Abstract
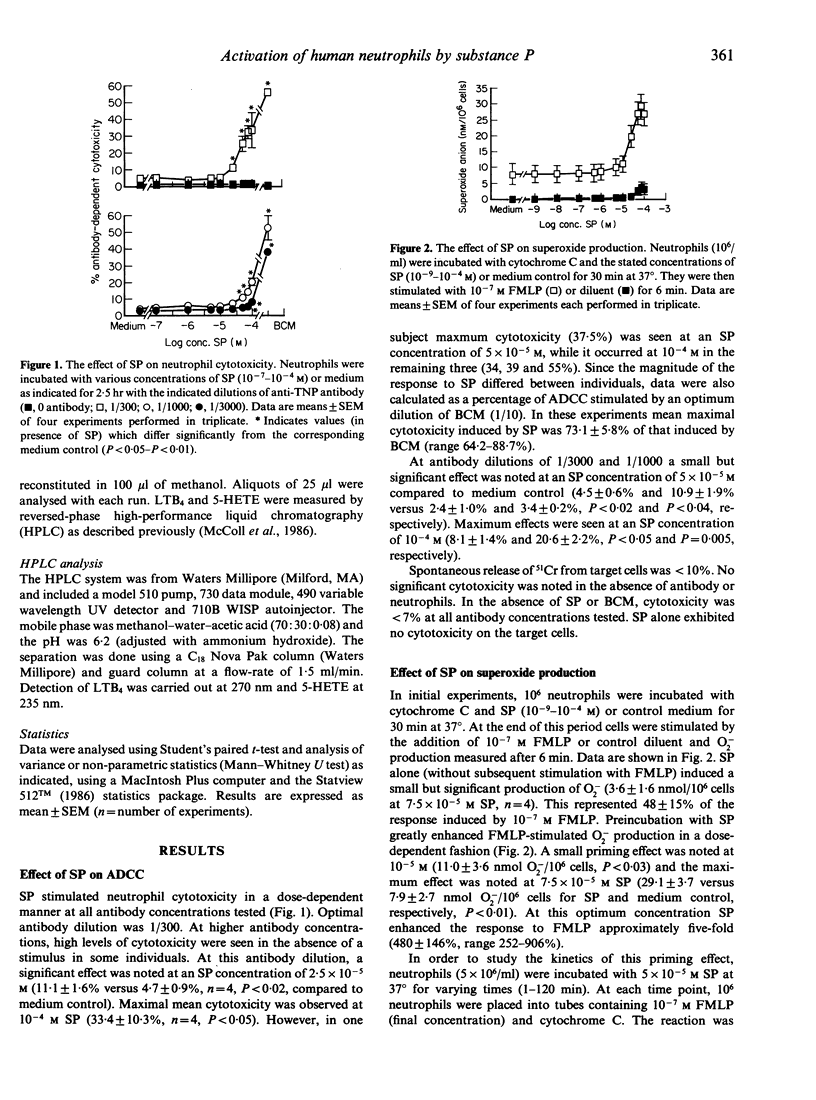
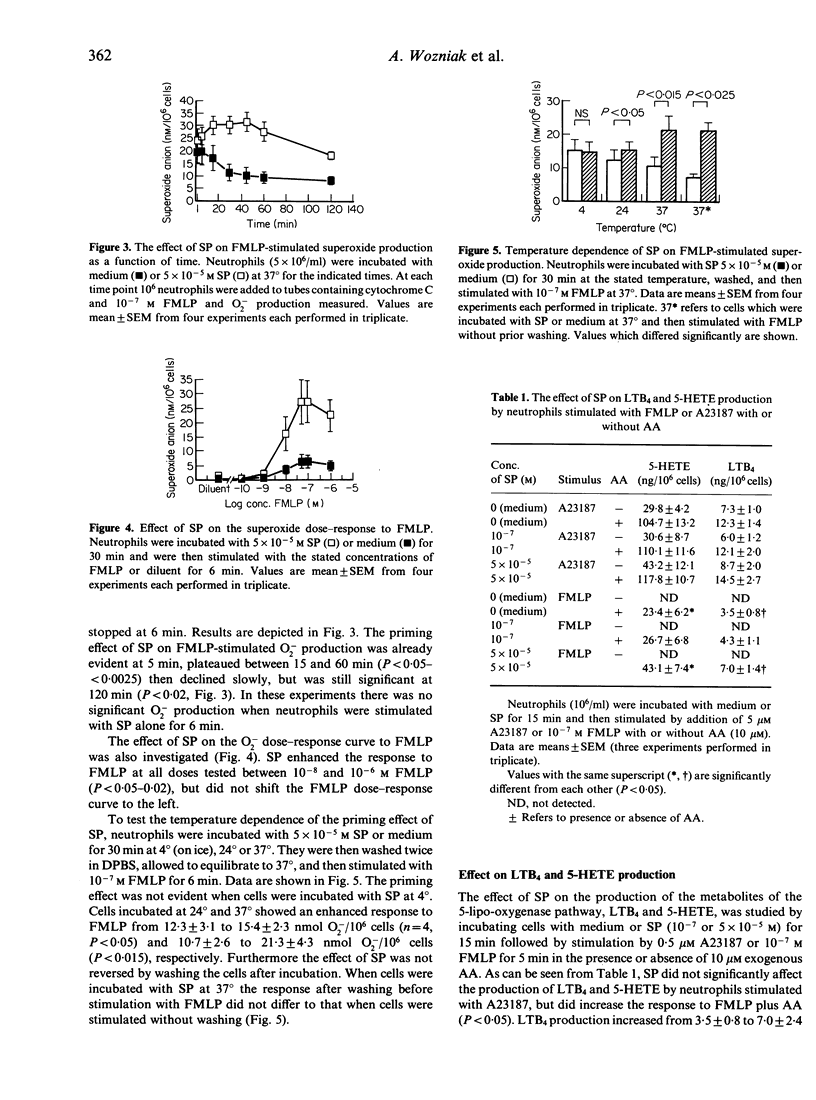
We show that the neuropeptide, substance P (SP), a putative mediator of neurogenic inflammation, is a potent regulator of mature, human neutrophil function. SP increased neutrophil cytotoxic activity against an antibody-coated target (P815 cells) in a dose-dependent manner. The maximal effect was noted at an SP concentration of 10(-4) M, when cytotoxicity increased from 4.7 +/- 0.9% to 33.4 +/- 10.3%. This effect was not due to toxicity of SP against the target cells and was antibody-dependent. The level of cytotoxic activity induced by SP was comparable to that described for a number of cytokines, such as GM-CSF, under identical assay conditions. SP-induced cytotoxicity was 73.1 +/- 5.8% of that produced by an optimum concentration of conditioned medium known to contain a number of cytokines which activate mature neutrophils. In addition, SP enhanced FMLP-stimulated superoxide anion production by neutrophils in a dose-dependent fashion. Neutrophils preincubated with medium or 7.5 x 10(-5) M SP and then stimulated with 10(-7) M FMLP produced 7.9 +/- 2.7 and 29.9 +/- 3.7 nmol superoxide anion/10(6) cells, respectively. This priming effect of SP was rapid in onset (less than 15 min) and was maximal from 15 to 60 min, after which it declined. It was not reversed by washing the cells and was temperature dependent. SP did not shift the dose-response curve to FMLP to the left, but it enhanced the response to FMLP in the concentration range 10(-8)-10(-6) M. Similarly SP enhanced LTB4 and 5-HETE production by FMLP-stimulated but not calcium ionophore-stimulated neutrophils. Therefore, these data provide evidence that SP regulates a number of neutrophil functions and suggests a mechanism whereby the nervous system may affect the immune response. Furthermore, the regulatory effects of SP on the neutrophil functions studied appear to be similar to those of a number of cytokines that have been previously implicated in inflammation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson Y. H., Lopez A. F., Marasco W. A., Lucas C. M., Wong G. G., Burns G. F., Vadas M. A. Recombinant human granulocyte-macrophage colony-stimulating factor (rH GM-CSF) regulates f Met-Leu-Phe receptors on human neutrophils. Immunology. 1988 Jul;64(3):519–525. [PMC free article] [PubMed] [Google Scholar]
- Atkinson Y. H., Marasco W. A., Lopez A. F., Vadas M. A. Recombinant human tumor necrosis factor-alpha. Regulation of N-formylmethionylleucylphenylalanine receptor affinity and function on human neutrophils. J Clin Invest. 1988 Mar;81(3):759–765. doi: 10.1172/JCI113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Goldman R., Stabinsky Y., Gottlieb P., Fridkin M., Teichberg V. I., Blumberg S. Enhancement of phagocytosis - a newly found activity of substance P residing in its N-terminal tetrapeptide sequence. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1445–1451. doi: 10.1016/0006-291x(80)90581-1. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. Asthma as an axon reflex. Lancet. 1986 Feb 1;1(8475):242–245. doi: 10.1016/s0140-6736(86)90777-4. [DOI] [PubMed] [Google Scholar]
- Dallegri F., Frumento G., Ballestrero A., Goretti R., Patrone F. Relationship between antibody-dependent tumour cell lysis and primary granule exocytosis by human neutrophils. Clin Exp Immunol. 1987 Nov;70(2):479–483. [PMC free article] [PubMed] [Google Scholar]
- Foreman J., Jordan C. Histamine release and vascular changes induced by neuropeptides. Agents Actions. 1983 Apr;13(2-3):105–116. doi: 10.1007/BF01967311. [DOI] [PubMed] [Google Scholar]
- Hartung H. P., Wolters K., Toyka K. V. Substance P: binding properties and studies on cellular responses in guinea pig macrophages. J Immunol. 1986 May 15;136(10):3856–3863. [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. D., Clark R., Devor M., Helms C., Moskowitz M. A., Basbaum A. I. Intraneuronal substance P contributes to the severity of experimental arthritis. Science. 1984 Nov 2;226(4674):547–549. doi: 10.1126/science.6208609. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Williamson D. J., Gamble J. R., Begley C. G., Harlan J. M., Klebanoff S. J., Waltersdorph A., Wong G., Clark S. C., Vadas M. A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986 Nov;78(5):1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Carson D. A., Vaughan J. H. Substance P activation of rheumatoid synoviocytes: neural pathway in pathogenesis of arthritis. Science. 1987 Feb 20;235(4791):893–895. doi: 10.1126/science.2433770. [DOI] [PubMed] [Google Scholar]
- Lotz M., Vaughan J. H., Carson D. A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988 Sep 2;241(4870):1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A., Brodin E., Rosell S., Folkers K. A substance P antagonist inhibits vagally induced increase in vascular permeability and bronchial smooth muscle contraction in the guinea pig. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1120–1124. doi: 10.1073/pnas.80.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco W. A., Showell H. J., Becker E. L. Substance P binds to the formylpeptide chemotaxis receptor on the rabbit neutrophil. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1065–1072. doi: 10.1016/0006-291x(81)90727-0. [DOI] [PubMed] [Google Scholar]
- McColl S. R., Betts W. H., Murphy G. A., Cleland L. G. Determination of 5-lipoxygenase activity in human polymorphonuclear leukocytes using high-performance liquid chromatography. J Chromatogr. 1986 Jun 13;378(2):444–449. doi: 10.1016/s0378-4347(00)80740-9. [DOI] [PubMed] [Google Scholar]
- Omann G. M., Allen R. A., Bokoch G. M., Painter R. G., Traynor A. E., Sklar L. A. Signal transduction and cytoskeletal activation in the neutrophil. Physiol Rev. 1987 Jan;67(1):285–322. doi: 10.1152/physrev.1987.67.1.285. [DOI] [PubMed] [Google Scholar]
- Payan D. G., McGillis J. P., Goetzl E. J. Neuroimmunology. Adv Immunol. 1986;39:299–323. doi: 10.1016/s0065-2776(08)60353-3. [DOI] [PubMed] [Google Scholar]
- Payan D. G. Receptor-mediated mitogenic effects of substance P on cultured smooth muscle cells. Biochem Biophys Res Commun. 1985 Jul 16;130(1):104–109. doi: 10.1016/0006-291x(85)90388-2. [DOI] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983 Jun;35(2):85–141. [PubMed] [Google Scholar]
- Serra M. C., Bazzoni F., Della Bianca V., Greskowiak M., Rossi F. Activation of human neutrophils by substance P. Effect on oxidative metabolism, exocytosis, cytosolic Ca2+ concentration and inositol phosphate formation. J Immunol. 1988 Sep 15;141(6):2118–2124. [PubMed] [Google Scholar]
- Shanahan F., Denburg J. A., Fox J., Bienenstock J., Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985 Aug;135(2):1331–1337. [PubMed] [Google Scholar]
- Stimler-Gerard N. P. Neutral endopeptidase-like enzyme controls the contractile activity of substance P in guinea pig lung. J Clin Invest. 1987 Jun;79(6):1819–1825. doi: 10.1172/JCI113023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thureson-Klein A., Hedqvist P., Ohlén A., Raud J., Lindbom L. Leukotriene B4, platelet-activating factor and substance P as mediators of acute inflammation. Pathol Immunopathol Res. 1987;6(3):190–206. doi: 10.1159/000157045. [DOI] [PubMed] [Google Scholar]
- Vadas M. A., Nicola N. A., Metcalf D. Activation of antibody-dependent cell-mediated cytotoxicity of human neutrophils and eosinophils by separate colony-stimulating factors. J Immunol. 1983 Feb;130(2):795–799. [PubMed] [Google Scholar]
- Wagner F., Fink R., Hart R., Dancygier H. Substance P enhances interferon-gamma production by human peripheral blood mononuclear cells. Regul Pept. 1987 Dec;19(5-6):355–364. doi: 10.1016/0167-0115(87)90177-7. [DOI] [PubMed] [Google Scholar]
- Watson S. P., Downes C. P. Substance P induced hydrolysis of inositol phospholipids in guinea-pig ileum and rat hypothalamus. Eur J Pharmacol. 1983 Sep 30;93(3-4):245–253. doi: 10.1016/0014-2999(83)90144-9. [DOI] [PubMed] [Google Scholar]
- Weening R. S., Wever R., Roos D. Quantitative aspects of the production of superoxide radicals by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):245–252. [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]


