Abstract
Nitric oxide (•NO) and •NO-derived reactive species rapidly react with lipids during both autocatalytic and enzymatic oxidation reactions to yield nitrated derivatives that serve as cell signaling molecules. Herein we report the synthesis, purification, characterization, and bioactivity of nitrolinoleate (LNO2). Nitroselenylation of linoleic acid yielded LNO2 that was purified by solvent extraction, silicic acid chromatography, and reverse-phase HPLC. Structural characterization was performed by IR spectroscopy, 15N-NMR, LC-negative ion electrospray mass spectroscopy (MS), and chemiluminescent nitrogen analysis. Quantitative MS analysis of cell and vessel LNO2 metabolism, using L[15N]O2 as an internal standard, revealed that LNO2 is rapidly metabolized by rat aortic smooth muscle (RASM) monolayers and rat thoracic aorta, resulting in nitrite production and up to 3-fold increases in cGMP (ED50 = 30 μM for RASM, 50 μM for aorta). LNO2 induced endothelium-independent relaxation of preconstricted rat aortic rings, which was unaffected by LG-nitro-l-arginine methyl ester addition and inhibited by the guanylate cyclase inhibitor 1H-[1,2,4] oxadiazole[4,3-a]quinoxalin-1-one and the •NO scavenger HbO2. These results reveal that synthetic LNO2, identical to lipid derivatives produced biologically by the reaction of •NO and •NO-derived species with oxidizing unsaturated fatty acids (e.g., linoleate), can transduce vascular signaling actions of •NO.
Nitric oxide (•NO) manifests diverse physiologic and pathologic actions beyond regulation of vascular relaxation, including the modulation of neurotransmission, inflammatory reactions, and cell proliferation (1). For example, •NO inhibits platelet function and leukocyte adherence to the vessel wall via stimulation of guanylate cyclase (GC)-mediated synthesis of cGMP (1, 2). •NO signaling is dictated in large part by heme protein reactions, with oxygen- and sulfur-dependent reactions of •NO also of significance in the transduction and termination of •NO bioactivity. For example, the reaction of •NO with thiol derivatives, molecular oxygen, lipid radicals, and superoxide (O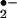 ) has a profound effect on •NO half-life, reactivity, and signaling properties. Secondary products of these reactions, including nitrite (NO
) has a profound effect on •NO half-life, reactivity, and signaling properties. Secondary products of these reactions, including nitrite (NO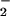 ), nitrito, nitrate ester, nitroso, and other nitrogen oxide derivatives can also activate GC after spontaneous decomposition, metabolism to •NO, or the formation of heme iron-reactive redox intermediates of •NO (3).
), nitrito, nitrate ester, nitroso, and other nitrogen oxide derivatives can also activate GC after spontaneous decomposition, metabolism to •NO, or the formation of heme iron-reactive redox intermediates of •NO (3).
Reactions of •NO with oxygen-derived species also yield products expressing non-cGMP-dependent actions. •NO reacts with oxygen to yield nitrogen dioxide (•NO2) (•NO2, k = 2 × 106 M−2 s−1), which in turn may further react with •NO to yield nitrogen trioxide (N2O3), a reaction that is accelerated in membranes and lipoproteins (4, 5). Peroxynitrite (ONOO−), formed from the diffusion-limited reaction of O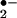 with •NO (k = 2 × 109 M−1 s−1), is a strong oxidant that mediates oxidation, nitrosation, and nitration reactions (6, 7). In terms of •NO signaling, ONOO− formation (i) diverts •NO away from direct GC activation (8, 9); (ii) directly activates GC (10); and (iii) reacts with biomolecules including uric acid, glucose, and glycerol to yield nitro- (RNO2) and nitrito- (RONO) adducts that display •NO-donating properties (11, 12). Acidic pH conditions will also protonate NO
with •NO (k = 2 × 109 M−1 s−1), is a strong oxidant that mediates oxidation, nitrosation, and nitration reactions (6, 7). In terms of •NO signaling, ONOO− formation (i) diverts •NO away from direct GC activation (8, 9); (ii) directly activates GC (10); and (iii) reacts with biomolecules including uric acid, glucose, and glycerol to yield nitro- (RNO2) and nitrito- (RONO) adducts that display •NO-donating properties (11, 12). Acidic pH conditions will also protonate NO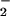 (pKa = 3.25), a predominant byproduct of aerobic •NO decomposition, to yield a series of reactive intermediates (13, 14). In aggregate, the aqueous and aerobic reactions of •NO in biological milieu yield a rich spectrum of potent nitrosating, nitrating, and oxidizing products that transduce •NO signaling (3).
(pKa = 3.25), a predominant byproduct of aerobic •NO decomposition, to yield a series of reactive intermediates (13, 14). In aggregate, the aqueous and aerobic reactions of •NO in biological milieu yield a rich spectrum of potent nitrosating, nitrating, and oxidizing products that transduce •NO signaling (3).
The reactions of •NO and its products in hydrophobic tissue compartments are important elements in the contribution of •NO to cell signaling and tissue pathobiology. Due to its small molecular radius and uncharged nature, the lipophilic •NO readily diffuses through both the hydrophilic surface and the more hydrophobic core of membranes and lipoproteins, with a diffusion coefficient of up to 2 × 10−5 cm2 s−1 (15, 16). There, •NO concentrates up to 20-fold and is more rapidly consumed by reaction with the molecular oxygen that also preferentially partitions in this compartment. This molecular “lens” effect, induced by •NO and O2 solvation in hydrophobic cell compartments, yields secondary •NO-derived species capable of oxidation, nitrosation, and nitration reactions (5). The catalysis of •NO oxidation may also occur in hydrophobic regions of proteins such as albumin (17).
•NO displays potent antioxidant and α-tocopherol-preserving actions by terminating propagation reactions catalyzed by both free- and enzyme-bound lipid alkoxyl and peroxyl radical intermediates (k = 2 × 109 M−1 s−1), yielding nitrated products (18–22). As with the nitration and nitrosation of amino acids and DNA bases observed in a variety of inflammatory diseases, multiple mechanisms account for the nitration of lipids by •NO-derived species such as •NO2, ONOO−, and HNO2 (22). During inflammatory conditions, lipid oxidation frequently occurs at an accelerated rate, yielding radical species that can serve as intermediates in lipid nitration. In acidic conditions, protonation of NO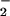 to HNO2 can mediate the nitration of polyunsaturated fatty acids and lipid hydroperoxides. Also, ONOO− has been observed to readily diffuse through membranes and lipoproteins to induce nitration of unsaturated fatty acids (23, 24). Nitronium ion (NO
to HNO2 can mediate the nitration of polyunsaturated fatty acids and lipid hydroperoxides. Also, ONOO− has been observed to readily diffuse through membranes and lipoproteins to induce nitration of unsaturated fatty acids (23, 24). Nitronium ion (NO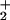 ) and •NO2 reaction with unsaturated fatty acids and the caged radical rearrangement of lipid peroxyl radical-•NO reaction intermediates can yield nitrated lipid products whose structure and function are incompletely characterized (24–26). Finally, peroxidases such as myeloperoxidase (MPO) can oxidize NO
) and •NO2 reaction with unsaturated fatty acids and the caged radical rearrangement of lipid peroxyl radical-•NO reaction intermediates can yield nitrated lipid products whose structure and function are incompletely characterized (24–26). Finally, peroxidases such as myeloperoxidase (MPO) can oxidize NO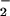 to •NO2, a species capable of catalyzing biomolecule nitration (27, 28).
to •NO2, a species capable of catalyzing biomolecule nitration (27, 28).
Herein, we report the synthesis, purification, and characterization of a nitrated lipid, nitrolinoleate (LNO2), that is structurally similar to products of the reaction of •NO2, ONOO−, HNO2, and MPO-catalyzed nitration of linoleic acid. The bioactivity of this nitrated lipid was investigated, revealing rapid metabolism by both smooth muscle cells and aortic segments to a species that yields NO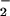 and stimulates cGMP production in both rat aortic smooth muscle cell (RASMC) monolayers and isolated rat thoracic aorta. The GC-activating property of LNO2 induced relaxation of isolated rat thoracic aorta via an 1H-[1,2,4] oxadiazole[4,3-a]quinoxalin-1-one (ODQ)- and HbO2-inhibitable mechanism, indicating that cGMP-dependent vasomotor actions of •NO can in part be transduced via the generation of nitrated fatty acids.
and stimulates cGMP production in both rat aortic smooth muscle cell (RASMC) monolayers and isolated rat thoracic aorta. The GC-activating property of LNO2 induced relaxation of isolated rat thoracic aorta via an 1H-[1,2,4] oxadiazole[4,3-a]quinoxalin-1-one (ODQ)- and HbO2-inhibitable mechanism, indicating that cGMP-dependent vasomotor actions of •NO can in part be transduced via the generation of nitrated fatty acids.
Experimental Procedures
Structural Analysis of LNO2.
The synthesis of LNO2 via nitroselenylation (29, 30) is published as supporting information on the PNAS web site, www.pnas.org. Mass spectroscopic analysis was performed on an API III triple-quadrupole mass spectrometer (PE-Sciex, Concord, ON, Canada) after reverse-phase HPLC on (i) a 100-× 2.1-mm i.d. Aquapore C8 column (Perkin–Elmer) with a linear 50–100% CH3OH gradient in 1% aqueous acetic acid at 0.2 ml/min. Under these conditions, nitration products with either m/z 324 or 340 eluted separately as single peaks, enabling integration and normalization with an added L[15N]O2 internal standard; or (ii) a 150- × 4.6-mm i.d. 5-μm C18 column (Microsorb, Rainin, MA) using a gradient of 50–90% B in A (A is 75:25:0.1 H2O/acetonitrile/acetic acid; B is 60:40:0.1 CH3OH/acetonitrile/acetic acid) over 20 min at a flow rate of 1 ml/min. The column eluent was split, with one-tenth going to the ionspray interface. Negative ion mass spectra were recorded with an orifice potential of −60 V. Daughter ion mass spectra were obtained by selecting the parent molecular ion with the first quadrupole, colliding it with a mixture of 10% N2–90% Ar in the second quadrupole and analyzing fragment ions in the third quadrupole.
For 15N-NMR analysis, L[15N]O2 was dissolved in 500 μl of CH3OH–d4. The spectrum was collected on a Bruker (Billerica, MA) AVANCE-600 spectrometer with the following acquisition parameters: 12,168 scans; 5-s recycle time; composite-pulse decoupling during the acquisition time (0.44 s); 25° pulse width; 37,037-Hz spectral width; and 300 K. Results are displayed as the chemical shift, and not chemical shielding, which has the opposite sign. Chemical shifts were referenced to 10% nitromethane in CH3OH. The 15N sensitivity of the AVANCE-600 NMR system was confirmed by using a solution of nitromethane in CH3OH. NMR data were further processed by using origin graph-fitting software from OriginLab (Northampton, MA). IR spectra were recorded for LNO2 by using a Bomem Michelson MB Series Fourier transform–IR spectrometer (ABB Bomem, Quebec, Canada). LNO2 (5 mg) was solvated in 500 μl of diethyl ether and distributed on a salt plate. The solvent was evaporated with a stream of nitrogen, and the spectrum was recorded. Re-extraction and rechromatography of LNO2 revealed no modification of the lipid derivative by analysis procedures.
Nitration of Linoleic Acid by MPO.
Linoleic acid (2 mM) was emulsified in 50 mM phosphate buffer/100 μM diethylenetriaminepentaacetic acid, pH 5, by sonicating 3 × 20 sec at 1-min intervals. Human MPO (50 μg) and 50 μM NO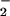 were added and reactions in 1 ml were initiated by addition of 100 μM H2O2 at 37°C four times over 1 h. Lipids were extracted, dissolved in MeOH, and subjected to HPLC fractionation with diode array and nitrogen monitoring.
were added and reactions in 1 ml were initiated by addition of 100 μM H2O2 at 37°C four times over 1 h. Lipids were extracted, dissolved in MeOH, and subjected to HPLC fractionation with diode array and nitrogen monitoring.
LNO2 Metabolism and Signaling.
LNO2 metabolism was determined in cell suspensions prepared from confluent 75-cm2 RASM monolayers maintained in Hanks' balanced salt solution (HBSS). After addition of 10 μM LNO2 solvated in CH3OH (<0.5% final CH3OH) for 0–60 min, reactions were terminated by addition of CHCl3/CH3OH (1:1, vol/vol) and heating at 60°C for 5 min. Then, 10 μM L[15N]O2 was added as an internal standard. Lipids were extracted with CHCl3/CH3OH/H2O, 1:1:0.9 (vol/vol), the organic phase collected, dried under a stream of N2 gas, redissolved in CH3OH, and the ratio L[14N]O2/L[15N]O2 determined by negative ion-mode MS. For vessel studies, adult male Sprague–Dawley rats weighing 250–300 g were used (Harlan Laboratories, Indianapolis, IN), with experimental procedures approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Descending thoracic aortas were excised, adhering tissue dissected away, and individual ring segments (4 mm) obtained. Ring segments were cut longitudinally and in some cases the endothelium removed by gentle scraping. LNO2 (50 μM) incubation and extraction conditions were as for RASMC monolayers, except Krebs–Henseleit buffer (NaHCO3 25.0/NaCl 118/KCl 4.7/MgSO4 1.2/NaH2PO4 1.2/CaCl2 1.2/glucose 5.6 mM, pH 7.4) was substituted for HBSS.
Additional Experimental Methods.
Description of materials and experimental procedures for cell culture, cGMP determination, vascular relaxation measurement, and statistical analysis are published as supporting information on the PNAS web site.
Results
Synthesis and Characterization of LNO2.
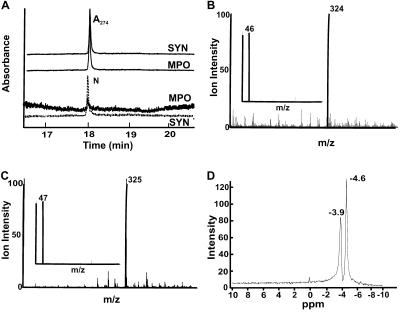
The nitroselenylation of linoleate yielded both nitration and peroxidation products that were purified to an LNO2-enriched fraction by solvent extraction and silica gel chromatography. Then, reverse-phase HPLC further resolved LNO2 and removed all non-nitrated contaminating lipids. The fraction eluting at ≈18 min (Fig. 1A) was retained for subsequent study, giving m/z 324 by negative ion mode electrospray MS, 45 mass units greater than the parent compound linoleate, m/z 279, inferring the addition of a nitro (NO2)- group (Fig. 1B). Tandem MS (MS/MS) yielded a daughter ion with m/z 46, supporting the addition of NO2 (Fig. 1B Inset). Analysis of products formed when substituting Na[15N]O2 for AgNO2 in LNO2 synthesis further verified NO2 addition to linoleate, with L[15N]O2 yielding an ion m/z 325 with a daughter ion of m/z 47 by MS/MS (Fig. 1C). Elemental analysis also supported incorporation of a −NO2 functional group into linoleate (not shown). The molar yield of LNO2 from nitroselenylation of linoleate was typically 5–7%, as quantified by chemiluminescent nitrogen analysis. This species shared similar chromatographic and mass spectral properties to linoleate nitration products formed by MPO-catalyzed oxidation of NO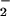 (Fig. 1A) and LNO2 species produced by ischemic, reperfused rodent liver (unpublished observations).
(Fig. 1A) and LNO2 species produced by ischemic, reperfused rodent liver (unpublished observations).
Fig 1.
Purification and analysis of LNO2. (A) Reverse-phase HPLC elution profiles of LNO2 generated by nitroselenylation (SYN) and exposure of linoleic acid (18:2) to MPO/H2O2/NO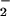 (MPO). Reverse-phase HPLC resolved the major nitrated linoleate derivative of m/z 324, eluting at a retention time of ≈18 min, with parallel UV and nitrogen detection confirming the presence of nitrated fatty acid. Control reactions omitting MPO revealed no detectable nitrated products. (B and C) Electrospray MS and MS/MS of LNO2 (B) and L[15N]O2 (C). The principal LNO2-containing HPLC-resolved fraction in A was analyzed by negative ion mode electrospray MS and yielded a molecular ion m/z = 324, indicative of LNO2. (Inset) The MS/MS fragmentation pattern of the m/z = 324 ion yielded a daughter ion, m/z = 46, indicative of an NO2 functional group. L[15N]O2 yielded an ion m/z = 325 with a daughter ion of m/z = 47 by MS/MS (C). (D) 15N NMR spectrum of LNO2. 15N NMR analysis of LNO2 gave a doublet chemical shift indicative of at least two positional isomers.
(MPO). Reverse-phase HPLC resolved the major nitrated linoleate derivative of m/z 324, eluting at a retention time of ≈18 min, with parallel UV and nitrogen detection confirming the presence of nitrated fatty acid. Control reactions omitting MPO revealed no detectable nitrated products. (B and C) Electrospray MS and MS/MS of LNO2 (B) and L[15N]O2 (C). The principal LNO2-containing HPLC-resolved fraction in A was analyzed by negative ion mode electrospray MS and yielded a molecular ion m/z = 324, indicative of LNO2. (Inset) The MS/MS fragmentation pattern of the m/z = 324 ion yielded a daughter ion, m/z = 46, indicative of an NO2 functional group. L[15N]O2 yielded an ion m/z = 325 with a daughter ion of m/z = 47 by MS/MS (C). (D) 15N NMR spectrum of LNO2. 15N NMR analysis of LNO2 gave a doublet chemical shift indicative of at least two positional isomers.
IR Spectroscopic Analysis of the HPLC-Purified m/z 324 LNO2 Fraction.
This analysis affirmed the identification of a nitro (LNO2) derivative of 18:2 and eliminated the presence of possible nitrito (LONO) or nitrate ester (LONO2) products. The IR spectrum of LNO2, when compared with that of 18:2, revealed absorbance maxima at 1,522 and 1,334 cm−1 (Table 1), corresponding to the asymmetric and symmetric stretches of nitroalkene N O bonds (30). No absorbance occurred in the 1,610–1,680 cm−1 region where the N
O bonds (30). No absorbance occurred in the 1,610–1,680 cm−1 region where the N O bonds of organic nitrites, and nitrates strongly absorb (31). When phosphatidylcholine is exposed to gaseous dinitrogen pentoxide (N2O5), bands appear at ≈1,640 and 1,550 cm−1, indicative of the asymmetric N
O bonds of organic nitrites, and nitrates strongly absorb (31). When phosphatidylcholine is exposed to gaseous dinitrogen pentoxide (N2O5), bands appear at ≈1,640 and 1,550 cm−1, indicative of the asymmetric N O bonds of RONO2 and RNO2, respectively (32), confirming the assignment herein of 1,522 cm−1 to the asymmetric N
O bonds of RONO2 and RNO2, respectively (32), confirming the assignment herein of 1,522 cm−1 to the asymmetric N O stretch of LNO2. The UV absorbance spectrum of 0.1 mM LNO2 showed absorbance at 270 nm and λmax = 202 nm, corresponding to aliphatic nitro compounds, whereas alkyl nitrites absorbed at 228 nm and have a low-intensity transition with six vibrational fine structure bands centered at ≈375 nm (not shown; ref. 33).
O stretch of LNO2. The UV absorbance spectrum of 0.1 mM LNO2 showed absorbance at 270 nm and λmax = 202 nm, corresponding to aliphatic nitro compounds, whereas alkyl nitrites absorbed at 228 nm and have a low-intensity transition with six vibrational fine structure bands centered at ≈375 nm (not shown; ref. 33).
Table 1.
IR spectroscopic analysis of linoleate and LNO2, units = cm−1
| Functional group stretch | Linoleate | LNO2 | Theoretical | Phosphatidylcholine |
|---|---|---|---|---|
| N–O of nitro (symmetric), RNO2 | — | 1,334 | 1,360–1,290 | — |
| N |
— | 1,522 | 1,550–1,500 | 1,550 |
| N |
— | — | 1,660–1,625 | 1,640 |
| N |
— | — | 1,680–1,610 | — |
| OH of carboxylic acid | ≈3,000 | ≈3,000 | — | — |
| C |
1,697 | 1,708 | — | 1,740 |
| C–H | 2,923, 2,854 | 2,929, 2,858 | 2,850–2,940 | — |
| C–C | 1,461, 1,413 | 1,462, 1,413 | 1,410–1,470 | — |
Additional structural insight was obtained by comparison of the HPLC-purified LNO2 fraction with the 15N-NMR spectrum of nitromethane (Fig. 1D). Again, 15N-NMR supported LNO2 formation over that of LONO. LNO2 had chemical shifts at −3.9 and −4.6 ppm (Fig. 1D) when externally referenced to nitromethane (0 ppm), suggesting the presence of positional isomers. If a nitrito derivative (LONO) were present, a distinct chemical shift would have appeared at ≈200 ppm (34).
Metabolism and Signaling Actions of LNO2.
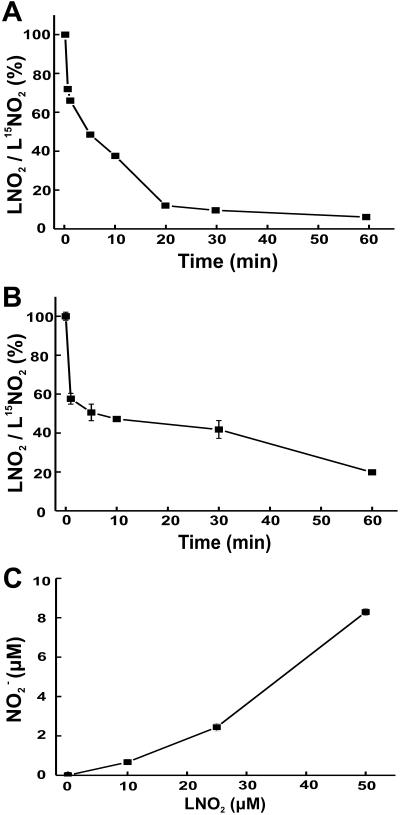
When added to RASMC monolayers or rat aortic segments in the dark, LNO2 (10 and 50 μM, respectively) was rapidly metabolized (Fig. 2 A and B). There was also a LNO2 concentration-dependent increase in medium NO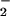 content after incubation of RASMC with LNO2, with 10–20% yields of NO
content after incubation of RASMC with LNO2, with 10–20% yields of NO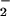 as a function of added LNO2 observed after 1-h incubation (Fig. 2C). The release of •NO by LNO2-treated cells and vessel segments was not detectable via electrochemical analysis having a limit of detection of ≈10 nM •NO. Control studies showed no decay of LNO2 in buffer alone at 37°C over similar time periods and no impact of light on LNO2 stability. Within 5 min, LNO2 was >50% consumed by both RASMC and aortic segments (Fig. 2 A and B), despite the temporal limitations dictated by the requirement for added LNO2 to diffuse to and become incorporated by vascular cells before metabolism. Saponification, extraction of cell lipids 10 min after LNO2 addition, and MS analysis did not reveal LNO2 esterification into more complex lipids over this time period. The stability of purified LNO2 to the alkaline conditions at 60°C used for saponification was also confirmed (not shown).
as a function of added LNO2 observed after 1-h incubation (Fig. 2C). The release of •NO by LNO2-treated cells and vessel segments was not detectable via electrochemical analysis having a limit of detection of ≈10 nM •NO. Control studies showed no decay of LNO2 in buffer alone at 37°C over similar time periods and no impact of light on LNO2 stability. Within 5 min, LNO2 was >50% consumed by both RASMC and aortic segments (Fig. 2 A and B), despite the temporal limitations dictated by the requirement for added LNO2 to diffuse to and become incorporated by vascular cells before metabolism. Saponification, extraction of cell lipids 10 min after LNO2 addition, and MS analysis did not reveal LNO2 esterification into more complex lipids over this time period. The stability of purified LNO2 to the alkaline conditions at 60°C used for saponification was also confirmed (not shown).
Fig 2.
Metabolism of LNO2 by smooth muscle cell monolayers (A) and isolated rat thoracic aorta (B). LNO2 (10 μM) was added as an ethanolic solution to the media of RASMC in HBSS, pH 7.4, 37°C (A) and in 50 μM concentration to 20 mg (wet weight) rat aortic vessel segments in Krebs–Henseleit buffer, pH 7.4, 37°C (B). Values are expressed as the proportional decrease in LNO2 at different incubation times to an L[15N]O2 internal standard added at the time of reaction termination. (C) NO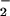 production by RASMC after 1-h incubation with LNO2. For A, data represent the mean of duplicate determinations of a representative experiment repeated three times (B and C). Data represent mean ± SEM, n = 3.
production by RASMC after 1-h incubation with LNO2. For A, data represent the mean of duplicate determinations of a representative experiment repeated three times (B and C). Data represent mean ± SEM, n = 3.
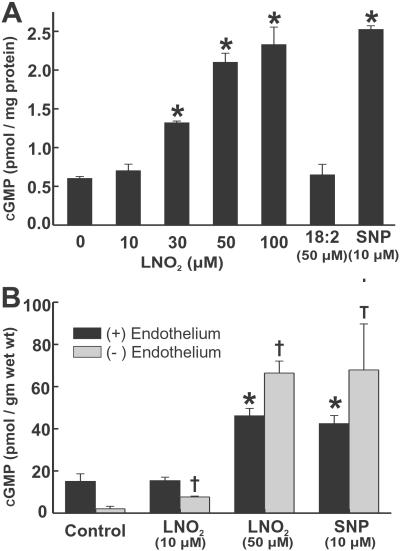
LNO2-Dependent Vasorelaxation.
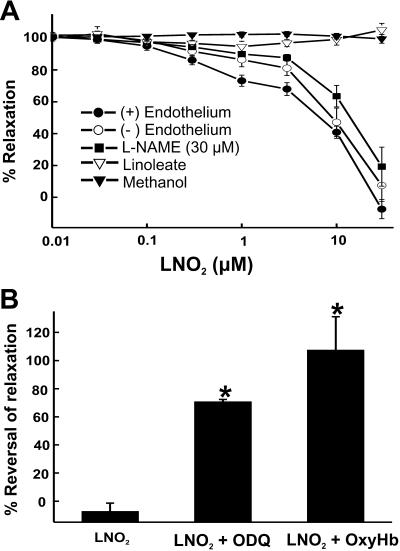
For both RASMC and aortic segments, LNO2 addition induced a concentration-dependent increase in cGMP. LNO2 (50 μM) increased the cGMP content of RASMC monolayers and aortic segments to an extent equivalent to that induced by 10 μM SNP (Fig. 3 A and B). Isometric tension analysis revealed LNO2 induced endothelium-independent vasorelaxation, with vehicle (CH3OH), the parent fatty acid (18:2), and exposure to light during LNO2 incubations having no effect (Fig. 4A). LG-nitro-l-arginine methyl ester addition and endothelial denudation did not affect LNO2-induced vasorelaxation, revealing that endogenous endothelial •NO synthesis was not stimulated by LNO2 (Fig. 4A). Vascular relaxation induced by LNO2 was inhibited by the GC inhibitor ODQ and the •NO scavenger oxyhemoglobin (oxyHb), indicating the participation of an •NO-like species in the vasomotor actions of LNO2 (Fig. 4B).
Fig 3.
LNO2 induces cGMP synthesis by RASMC monolayers (A) and rat thoracic aorta segments (B) in a concentration-dependent manner. Data are expressed as mean ± SEM, n = 3, except for endothelium(−) aorta treated with 50 μM LNO2 and endothelium(+) aorta treated with 10 μM SNP, where n = 2. In A, * represents P < 0.05, for RASMC monolayers vs. control conditions (addition of 0.05% CH3OH). In B, * and † represent P < 0.05 vs. control conditions [* for endothelium(+) and † for endothelium(−) aortic segments].
Fig 4.
LNO2 stimulates relaxation of rat thoracic aortic rings. LNO2 (3–30 μM) induced significant relaxation of endothelium-intact, endothelium-denuded, and LG-nitro-l-arginine methyl ester-pretreated thoracic aorta rings compared with controls (3–30 μM linoleate or equivalent amounts of solvating CH3OH added for each lipid concentration used) (A). ODQ (30 μM) and oxyHb (10 μM) addition significantly reversed LNO2 (30 μM)-induced relaxation of isolated thoracic aorta (B). Data are expressed as mean ± SEM, n = 6, with * representing P < 0.05 vs. LNO2 addition.
Discussion
Enzymatically oxidized lipid derivatives (eicosanoids) play an indispensable signaling role in diverse tissue compartments and cell types (35–38). Also, the nonenzymatic oxidation of free and esterified arachidonic acid yields isoprostane and isoleukotriene derivatives that contribute to inflammatory signaling and injury processes (39). These isoeicosanoids potently affect vascular, renal, and inflammatory cell function and are produced at increased rates when membrane and lipoprotein lipids are oxidized by reactive inflammatory mediators and xenobiotics including hydroperoxides, ONOO−, transition metals, cigarette smoke, photochemical air pollution, and other products of organic combustion (39–42). Oxides of nitrogen have long been recognized to catalyze lipid oxidation and nitration, on the basis of investigations focused on understanding pathologic processes associated with the •NO and •NO2 components of cigarette smoke and polluted air (19, 43–45). Also, bioactive isoeicosanoid derivatives have been described in lipid systems exposed to •NO2 and ONOO− (40, 41). This foundation of knowledge encourages that oxides of nitrogen may similarly catalyze formation of the nitrated fatty acid signaling species reported herein, yielding a class of diffusible mediators that represent the convergence of eicosanoid, isoeicosanoid, and ⋅NO signaling pathways.
Since the description of •NO as a ubiquitous signaling molecule, there have been a number of linkages reported between eicosanoid and •NO signaling pathways. First, there is comodulation of the expression of •NO and eicosanoid biosynthetic enzymes, with prostaglandins modulating •NO synthase expression and •NO modulating prostaglandin H synthase (PGHS) expression (46, 47). Also, •NO reacts at almost diffusion-limited rates with enzyme-bound lipid peroxyl radical intermediates during eicosanoid metabolism, leading to an inhibition of lipoxygenase- and PGHS-derived eicosanoid formation (48–50). During the PGHS catalytic cycle, •NO-derived species can also react with and impair the catalytic function of enzyme tyrosyl radical and heme peroxidase intermediates (51). Conversely, ONOO− serves to activate PGHS (47, 51). This interaction of •NO and eicosanoid signaling pathways is exemplified by studies of NOS2−/− mice, wherein endogenous rates of tissue •NO production modulate tissue F2-isoprostane, prostaglandin E2, and thromboxane B2 levels (52). Concomitantly, the reactions of •NO with intermediates of lipoxygenase- and PGHS-dependent eicosanoid synthesis catalytically consume •NO to a degree that both platelet and monocyte function are altered (48, 50).
The nitrated lipids formed by •NO-derived species mediate a combination of direct and cAMP- and cGMP-dependent signaling actions, depending on cell type and the response variable. LNO2 potently inhibits neutrophil activation [O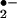 production, N-formyl-methionyl-leucyl-phenylalanine (fMLP)-stimulated Ca+2 influx, degranulation, and CD11b expression] via non-cGMP-dependent mechanisms (53). Also, LNO2 inhibits thrombin-mediated platelet aggregation by cAMP-dependent attenuation of Ca+2 mobilization and phosphorylation of vasodilator-stimulated phosphoprotein at serine 157. This occurs via dual regulation of adenylyl cyclase and phosphodiesterase-3 activities (54). In both platelets and neutrophils, there was no evidence for a mediation of cell responses to LNO2 by •NO- or cGMP-dependent mechanisms. In contrast, vascular cells were observed herein to rapidly metabolize LNO2 to an oxyHb-inhibitable species that activated GC and induced relaxation of aortic rings. Also, the GC inhibitor ODQ prevented LNO2-induced vasorelaxation, supporting the precept that a •NO or nitrosothiol-like species mediates the vascular actions of LNO2 (Fig. 4B). Endothelium was not critical for transducing vasoactive actions of LNO2, because inhibition of NOS and denudation of endothelium revealed similar extents of cGMP elevation and vessel relaxation. The ability of smooth muscle cells to metabolize LNO2 to a •NO-like cGMP-dependent species is distinctive when compared with the platelet and neutrophil signaling actions of LNO2. Nitrated organic esters, such as urate, glucose, and glycerol derivatives, are at least in part metabolized to •NO via the reductase action of mitochondrial aldehyde dehydrogensae (55). Nitrosamines, NONOates, nitrosothiols, nitroprusside, organic nitrites (RONO, e.g., isopentyl nitrite), and vicinal nitrohydroxy-eicosanoid derivatives spontaneously liberate •NO in a biological milieu via mechanisms sometimes stimulated by metal centers or thiols (55–58). Because we did not observe spontaneous liberation of •NO from LNO2 in the absence or presence of light or thiols, the present data show a requirement for vascular cell metabolism during tissue generation of •NO-like species from the more stable allylic nitro adduct of LNO2.
production, N-formyl-methionyl-leucyl-phenylalanine (fMLP)-stimulated Ca+2 influx, degranulation, and CD11b expression] via non-cGMP-dependent mechanisms (53). Also, LNO2 inhibits thrombin-mediated platelet aggregation by cAMP-dependent attenuation of Ca+2 mobilization and phosphorylation of vasodilator-stimulated phosphoprotein at serine 157. This occurs via dual regulation of adenylyl cyclase and phosphodiesterase-3 activities (54). In both platelets and neutrophils, there was no evidence for a mediation of cell responses to LNO2 by •NO- or cGMP-dependent mechanisms. In contrast, vascular cells were observed herein to rapidly metabolize LNO2 to an oxyHb-inhibitable species that activated GC and induced relaxation of aortic rings. Also, the GC inhibitor ODQ prevented LNO2-induced vasorelaxation, supporting the precept that a •NO or nitrosothiol-like species mediates the vascular actions of LNO2 (Fig. 4B). Endothelium was not critical for transducing vasoactive actions of LNO2, because inhibition of NOS and denudation of endothelium revealed similar extents of cGMP elevation and vessel relaxation. The ability of smooth muscle cells to metabolize LNO2 to a •NO-like cGMP-dependent species is distinctive when compared with the platelet and neutrophil signaling actions of LNO2. Nitrated organic esters, such as urate, glucose, and glycerol derivatives, are at least in part metabolized to •NO via the reductase action of mitochondrial aldehyde dehydrogensae (55). Nitrosamines, NONOates, nitrosothiols, nitroprusside, organic nitrites (RONO, e.g., isopentyl nitrite), and vicinal nitrohydroxy-eicosanoid derivatives spontaneously liberate •NO in a biological milieu via mechanisms sometimes stimulated by metal centers or thiols (55–58). Because we did not observe spontaneous liberation of •NO from LNO2 in the absence or presence of light or thiols, the present data show a requirement for vascular cell metabolism during tissue generation of •NO-like species from the more stable allylic nitro adduct of LNO2.
Synthetic LNO2 displayed chromatographic elution characteristics similar to the species produced by nitration of linoleate by HNO2, ONOO−, and exposure to a MPO/NO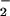 /H2O2 oxidation system (Fig. 1A and data not shown). Identity of these products as LNO2 was affirmed by internally consistent mass, IR, and 15N-NMR spectral properties. MS verified the presence of a parent compound with m/z = 324 and an MS/MS daughter ion of m/z = 46. The IR spectrum of LNO2, when compared with that of 18:2, displayed absorbance maxima at 1,334 and 1,522 cm−1, corresponding to the symmetric and asymmetric stretches of the N
/H2O2 oxidation system (Fig. 1A and data not shown). Identity of these products as LNO2 was affirmed by internally consistent mass, IR, and 15N-NMR spectral properties. MS verified the presence of a parent compound with m/z = 324 and an MS/MS daughter ion of m/z = 46. The IR spectrum of LNO2, when compared with that of 18:2, displayed absorbance maxima at 1,334 and 1,522 cm−1, corresponding to the symmetric and asymmetric stretches of the N O bonds of a nitroalkene. No maxima appeared in the 1,610- to 1,680-cm−1 region where the N
O bonds of a nitroalkene. No maxima appeared in the 1,610- to 1,680-cm−1 region where the N O bonds of organic nitrites and nitrates would strongly absorb (31). The 15N-NMR spectrum of LNO2 had chemical shifts at −3.9 and −4.6 ppm when externally referenced to nitromethane (0 ppm), suggesting the presence of at least two positional isomers. Other related compounds with −NO2 functional groups have shifts between ±30 ppm when referenced to nitromethane (34). The presence of the nitrito derivative (LONO) was eliminated, due to the absence of both a characteristic IR spectrum and the expected 15N-NMR chemical shift at ≈200 ppm (34). Nitrating species such as •NO2 can react with unsaturated fatty acids by both addition and H-atom abstraction reactions, events that are influenced by •NO2 concentration and solvent polarity (26). Thus, present effort is directed toward the characterization of nitrated lipids of different acyl chain lengths and unsaturation with respect to allylic and nitro orientations, the concomitant presence in some products of hydroxyl, peroxyl, or nitrate ester derivatives, and the purification of specific positional isomers in quantities sufficient for assessing relative bioactivities of key isomers. In this regard, progress has been made in defining the structural characteristics of polyunsaturated acyl esters (e.g., organic-solvated methyl linoleate and methyl arachidonate) exposed to acidic NO
O bonds of organic nitrites and nitrates would strongly absorb (31). The 15N-NMR spectrum of LNO2 had chemical shifts at −3.9 and −4.6 ppm when externally referenced to nitromethane (0 ppm), suggesting the presence of at least two positional isomers. Other related compounds with −NO2 functional groups have shifts between ±30 ppm when referenced to nitromethane (34). The presence of the nitrito derivative (LONO) was eliminated, due to the absence of both a characteristic IR spectrum and the expected 15N-NMR chemical shift at ≈200 ppm (34). Nitrating species such as •NO2 can react with unsaturated fatty acids by both addition and H-atom abstraction reactions, events that are influenced by •NO2 concentration and solvent polarity (26). Thus, present effort is directed toward the characterization of nitrated lipids of different acyl chain lengths and unsaturation with respect to allylic and nitro orientations, the concomitant presence in some products of hydroxyl, peroxyl, or nitrate ester derivatives, and the purification of specific positional isomers in quantities sufficient for assessing relative bioactivities of key isomers. In this regard, progress has been made in defining the structural characteristics of polyunsaturated acyl esters (e.g., organic-solvated methyl linoleate and methyl arachidonate) exposed to acidic NO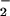 , pure •NO in the presence of oxygen and a wide range of gaseous •NO2 concentrations (25, 26, 44, 45).
, pure •NO in the presence of oxygen and a wide range of gaseous •NO2 concentrations (25, 26, 44, 45).
The in vivo production of nitrated free and esterified fatty acids is expected from the occurrence of multiple pathways for generating nitrating intermediates during cell signaling and inflammatory conditions (6, 59). For example, the generation of nitro derivatives of DNA bases, carbohydrates, free tyrosine, and protein tyrosine residues has been observed in >40 disease processes (59). The parallel discovery of enzymatic pathways mediating the reduction or denitration of protein 3-nitrotyrosine derivatives (60) also infers that regulable signaling events can be a consequence of biomolecule nitration. Determination of the in vivo presence of nitrated fatty acids, while described for human plasma (61), bovine cardiac muscle phospholipids (56), human atherosclerotic lesions, and ischemic/reperfused rodent liver (unpublished observations), is challenged by complications induced by extraction procedures and a rapid metabolism of LNO2 by vascular tissues during preparative procedures. There is also significant risk for artifactual de novo LNO2 production during acidic extraction or analytical procedures when adventitious NO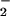 is present (24, 56, 61). In this regard, it has been observed that pH 4–4.5 lipid extraction or analytical conditions is sufficient to stimulate NO
is present (24, 56, 61). In this regard, it has been observed that pH 4–4.5 lipid extraction or analytical conditions is sufficient to stimulate NO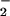 -mediated linoleate nitration (24). Once plasma and tissue concentrations of nitrated lipids are better defined, a further challenge exists in the translation of concentration-response observations derived from in vitro studies of LNO2 signaling to the prediction of in vivo organ-level responses. It is noted that solutions of lipophilic and amphipathic molecules added to in vitro test systems must diffuse through an aqueous milieu and partition into membranes and lipoproteins, as well as bind to hydrophobic regions of target proteins, before exerting signaling actions or undergoing metabolism. In this regard, a strong precedent exists for much greater concentrations of exogenously supplied lipid signaling molecules being required, as compared with endogenous production, for activation of a response element such as the peroxisome proliferator-activated receptor-γ (62).
-mediated linoleate nitration (24). Once plasma and tissue concentrations of nitrated lipids are better defined, a further challenge exists in the translation of concentration-response observations derived from in vitro studies of LNO2 signaling to the prediction of in vivo organ-level responses. It is noted that solutions of lipophilic and amphipathic molecules added to in vitro test systems must diffuse through an aqueous milieu and partition into membranes and lipoproteins, as well as bind to hydrophobic regions of target proteins, before exerting signaling actions or undergoing metabolism. In this regard, a strong precedent exists for much greater concentrations of exogenously supplied lipid signaling molecules being required, as compared with endogenous production, for activation of a response element such as the peroxisome proliferator-activated receptor-γ (62).
In summary, a nitrated lipid derivative has been synthesized and purified that is similar in basic structure and chromatographic behavior to nitrated lipid species generated by •NO-dependent signaling and inflammatory reactions. The cGMP-dependent vasomotor effects of this derivative in smooth muscle cells reveal that nitrated membrane and lipoprotein lipids can uniquely transduce •NO signaling reactions. We have also recently observed antiplatelet and neutrophil-inhibitory actions of LNO2 that are not exclusively cGMP-dependent (53, 54). These collective properties reveal that nitrated lipids, produced in greater rates and quantities during inflammation, can mediate the attenuation of inflammation or promotion of tissue repair by down-regulation of key signaling elements and events.
Supplementary Material
Acknowledgments
V.B.O. is supported by the British Heart Foundation and the Wellcome Trust and is a Wellcome Trust RCD Fellow. This work was supported by the National Institutes of Health (RO1-HL64937, RO1-HL 58115, P6-HL58418, and NCI CA13148).
Abbreviations
ODQ, 1H-[1,2,4] oxadiazole[4,3-a]quinoxalin-1-one
GC, guanylate cyclase
LNO2, nitrolinoleate
MPO, myeloperoxidase
PGHS, prostaglandin H synthase
RASMC, rat aortic smooth muscle cells
MS, mass spectroscopy
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ignarro L. J. (2000) in Nitric Oxide Biology and Pathobiology, ed. Ignarro, L. J. (Academic, San Diego), pp. 3–19.
- 2.Moncada S. & Higgs, A. (1993) N. Engl. J. Med. 329, 2002-2012. [DOI] [PubMed] [Google Scholar]
- 3.Ignarro L. J., Lippton, H., Edwards, J. C., Baricos, W. H., Hyman, A. L., Kadowitz, P. J. & Gruetter, C. A. (1981) J. Pharmacol. Exp. Ther. 218, 739-749. [PubMed] [Google Scholar]
- 4.Liu X., Miller, M. J. S., Joshi, M. S., Thomas, D. D. & Lancaster, J. R., Jr. (1998) Proc. Natl. Acad. Sci. USA 95, 2175-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas D. D., Liu, X., Kantrow, S. P. & Lancaster, J. R., Jr. (2001) Proc. Natl. Acad. Sci. USA 98, 355-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckman J. S., Beckman, T. W., Chen, J., Marshall, P. A. & Freeman, B. A. (1990) Proc. Natl. Acad. Sci. USA 87, 1620-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radi R., Denicola, A., Alvarez, B., Ferrer-Sueta, G. & Rubbo, H. (2000) in Nitric Oxide Biology and Pathobiology, ed. Ignarro, L. J. (Academic, San Diego), pp. 57–82.
- 8.Rubanyi G. M., Ho, E. H., Cantor, E. H., Lumma, W. C. & Botelho, P. (1991) Biochem. Biophys. Res. Commun. 181, 1392-1397. [DOI] [PubMed] [Google Scholar]
- 9.White C. R., Brock, T. A., Chang, L., Crapo, J., Briscoe, P., Ku, D., Bradley, W. A., Gianturco, S. H., Gore, J., Freeman, B. A. & Tarpey, M. M. (1994) Proc. Natl. Acad. Sci. USA 91, 1044-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarpey M. M., Beckman, J. S., Ischiropoulos, H., Gore, J. Z. & Brock, T. A. (1995) FEBS Lett. 364, 314-318. [DOI] [PubMed] [Google Scholar]
- 11.White C. R., Moellering, D., Patel, R. P., Kirk, M., Barnes, S. & Darley-Usmar, V. M. (1997) Biochem. J. 328, 517-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skinner K. A., White, C. R., Patel, R., Tan, S., Barnes, S., Kirk, M., Darley-Usmar, V. & Parks, D. A. (1998) J. Biol. Chem. 273, 24491-24497. [DOI] [PubMed] [Google Scholar]
- 13.Napolitano A., Camera, E., Picardo, M. & d'Ischia, M. (2000) J. Org. Chem. 65, 4853-4860. [DOI] [PubMed] [Google Scholar]
- 14.Napolitano A., Camera, E., Picardo, M. & d'Ischia, M. (2002) J. Org. Chem. 67, 1125-1132. [DOI] [PubMed] [Google Scholar]
- 15.Denicola A., Batthyany, C., Lissi, E., Freeman, B. A., Rubbo, H. & Radi, R. (2002) J. Biol. Chem. 277, 932-936. [DOI] [PubMed] [Google Scholar]
- 16.Denicola A., Souza, J. M., Radi, R. & Lissi, E. (1996) Arch. Biochem. Biophys. 328, 208-212. [DOI] [PubMed] [Google Scholar]
- 17.Rafikova O., Rafikov, R. & Nudler, E. (2002) Proc. Natl. Acad. Sci. USA 99, 5913-5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padmaja S. & Huie, R. E. (1993) Biochem. Biophys. Res. Commun. 195, 539-544. [DOI] [PubMed] [Google Scholar]
- 19.Rubbo H., Radi, R., Trujillo, M., Telleri, R., Kalyanaraman, B., Barnes, S., Kirk, M. & Freeman, B. A. (1994) J. Biol. Chem. 269, 26066-26075. [PubMed] [Google Scholar]
- 20.O'Donnell V. B., Chumley, P. H., Hogg, N., Bloodsworth, A., Darley-Usmar, V. M. & Freeman, B. A. (1997) Biochemistry 36, 15216-15223. [DOI] [PubMed] [Google Scholar]
- 21.Rubbo H., Radi, R., Anselmi, D., Kirk, M., Barnes, S., Butler, J., Eiserich, J. P. & Freeman, B. A. (2000) J. Biol. Chem. 275, 10812-10818. [DOI] [PubMed] [Google Scholar]
- 22.O'Donnell V. B. & Freeman, B. A. (2001) Circ. Res. 88, 12-21. [DOI] [PubMed] [Google Scholar]
- 23.Denicola A., Souza, J. M. & Radi, R. (1998) Proc. Natl. Acad. Sci. USA 95, 3566-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Donnell V. B., Eiserich, J. P., Chumley, P. H., Jablonsky, M. J., Krishna, N. R., Kirk, M., Barnes, S., Darley-Usmar, V. M. & Freeman, B. A. (1999) Chem. Res. Toxicol. 12, 83-92. [DOI] [PubMed] [Google Scholar]
- 25.d'Ischia M. (1996) Tetrahedron Lett. 37, 5773-5774. [Google Scholar]
- 26.d'Ischia M., Rega, N. & Barone, V. (1999) Tetrahedron 55, 9297-9308. [Google Scholar]
- 27.Baldus S., Eiserich, J. P., Mani, A., Castro, L., Figueroa, M., Chumley, P., Ma, W., Tousson, A., White, C. R., Bullard, D. C., et al. (2001) J. Clin. Invest. 108, 1759-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eiserich J. P., Baldus, S., Brennan, M., Ma, W., Zhang, C., Tousson, A., Castro, L., Lusis, A. J., Nauseef, W. M., White, C. R. & Freeman, B. A. (2002) Science 296, 2391-2394. [DOI] [PubMed] [Google Scholar]
- 29.Hamaya T., Tomoda, S., Takeuchi, Y. & Nomura, Y. (1982) Tetrahedron Lett. 23, 4733-4734. [Google Scholar]
- 30.Seebach D., Calderari, G. & Knochel, P. (1985) Tetrahedron 41, 4861-4872. [Google Scholar]
- 31.Silverstein R. M., Bassler, G. C. & Morrill, T. C., (1991) Spectrometric Identification of Organic Compounds (Wiley, New York), pp. 91–133.
- 32.Finlayson-Pitts B. J., Sweetman, L. L. & Weissbart, B. (1987) Toxicol. Appl. Pharmacol. 89, 438-448. [DOI] [PubMed] [Google Scholar]
- 33.Ungnade H. E. & Smiley, R. A. (1956) J. Inorg. Chem. 21, 993-996. [Google Scholar]
- 34.Webb G. A., (1993) Annual Reports on NMR Spectroscopy (Academic, New York), Vol. 25, pp. 66–67. [Google Scholar]
- 35.Vane J. R. & Botting, R. M. (1995) Am. J. Cardiol. 75, 3A-10A. [DOI] [PubMed] [Google Scholar]
- 36.Moncada S. & Vane, J. R. (1979) Pharmacol. Rev. 30, 293-331. [PubMed] [Google Scholar]
- 37.Davidge S. T. (2001) Circ. Res. 89, 650-660. [DOI] [PubMed] [Google Scholar]
- 38.Dusting G. J., Moncada, S. & Vane, J. R. (1979) Prog. Cardiovasc. Dis. 21, 405-430. [DOI] [PubMed] [Google Scholar]
- 39.Roberts L. J., II & Morrow, J. D. (1997) Biochim. Biophys. Acta 1345, 121-135. [DOI] [PubMed] [Google Scholar]
- 40.Moore K. P., Darley-Usmar, V., Morrow, J. & Roberts, L. J., II (1995) Circ. Res. 77, 335-341. [DOI] [PubMed] [Google Scholar]
- 41.Long N. C., Suh, J., Morrow, J. D., Schiestl, R. H., Murthy, G. G. K., Brain, J. D. & Frei, B. (2001) J. Appl. Physiol. 91, 1694-1700. [DOI] [PubMed] [Google Scholar]
- 42.Morrow J. D., Frei, B., Longmire, A. W., Gaziano, M., Lynch, S. M., Shyr, Y., Strauss, W. E., Oates, J. A. & Roberts, L. J., II (1995) N. Engl. J. Med. 332, 1198-1203. [DOI] [PubMed] [Google Scholar]
- 43.Pryor W. A. & Lightsey, J. W. (1981) Science 214, 435-437. [DOI] [PubMed] [Google Scholar]
- 44.Gallon A. A. & Pryor, W. A. (1994) Lipids 29, 171-176. [DOI] [PubMed] [Google Scholar]
- 45.Gallon A. A. & Pryor, W. A. (1993) Lipids 28, 125-133. [DOI] [PubMed] [Google Scholar]
- 46.Tetsuka T., Daphna-Iken, D., Srivastava, S. K., Baier, L. D., DuMaine, J. & Morrison, A. R. (1994) Proc. Natl. Acad. Sci. USA 91, 12168-12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodwin D. C., Landino, L. M. & Marnett, L. J. (1999) FASEB J. 13, 1121-1136. [DOI] [PubMed] [Google Scholar]
- 48.O'Donnell V. B., Coles, B., Lewis, M. J., Crews, B. C., Marnett, L. J. & Freeman, B. A. (2000) J. Biol. Chem. 275, 38239-38244. [DOI] [PubMed] [Google Scholar]
- 49.O'Donnell V. B., Taylor, K. B., Parthasarathy, S., Kuhn, H., Koesling, D., Friebe, A., Bloodsworth, A., Darley-Usmar, V. M. & Freeman, B. A. (1999) J. Biol. Chem. 274, 20083-20091. [DOI] [PubMed] [Google Scholar]
- 50.Coffey M. J., Natarajan, R., Chumley, P. H., Coles, B., Thimmalapura, P., Nowell, M., Kuhn, H., Lewis, M. J., Freeman, B. A. & O'Donnell, V. B. (2001) Proc. Natl. Acad. Sci. USA 98, 8006-8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodwin D. C., Landino, L. M. & Marnett, L. J. (1999) Drug Metab. Rev. 31, 273-294. [DOI] [PubMed] [Google Scholar]
- 52.Marnett L. J., Wright, T. L., Crews, B. C., Tannenbaum, S. R. & Morrow, J. D. (2000) J. Biol. Chem. 275, 13427-13430. [DOI] [PubMed] [Google Scholar]
- 53.Coles B., Bloodsworth, A., Clark, S. R., Lewis, M. J., Cross, A. R., Freeman, B. A. & O'Donnell, V. B. (2002) Circ. Res. 91, 375-381. [DOI] [PubMed] [Google Scholar]
- 54.Coles B., Bloodsworth, A., Eiserich, J. P., Coffey, M. J., McLoughlin, R. M., Giddings, J. C., Lewis, M. J., Haslam, R. J., Freeman, B. A. & O'Donnell, V. B. (2002) J. Biol. Chem. 277, 5832-5840. [DOI] [PubMed] [Google Scholar]
- 55.Chen Z., Zhang, J. & Stamler, J. S. (2002) Proc. Natl. Acad. Sci. USA 99, 8306-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balazy M., Iesaki, T., Park, J. L., Jiang, H., Kaminski, P. M. & Wolin, M. S. (2001) J. Pharmacol. Exp. Ther. 299, 1-9. [PubMed] [Google Scholar]
- 57.Barnett D. J., McAninly, J. & Williams, D. L. H. (1994) J. Chem. Soc. Perkin Trans. 2, 1131-1133. [Google Scholar]
- 58.Williams D. L. H. (1996) Transition Met. Chem. 21, 189-191. [Google Scholar]
- 59.Greenacre S. A. & Ischiropoulos, H. (2001) Free Radical Res. 34, 541-581. [DOI] [PubMed] [Google Scholar]
- 60.Kamisaki Y., Wada, K., Bian, K., Balabanli, B., Davis, K., Martin, E., Behbod, F., Lee, Y. & Murad, F. (1998) Proc. Natl. Acad. Sci. USA 95, 11584-11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lima E. S., Mascio, P. D., Rubbo, H. & Abdalla, D. S. P. (2002) Biochemistry 41, 10717-10722. [DOI] [PubMed] [Google Scholar]
- 62.Huang J. T., Welch, J. S., Ricote, M., Binder, C. J., Willson, T. M., Kelly, C., Witztum, J. L., Funk, C. D., Conrad, D. & Glass, C. K. (1999) Nature 400, 378-382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.