Abstract
Essential micronutrient selenium is excreted into the urine and/or expired after being transformed to methylated metabolites. Monomethylated selenium is excreted into the urine in response to a supply within the required to low-toxic range, whereas tri- and dimethylated selenium increase with excessive supply at a toxic dose. Here we show that the major urinary selenium metabolite within the required to low-toxic range is a selenosugar. The structure of 1β-methylseleno-N-acetyl-d-galactosamine was deduced from the spectroscopic data and confirmed by chemical synthesis. This metabolite was also detected in the liver, and an additional metabolite increased with inhibition of methylation. The latter metabolite was again a selenosugar conjugated with glutathione instead of a methyl group and was assumed to be a precursor for methylation to the former metabolite. A metabolic pathway for the urinary excretion of selenium, i.e., from the glutathione-S-conjugated selenosugar to the methylated one, was proposed. Urinary monomethylated (selenosugar) and trimethylated selenium can be used as specific indices that increase within the required to low-toxic range and with a distinct toxic dose, respectively.
Selenium (Se) is an ultra-trace element indispensable for normal functioning of the body (1, 2). However, it is also known to be highly toxic, and the boundary between adequate and toxic levels is extremely narrow. The nutritional supply of Se depends on the concentration of Se in foods, and populations inhabiting areas with a low or high concentration of it in soil are at risk as to a deficiency or excess of it worldwide (3, 4). In addition, Se is becoming popular as a supplement for a nutritional demand (5) and also for preventing cancer (6).
Both inorganic (selenite and selenate) and organic forms of Se (selenocysteine and selenomethionine) can be used as nutritional sources, the former being reduced to the assumed intermediate, selenide (7), whereas the latter are transformed to the same assumed intermediate by lyase (8, 9). Selenide is assumed to be used for the synthesis of selenoproteins by the specific codon for selenocysteine (10, 11). Se released from degraded selenoproteins and surplus Se are excreted after being methylated to monomethylated Se, dimethylated Se, and trimethylated Se (12, 13). Thus, selenide is assumed not only to be a common intermediate but also a checkpoint intermediate for utilization for selenoprotein synthesis and for the excretion of methylated Se (14).
Monomethylated Se is excreted as the major form into the urine with deficient to low-toxic doses of Se. When monomethylated Se reaches a plateau in the urine, trimethylated Se in the urine and expired dimethylated Se increase (12, 13). Although monomethylated Se has been believed to be methylselenol, doubts as to its structure in relation to the reactive selenol group have been raised. Meanwhile, we have made several unpublished findings regarding the urinary Se metabolite and its relation with Se metabolites in the liver.
The HPLC/inductively coupled argon plasma mass-spectrometry (ICP-MS) and HPLC/electrospray ionization tandem mass-spectrometry (ESI-MS/MS) methods have been used efficiently for the speciation of bio-trace elements (15, 16), and our recent work indicated that the major urinary metabolite (hepatic metabolite B) is a selenosugar, Se-methyl-N-acetylselenohexosamine (17). In the present study, we determined the chemical structures of two selenosugars and proposed their biological roles as intermediates in the metabolism of Se leading to urinary excretion.
Experimental Procedures
Apparatus.
ICP-MS (HP4500, Yokogawa Analytical Systems, Musashino, Japan), HPLC (PU 610, GL Sciences, Tokyo), 1H (600-MHz) and 13C NMR (150-MHz, JEOL JNM-ECP 600, Tokyo), and ESI-MS/MS (API3000, Applied Biosystems- Tokyo) were performed under standard conditions.
Partial Purification of the Major Urinary Metabolite.
Male rats of the Wistar strain, 8 weeks of age, were fed a standard diet (CE-2, CLEA Japan, Osaka) and water containing sodium selenite at a concentration of 2 μg of Se per ml, and 24-h urine was collected with a metabolic cage. A 325-ml portion of the Se-enriched urine specimen (2.0 μg of Se per ml) was incubated with 0.5 g of urease at 45°C for 30 min, and then the incubation mixture was extracted with methanol to reduce urea and salts in the urine sample. The resulting extract was subjected to silica gel open-column chromatography (Merck, 230–400 mesh), and then the column was eluted with 0–100% methanol-chloroform or methanol-ethyl acetate gradient by monitoring Se with ICP-MS. Methanol-chloroform (40–50%) and methanol-ethyl acetate (40%) fractions gave the highest Se concentration. The resulting solution was applied to a gel-filtration HPLC column (GS-220 HQ of 7.6 × 300 mm, with a guard column, 7.6 × 50 mm; Showa Denko, Tokyo), and the column was eluted with deionized water, the Se-containing fraction being combined and lyophilized for NMR spectrometry.
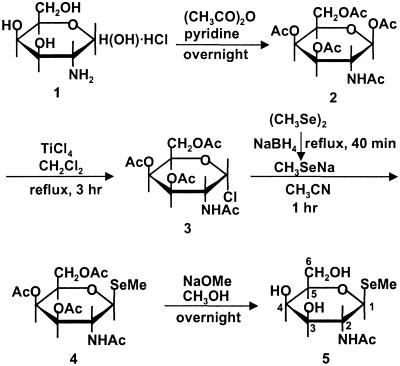
Chemical Synthesis of the Major Urinary Selenosugar (Fig. 1).
Fig 1.
Chemical synthesis of the major urinary selenosugar.
1α-Chlorogalactosamine tetraacetate (3) (18, 19) in Fig. 1 was synthesized from d-galactosamine hydrochloride (1) through the pentaacetate (2) with 47 and 70% yields, respectively, and then reacted with sodium methylselenolate, generated in situ through the reduction of dimethyldiselenide, in acetonitrile at room temperature for 1 h to give the 1β-methylseleno derivative (4) in a 73% yield as a crystalline material (m.p. 218–220°C). Acetyl groups on the tetraacetate (4) were removed with a catalytic amount of sodium methoxide to obtain the selenosugar (5) in an 88% yield, which showed m.p. 255–257°C (decomposition), [α]D24 +16.4 (c 0.51, H2O). The structure was confirmed by fast atomic bombardment MS (calcd. for C9H18O5NSe, 300.0350; found, 300.0346) and 1H NMR (600 MHz, CD3OD) (Table 1).
Table 1.
1H NMR spectral data for the urinary Se metabolite and synthetic selenosugar (CD3OD, 600 MHz)
| Position
|
δ (No. of protons, multiplicity, coupling constants) | |
|---|---|---|
| Urinary Se metabolite | Synthetic selenosugar | |
| 1 | 4.61 (1H, d, J=10.4 Hz) | 4.61 (1H, d, J=10.4 Hz) |
| 2 | 4.13 (1H, t, J=10.4 Hz) | 4.13 (1H, t, J=10.4 Hz) |
| 3 | 3.54 (1H, dd, J=10.4, 3.3 Hz) | 3.54 (1H, dd, J=10.1, 3.3 Hz) |
| 4 | 3.89 (1H, d, J=3.0 Hz) | 3.89 (1H, d, J=3.3 Hz) |
| 5 | 3.49 (1H, dd, J=6.0, 5.9 Hz) | 3.49 (1H, dd, J=6.0, 5.5 Hz) |
| 6 | 3.76 (1H, dd, J=11.5, 6.9 Hz) | 3.76 (1H, dd, J=11.5, 6.9 Hz) |
| 3.69 (1H, dd, J=11.5, 5.2 Hz) | 3.69 (1H, dd, J=11.5, 5.2 Hz) | |
| 1-Me | 2.08 (3H, s) | 2.08 (3H, s) |
| 2-Ac | 1.96 (3H, s) | 1.96 (3H, s) |
The positions of protons are shown in Fig. 1.
1β-Methylseleno-N-acetyl-d-galactosamine.
Preparation of Hepatic Se Metabolite A Fraction.
Male rats of the Wistar strain, 8 weeks of age, were injected i.p. with periodate-oxidized adenosine (20) at a dose of 100 μmol/kg body weight and then received an i.v. injection of selenite at a dose of 100 μg of Se per kg body weight 30 min after the periodate-oxidized adenosine injection. The liver supernatant obtained at 105,000 × g for 1 h was subjected to ultrafiltration on a DIAFLO ultrafiltration membrane (YM10, Amicon) to separate metabolite A from high molecular weight proteins, and then the filtrate was lyophilized. The concentrated filtrate was applied to a gel-filtration column (GS-320 7G of 7.6 × 500 mm, with a guard column, 7.6 × 50 mm) and then eluted with 10 mM ammonium acetate, pH 7.4. The Se metabolite A fraction was applied to an anion-exchange column (PRP-X100, Hamilton) and then eluted with 100 mM ammonium acetate plus 2% methanol, pH 7.4. The Se-containing fraction was subjected to HPLC/ESI-MS/MS.
Analytical Procedures.
For HPLC/ICP-MS analysis, a 20-μl aliquot of a sample solution was applied to a GS-220 HQ or GS-320 7G column, and then the column was eluted with deionized water at a flow rate of 0.6 ml/min or 10 mM ammonium acetate, pH 7.4, at a flow rate of 1.0 ml/min, respectively. For HPLC/ESI-MS/MS analysis, a 10-μl aliquot of a sample solution was applied to a GS-320 HQ column (7.6 × 300 mm, with a guard column, 7.6 × 50 mm), and then the column was eluted with deionized water at a flow rate of 0.6 ml/min. Introduction into the ESI MS was time-separated for Se elution by using a four-way valve, i.e., the eluate from 12.7 to 13.4 min was introduced for both the positive- and negative-ion modes.
Results
Chemical Structure of the Major Urinary Se Metabolite.
The major urinary Se metabolite in rats was proposed to be Se-methyl-N-acetylselenohexosamine in our previous article (17). Although Se in normal human urine was too low to speciate Se species, the same Se peak was detected on a gel-filtration column (GS-320 7G) with the HPLC/ICP-MS method (data not shown) after feeding a supplement containing 50 μg of Se per tablet, indicating that the selenosugar may be a common urinary metabolite for the excretion of Se in mammals.
The 1H NMR spectrum of the nearly purified urinary metabolite exhibited two characteristic methyl signals ascribable to the CH3Se group at the C1 position and the CH3CONH group at the C2 position in the hexosamine unit (as shown in Table 1). Furthermore, the coupling constant of protons between C3 and C4 positions indicated that the hexosamine unit is of the galactosamine type. Proton-decoupling experiments as well as analysis of the multiplicity of all the proton signals in addition to 1H–13C correlation studies suggested that the major urinary metabolite is a previously uncharacterized selenosugar, 1β-methylseleno-N-acetyl-d-galactosamine.
The proposed structure was confirmed by chemical synthesis (as shown in Fig. 1). 1H NMR data in Table 1 were indistinguishable between the urinary Se metabolite and the synthetic selenosugar. Furthermore, 13C NMR (150 MHz, CD3OD) for the synthetic selenosugar was reasonably assigned as 2.29 (−SeCH3), 22.98 (CH3 of −NHCOCH3), 49.47 (C-2), 62.76 (C-6), 69.92 (C-4), 74.22 (C-3), 80.85 (C-1), 81.96 (C-5), and 173.88 (CO of −NHCOCH3).
Characterization of the Se Metabolites in the Liver.
Labeled Se taken up in the form of selenite is transformed to two kinds of Se metabolites in the liver, the former (metabolite A) on a gel-filtration HPLC column disappearing faster from the liver than the latter (metabolite B) (21, 22). Metabolite B was suggested to be same as the major urinary metabolite by means of the HPLC/ICP-MS method. The relative peak intensity of the two Se metabolites (A/B) decreased with time. Furthermore, metabolite A became the major one when the transformation was inhibited in the presence of an inhibitor of methylation, periodate-oxidized adenosine, suggesting that metabolite A is a precursor, and it is transformed to a methylated Se metabolite (metabolite B) for excretion in the urine. Metabolite A was transformed chemically with methyl iodide to metabolite B by incubating a liver supernatant containing metabolite A with methyl iodide (20 μl of 1.0 M CH3I in CH3OH in 980 μl of a 20% liver supernatant).
Although selenite is readily reduced by glutathione (GSH), metabolite B was not detected by incubation of selenite with a liver homogenate in the presence of GSH, ATP, UTP, glucosamine, galactosamine, and so on, suggesting that metabolite B is not produced under the present analytical procedures.
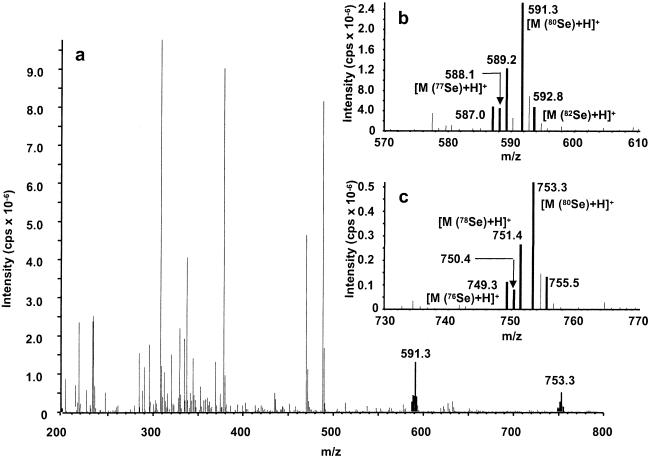
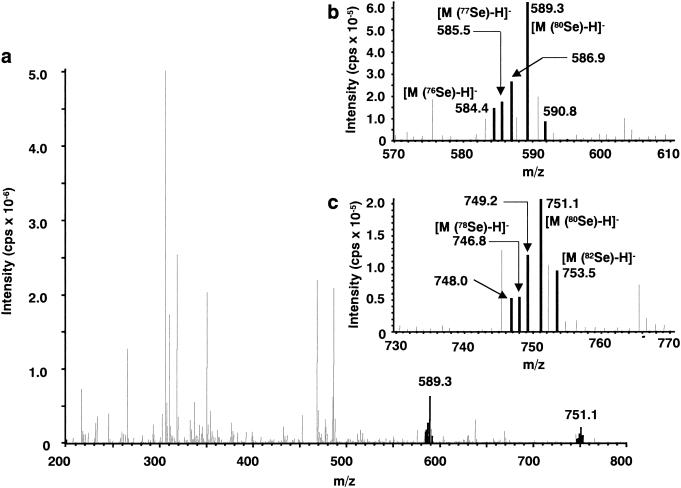
The partially purified metabolite A fraction (2 μg of Se per ml) was subjected to HPLC/ESI-MS analysis. Although contaminants were present in the sample, Se-containing metabolites were identified from the characteristic isotope pattern for Se, Se-76 (9.36%), −77 (7.63), −78 (23.8), −80 (49.6), and −82 (8.73). Characteristic signals were detected at m/z 591 and 753 for 80Se in the positive-ion mode (as demonstrated in Fig. 2). In the negative-ion mode, signals were detected at m/z 589 and 751 for 80Se (Fig. 3). These observations indicated that m/z 591 and 753 correspond to [M+H]+, and m/z 589 and 751 correspond to [M-H]−. These data suggest that the metabolite A peak contains two Se metabolites with molecular weights of 590 and 752 for 80Se.
Fig 2.
Electrospray-positive mass spectrum of hepatic Se metabolite A. The metabolite A fraction was separated on a Shodex Asahipak GS-320 HQ column by elution with 10 mM ammonium acetate, pH 7.4. (a) Full-scan spectrum for the parent ion. (b and c) Enlarged parent ions comprising the Se-characteristic isotope pattern.
Fig 3.
Electrospray-negative mass spectrum of hepatic Se metabolite A. The metabolite A fraction was separated on a Shodex Asahipak GS-320 HQ column by elution with 10 mM ammonium acetate, pH 7.4. (a) Full-scan spectrum for the parent ion. (b and c) Enlarged parent ions comprising the Se-characteristic isotope pattern.
Proposed Structure of Se Metabolite A in the Liver.
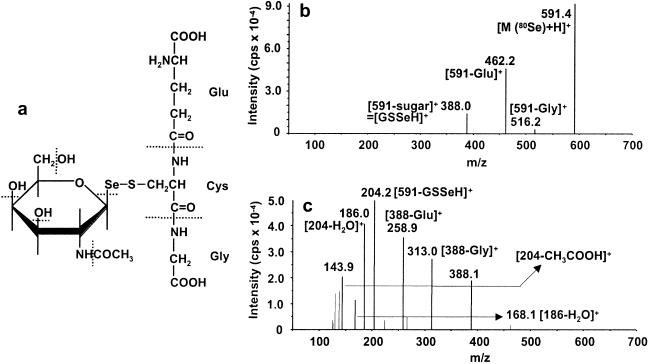
Parent-ion 591 in the first quadruple was introduced into the second quadruple to dissociate the parent ion and detect the fragment ions in the third quadruple. Although fragment ions were detected for parent-ion 591 depending on collision energy, those for parent-ion 753 were not detected under the present conditions, and further information is required to identify the bigger parent ion.
The most abundant fragment ion was detected at m/z 204 for parent-ion 591 with elevated collision energy (as shown in Fig. 4c). In our previous report for the urinary metabolite (17), the same major fragment ion as in the present one was detected and assigned to be the fragment ion (parent-ion −SeCH3), suggesting that metabolite A has the same selenosugar structure as that of metabolite B (Fig. 4c). Then it was assumed that m/z 388 is the fragment ion (parent-ion −aminosugar) instead of SeCH3.
Fig 4.
Collision-induced dissociation mass spectra (ESI-MS/MS) of the Se-containing positive molecular ions for hepatic Se metabolite A with m/z 591. (a) The proposed structure for hepatic Se metabolite A. Dissociation of the major Se-containing molecular ion, i.e., 591 (80Se), was induced in the collision cell with 20- (b) and 40-eV (c) collision energy, and the corresponding fragment ions were detected with the second mass spectrometer.
As a candidate structure for the fragment ion 308 (388 − 80Se) instead of 15 [CH3] in metabolite B, GSH (γ-glutamylcysteinylglycine) (parent ion = 308) was deduced from the fragment ions between the hepatic Se metabolite A (parent ion = 591) and GSH (parent ion = 308) (MS data for GSH were not shown); (462 = 591 − Glu) vs. (179 = 308 − Glu), (516 = 591 − Gly) vs. (233 = 308 − Gly) at 20-eV (1 eV = 1.602 × 10−19 J) collision energy. Furthermore, fragment ions also were detected for parent-ion 591 at m/z 313 (GSSeH − Gly) and 259 (GSSeH − Glu) at 40-eV collision energy. These data suggest that metabolite A is also a selenosugar conjugated with GSH instead of methyl group in metabolite B (as depicted in Fig. 4a).
Discussion
This article shows that the major urinary Se metabolite is an Se compound, selenosugar, 1β-methylseleno-N-acetyl-d-galactosamine (compound 5 in Fig. 1). Although Se-containing sugars were suggested to be present naturally from the incorporation of radiolabeled Se into the glycogen fraction in the liver of rats (23) and into the carbohydrate fraction in a plant (24), precise data such as chemical structures have not been studied. Therefore, the present selenosugar is a natural Se product determined unequivocally for its chemical structure.
The same selenosugar as in rats was detected in humans, suggesting that the essential element Se is excreted into the urine commonly in mammals in the form of this selenosugar within the required to low-toxic range, i.e., doses lower than the toxic level. Because Se of toxic doses is known to be excreted into urine in the form of trimethylselenonium ion (trimethylated Se) and then into breath in the form of dimethylselenide (dimethylated Se), the two urinary metabolites, selenosugar and trimethylated Se, can be good indicators for the Se demand within the required to low-toxic range and beyond the toxic dose.
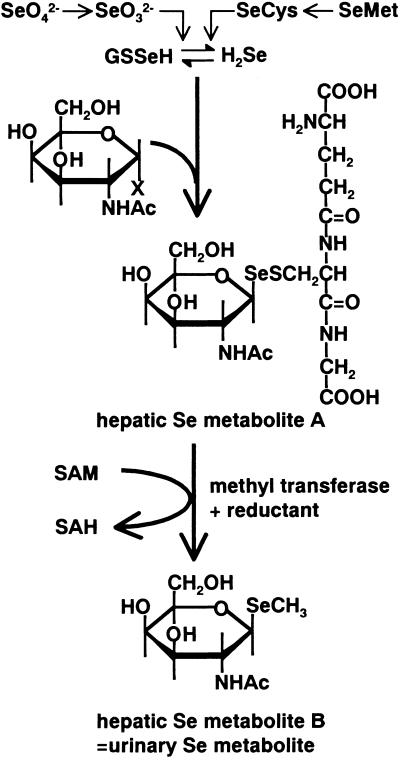
The present results also demonstrate that the same selenosugar as the major urinary Se metabolite is present in the liver (metabolite B) together with the selenosugar conjugated with GSH instead of a methyl group (metabolite A; Fig. 4a). The time-related change in the peak intensity for the two selenosugars and the inhibition of the transformation from metabolite A to metabolite B by a methylation inhibitor, periodate-oxidized adenosine, suggested that metabolite A is a precursor of metabolite B. Thus, the reductive methylation reaction seems to occur at this stage of the metabolic pathway in the presence of a methyl donor, i.e., (selenosugar)-Se-SG → (selenosugar)-Se-CH3 (Fig. 5), which was inhibited by an inhibitor of methylation.
Fig 5.
Proposed metabolic pathway for selenosugars. GSSeH, GSH selenopersulfide; SeCys, selenocysteine; SeMet, selenomethionine; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine.
Although methylation masks active selenol groups and hence offers protection against the toxic reaction, it may not be a beneficial metabolic pathway for the body because toxic and lipophilic monomethylselenol and dimethylselenide are produced. On the other hand, selenosugars are highly water-soluble, and their active selenol groups can be masked by GSH or a methyl group. Therefore, selenosugars seem to be more reasonable and safer metabolites than methylated metabolites containing selenol groups. Hence, the metabolic pathway proposed in Fig. 5 can be accepted as a logical route for the excretion of Se after the checkpoint Se metabolite. However, it cannot be explained reasonably why and how N-acetyl galactosamine is selected as a component of selenosugars. The biological significance of the selenosugar and its biological role in the metabolism of essential element Se remain to be determined.
Although selenosugars were determined as a natural product for the first time, arsenosugars have been widely detected in marine organisms such as plant (25), crustacea (26), and mollusk (27). Arsenosugars have a ribose frame with an arseno group at the C5 position, whereas selenosugars have a hexosamine frame with a seleno group at the C1 position. Arsenosugars are not synthesized in mammals but metabolized in mammals as xenobiotics to the urinary form of dimethylarsinic acid (28), whereas selenosugars are synthesized in mammals and excreted in this form into the urine. Thus, arsenosugars and selenosugars are distinct not only in their chemical structures, ribose vs. N-acetylgalactosamine, but also in their synthesis and metabolism in mammals.
Acknowledgments
We acknowledge the financial support of Ministry of Education, Culture, Science, Sports, and Technology of Japan Grants 12470509 and 14657587.
Abbreviations
ICP-MS, inductively coupled argon plasma mass spectrometry
ESI-MS/MS, electrospray ionization tandem mass spectrometry
GSH, glutathione
References
- 1.Stadtman T. C. (1974) Science 183, 915-922. [DOI] [PubMed] [Google Scholar]
- 2.Burk R. F. (1978) World Rev. Nutr. Diet. 30, 88-106. [DOI] [PubMed] [Google Scholar]
- 3.Yang G. Q., Ge, K. Y., Chen, J. S. & Chen, X. S. (1988) World Rev. Nutr. Diet. 55, 98-152. [DOI] [PubMed] [Google Scholar]
- 4.Wilber C. G. (1980) Clin. Toxicol. 17, 171-230. [DOI] [PubMed] [Google Scholar]
- 5.Fox J. M. (1992) Methods Find. Exp. Clin. Pharmacol. 14, 275-287. [PubMed] [Google Scholar]
- 6.Burk R. F. (2001) Curr. Opin. Gastroenterol. 17, 162-166. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh H. S. & Ganther, H. E. (1975) Biochemistry 14, 1632-1636. [DOI] [PubMed] [Google Scholar]
- 8.Esaki N., Nakamura, T., Tanaka, H. & Soda, K. (1982) J. Biol. Chem. 257, 4386-4391. [PubMed] [Google Scholar]
- 9.Lacourciere G. M., Mihara, H., Kurihara, T., Esaki, N. & Stadtman, T. C. (2000) J. Biol. Chem. 275, 23769-23773. [DOI] [PubMed] [Google Scholar]
- 10.Forchhammer K., Leinfelder, W. & Bock, A. (1989) Nature 342, 453-456. [DOI] [PubMed] [Google Scholar]
- 11.Berry M. J., Banu, L., Chen, Y. Y., Mandel, S. J., Kieffer, J. D., Harney, J. W. & Larsen, P. R. (1991) Nature 353, 273-276. [DOI] [PubMed] [Google Scholar]
- 12.Itoh M. & Suzuki, K. T. (1997) Arch. Toxicol. 71, 461-466. [DOI] [PubMed] [Google Scholar]
- 13.Ganther H. E. (1986) J. Am. Coll. Toxicol. 5, 1-5. [Google Scholar]
- 14.Suzuki K. T. & Ogra, Y. (2001) Phosphorus Sulfur Silicon Relat. Elem. 171, 135-169. [Google Scholar]
- 15.Lobinski R. & Szpunar, J. (1999) Anal. Chim. Acta 400, 321-332. [Google Scholar]
- 16.Lobinski R., Edmonds, J., Suzuki, K. T. & Uden, P. (2000) Pure Appl. Chem. 72, 447-461. [Google Scholar]
- 17.Ogra Y., Ishiwata, K., Takayama, H., Aimi, N. & Suzuki, K. T. (2002) J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 767, 301-312. [DOI] [PubMed] [Google Scholar]
- 18.Tarasiejska Z. & Jeanloz, R. W. (1958) J. Am. Chem. Soc. 80, 6325-6327. [Google Scholar]
- 19.Baker B. R., Joseph, J. P., Schaub, R. E. & Williams, J. H. (1954) J. Org. Chem. 19, 1786-1792. [Google Scholar]
- 20.Hoffman J. L. (1980) Arch. Biochem. Biophys. 205, 132-135. [DOI] [PubMed] [Google Scholar]
- 21.Shiobara Y., Ogra, Y. & Suzuki, K. T. (1999) Analyst 124, 1237-1241. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi Y., Ogra, Y. & Suzuki, K. T. (2001) J. Chromatogr. B Analyt. Technol. Biomed. Sci. Appl. 760, 73-81. [DOI] [PubMed] [Google Scholar]
- 23.Zingaro R. A. (1975) Chem. Scr. 8A, 51-57. [Google Scholar]
- 24.Zingaro R. A. & Price, J. E. (1977) J. Carbohydr. Nucleosides Nucleotides 4, 271-280. [Google Scholar]
- 25.Edmonds J. S. & Francesconi, K. A. (1981) Nature 289, 602-604. [Google Scholar]
- 26.Edmonds J. S., Shibata, Y., Francesconi, K. A., Yoshinaga, J. & Morita, M. (1992) Sci. Total Environ. 122, 321-335. [DOI] [PubMed] [Google Scholar]
- 27.Lai V. W. N., Cullen, W. R. & Ray, S. (1999) Mar. Chem. 66, 81-89. [Google Scholar]
- 28.Ma M. & Le, X. C. (1998) Clin. Chem. 44, 539-550. [PubMed] [Google Scholar]