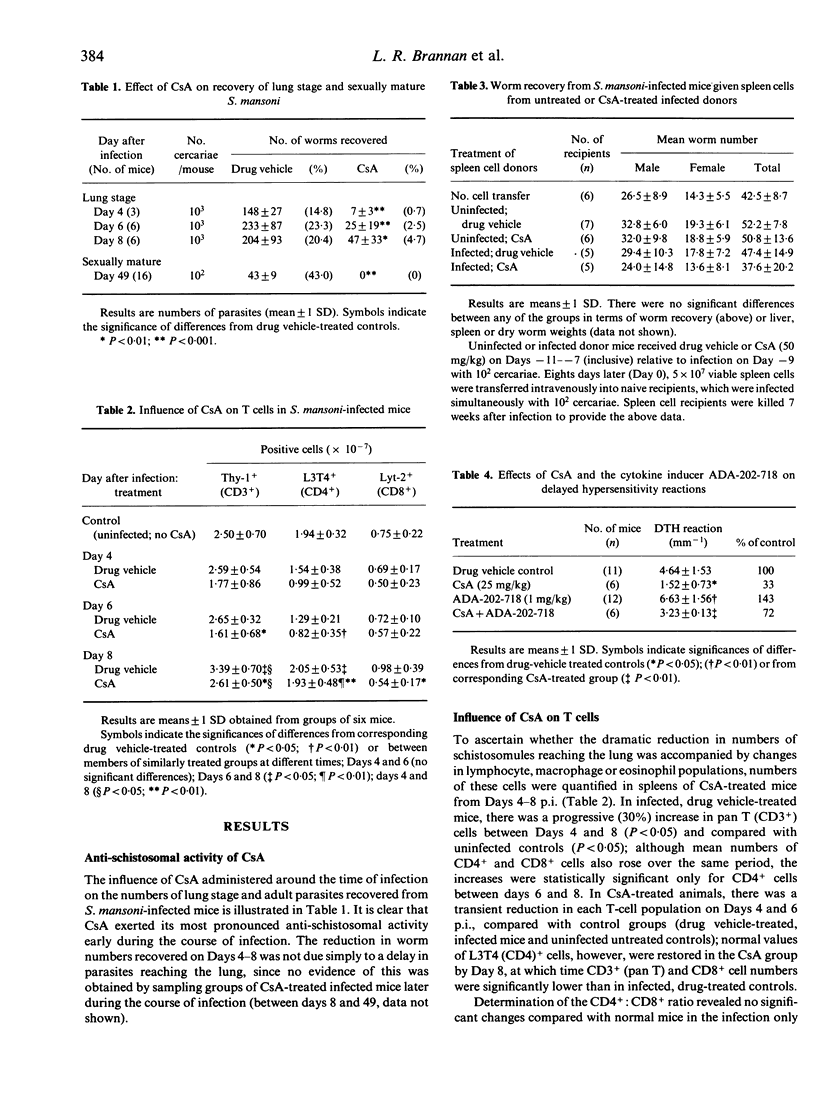
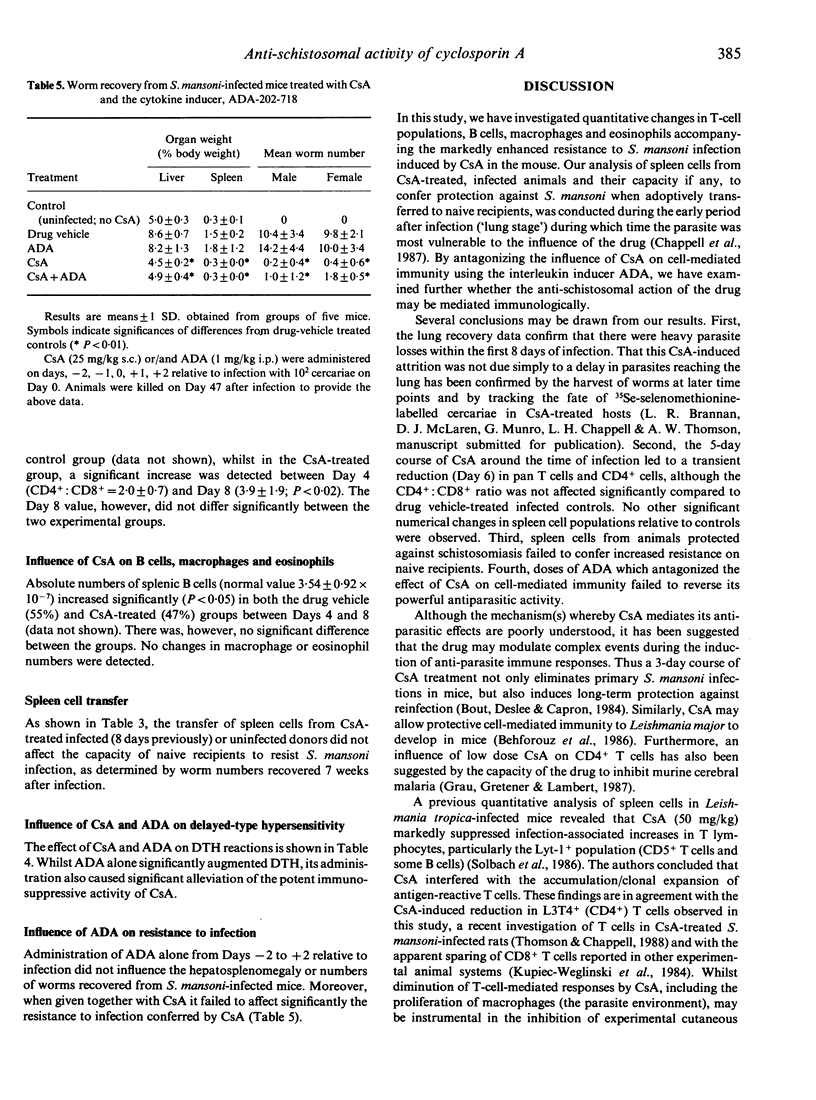
Mice were treated with 5-day courses of cyclosporin A (CsA) around the time of infection with Schistosoma mansoni. Recovery of lung-stage worms 4-8 days post-infection (p.i.) was substantially reduced (80%) and no sexually mature adults were recovered from the hepatic portal system at 7 weeks p.i. Flow cytometric analysis of spleen cells from CsA-treated animals during the period of maximal parasite attrition revealed transient reductions in CD3+ and CD4+ cells and in the CD4+: CD8+ ratio compared with drug vehicle-treated, infected controls. No significant numerical changes in B cells, macrophages or eosinophils were detected relative to vehicle-treated infected mice. Transfer of spleen cells from CsA-treated donors 8 days after infection failed to confer increased resistance to S. mansoni infection on untreated recipients. Moreover, concomitant administration of CsA and an inducer of interleukin-2 production (ADA-202-718) did not interfere with the anti-schistosomal effect of CsA. Despite our incomplete understanding of the in vivo properties of CsA and reports of its paradoxical effects on immune responses, these new data indicate that the influence of CsA in schistosomiasis is unlikely to be mediated by modulation of host cell mediated immunity. This contrasts with certain other anti-parasitic effects of CsA which appear to be mediated by an action on T cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bout D. T., Deslee D., Capron A. R. Protection against schistosomiasis produced by cyclosporin A. Am J Trop Med Hyg. 1984 Jan;33(1):185–186. doi: 10.4269/ajtmh.1984.33.185. [DOI] [PubMed] [Google Scholar]
- Bout D., Deslèe D., Capron A. Antischistosomal effect of cyclosporin A: cure and prevention of mouse and rat schistosomiasis mansoni. Infect Immun. 1986 Jun;52(3):823–827. doi: 10.1128/iai.52.3.823-827.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida M., Knop J. Effect of cyclosporin A on the T-effector and T-suppressor cell response in contact sensitivity. Immunology. 1986 Dec;59(4):503–507. [PMC free article] [PubMed] [Google Scholar]
- Chappell L. H., Thomson A. W., Barker G. C., Smith S. W. Dosage, timing, and route of administration of cyclosporin A and nonimmunosuppressive derivatives of dihydrocyclosporin A and cyclosporin C against Schistosoma mansoni in vivo and in vitro. Antimicrob Agents Chemother. 1987 Oct;31(10):1567–1571. doi: 10.1128/aac.31.10.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier A., Tutschka P. J., Farmer E. R., Santos G. W. Graft-versus-host disease in cyclosporin A-treated rats after syngeneic and autologous bone marrow reconstitution. J Exp Med. 1983 Jul 1;158(1):1–8. doi: 10.1084/jem.158.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Gretener D., Lambert P. H. Prevention of murine cerebral malaria by low-dose cyclosporin A. Immunology. 1987 Aug;61(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Hess A. D., Tutschka P. J., Santos G. W. Effect of cyclosporin A on human lymphocyte responses in vitro. III. CsA inhibits the production of T lymphocyte growth factors in secondary mixed lymphocyte responses but does not inhibit the response of primed lymphocytes to TCGF. J Immunol. 1982 Jan;128(1):355–359. [PubMed] [Google Scholar]
- Hiestand P. C., Strasser M. Immunostimulatory properties of ethylene-2,2' -bis(dithio)bis(ethanol) and related compounds in vivo. Int J Immunopharmacol. 1985;7(1):141–151. doi: 10.1016/0192-0561(85)90019-0. [DOI] [PubMed] [Google Scholar]
- Kaibara N., Hotokebuchi T., Takagishi K., Katsuki I. Paradoxical effects of cyclosporin A on collagen arthritis in rats. J Exp Med. 1983 Dec 1;158(6):2007–2015. doi: 10.1084/jem.158.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh L. C., Morrice L. M., Udagawa Y., Thomson A. W. Influence of tumour carriage on the production of lymphokines affecting macrophage behaviour. Immunology. 1986 Mar;57(3):359–365. [PMC free article] [PubMed] [Google Scholar]
- Schiltknecht E., Ada G. L. The generation of effector T cells in influenza A-infected, cyclosporine A-treated mice. Cell Immunol. 1985 Oct 15;95(2):340–348. doi: 10.1016/0008-8749(85)90321-1. [DOI] [PubMed] [Google Scholar]
- Smithers S. R., Gammage K. Recovery of Schistosoma mansoni from the skin, lungs and hepatic portal system of naive mice and mice previously exposed to S. mansoni: evidence for two phases of parasite attrition in immune mice. Parasitology. 1980 Apr;80(2):289–300. doi: 10.1017/s0031182000000755. [DOI] [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965 Nov;55(4):695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- Solbach W., Forberg K., Kammerer E., Bogdan C., Röllinghoff M. Suppressive effect of cyclosporin A on the development of Leishmania tropica-induced lesions in genetically susceptible BALB/c mice. J Immunol. 1986 Jul 15;137(2):702–707. [PubMed] [Google Scholar]
- Thomson A. W., Chappell L. H. Immunophenotypic analysis of blood and spleen lymphocyte subsets in rats protected against schistosomiasis by cyclosporin A. Immunol Lett. 1988 Feb;17(2):169–172. doi: 10.1016/0165-2478(88)90086-7. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Milton J. I., Aldridge R. D., Davidson R. J., Simpson J. G. Inhibition of drug-induced eosinophilia by cyclosporin A. Scand J Immunol. 1986 Aug;24(2):163–170. doi: 10.1111/j.1365-3083.1986.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Moon D. K., Inoue Y., Geczy C. L., Nelson D. S. Modification of delayed-type hypersensitivity reactions to ovalbumin in cyclosporin A-treated guinea-pigs. Immunology. 1983 Feb;48(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- Thomson A. W., Smith S. W., Chappell L. H. Cyclosporin A: immune suppressant and antiparasitic agent. Parasitol Today. 1986 Oct;2(10):288–290. doi: 10.1016/0169-4758(86)90141-9. [DOI] [PubMed] [Google Scholar]
- Webster L. M., Thomson A. W. Augmentation of delayed-type hypersensitivity to high dose sheep erythrocytes by cyclosporin A in the mouse: influence of drug dosage and route of administration and analysis of spleen cell populations. Clin Exp Immunol. 1988 Jan;71(1):149–154. [PMC free article] [PubMed] [Google Scholar]


