Abstract
Human telomerase is a reverse-transcriptase enzyme that synthesizes the multikilobase repeating hexamer telomere sequence (TTAGGG)n at the ends of chromosomes. Here we describe a designed approach to mimicry of telomerase, in which synthetic DNA nanocircles act as essentially infinite catalytic templates for efficient synthesis of long telomeres by DNA polymerase enzymes. Results show that the combination of a nanocircle and a DNA polymerase gives a positive telomere-repeat amplification protocol assay result for telomerase activity, and similar to the natural enzyme, it is inhibited by a known telomerase inhibitor. We show that artificial telomeres can be engineered on human chromosomes by this approach. This strategy allows for the preparation of synthetic telomeres for biological and structural study of telomeres and proteins that interact with them, and it raises the possibility of telomere engineering in cells without expression of telomerase itself. Finally, the results provide direct physical support for a recently proposed rolling-circle mechanism for telomerase-independent telomere elongation.
Keywords: rolling-circle replication, primer extension, telomerase, TRAP assay
The telomerase enzyme synthesizes the multikilobase repeating hexamer telomere sequence (TTAGGG)n at the ends of chromosomes (1–3). The protein component shares significant sequence homology with known reverse transcriptases. Unlike common reverse transcriptases, however, telomerase is a ribonucleoprotein in which the associated RNA fragment (human telomerase RNA) acts as the template for addition of this G-rich sequence. To make multiple repeats, the enzyme shuttles every six nucleotides to initiate a new hexamer. Although the protein and RNA components of human telomerase have been cloned, telomerase is difficult to reconstitute in pure form. Standard telomerase preparations from cell extracts have low activity and purity and only moderate processivity. As a result, it is difficult to prepare G-rich telomere repeats for structural and functional studies. Here we describe an approach to functional mimicry of telomerase by providing a synthetic template for common DNA polymerases that encodes essentially infinite telomere repeats. This synthetic template consists of a nanometer-scale circular DNA encoding the telomeric G-rich strand. This telomerase-mimicking system allows for simple and efficient synthesis of long telomere repeats.
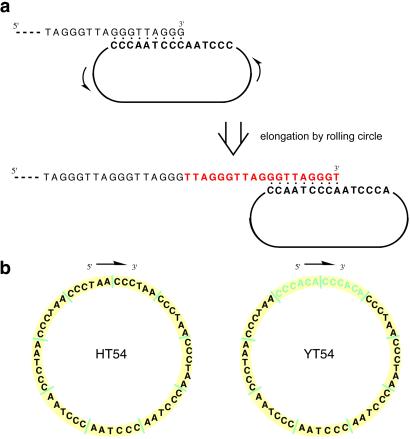
Replication of very small circular DNA templates by polymerase enzymes has been shown to result in the synthesis of repeating DNAs complementary to the circle (4, 5). Small (nanometer-scale) circular single-stranded DNAs as short as 18 nt are known to act as substrates for a number of DNA polymerases, and rolling-circle amplification on such templates recently has become broadly useful as an isothermal amplification method (6–8). The only requirements for successful rolling replication with such small circles are a DNA polymerase, nucleoside triphosphates, and a primer 3′ end complementary to a portion of the circle. We recognized that a primer representing a telomere 3′-overhanging end sequence might be a substrate for extension by the rolling-circle mechanism if a DNA nanocircle were complementary to the 3′ end. Elongation of this primer (Fig. 1) would result in extension of the telomere sequence isothermally, mimicking the action of telomerase itself. This extension is biologically relevant, because telomere length is a primary determinant of replicative life span of human cells (9, 10). Moreover, the ability to readily synthesize long telomeric repeats should facilitate structural and functional studies of telomeres greatly.
Fig 1.
Mechanism and sequences involved in telomerase mimicry with DNA nanocircles. (a) Illustration of binding of nanocircle template to telomeric DNA 3′ end, acting as a primer, and polymerase-catalyzed elongation by rolling of the circle to produce new telomeric repeats. (b) Sequences of nanocircles in this study.
Materials and Methods
Telomere-Encoding Nanocircles.
DNA circles were synthesized and purified from linear precursors according to published procedures (11). Circularity was confirmed by nicking with S1 endonuclease; gel electrophoretic analysis of the products of S1 cleavage showed mobility identical to that of a linear 54-mer of the same sequence. [In brief, this problem arises because closure by ligase requires the addition of a short DNA splint complementary to the ends, bringing the reactive ends together. In a perfect repeat, such a splint will bind not to the ends but rather to the intact repeats (12).] The HT54 nanocircle required special base-protection strategies and was prepared as described (12). All synthetic oligonucleotides were purified by denaturing PAGE.
Telomere Primer Extension.
All extension reactions contained 100 nM circle, 100 μM 18-mer primer (5′-TTAGGGTTAGGGTTAGGG-3′), and 1 mM each of dATP, dCTP, dGTP, and dTTP in a total reaction volume of 25 μl. For calf thymus DNA polymerase α (pol α, Chimerx, Milwaukee, WI), reactions contained 60 mM Tris⋅HCl (pH 8.0), 5 mM MgCl2, 0.3 mg/ml BSA, 1 mM DTT, 0.1 mM spermidine, and 0.15 units/μl pol α. Reactions using human DNA polymerase β (pol β, Chimerx) contained 50 mM Tris⋅HCl (pH 8.7), 5 mM MgCl2, 100 mM KCl, 0.4 mg/ml BSA, 1 mM DTT, and 0.16 units/μl pol β. Reactions using exo minus Klenow fragment of DNA pol I (KF−, United States Biochemical) contained 50 mM Tris⋅HCl (pH 7.5), 10 mM MgCl2, 1 mM DTT, 50 μg/ml BSA, and 0.4 units/μl KF−. For Sequenase 2.0 (exonuclease-free T7 DNA polymerase, United States Biochemical), reactions contained 40 mM Tris⋅HCl (pH 7.5), 20 mM MgCl2, 0.2 mM DTT, and 0.52 units/μl Sequenase. Reactions using thermophilic Deep Vent DNA polymerase (DV, New England Biolabs) contained 10 mM KCl, 10 mM (NH4)2SO4, 20 mM Tris⋅HCl (pH 8.8), 2.0 mM MgSO4, 0.1% Triton X-100, and 0.08 units/μl DV. The DV reactions were incubated at 70°C, whereas all other extension reactions were carried out at 37°C. All reactions proceeded for 3 h and were stopped by the addition of an equal volume of PAGE loading buffer (10 mM EDTA in formamide). Reaction mixtures then were run on 20% denaturing PAGE gels at 30 W for 2 h.
DNA sequencing of single-stranded circle-extension products was carried out after extension with pol β. Polymerase reaction conditions were as follows: 100 nM circle/100 nM primer/1 mM each of dATP, dTTP, dCTP and dGTP (Roche Biosciences, Palo Alto, CA)/8 units of human DNA pol β (Chimerx)/reaction buffer (50 mM Tris⋅HCl (pH 8.7)/10 mM MgCl2/100 mM KCl/0.2 mg/ml BSA/1.0 mM DTT) were incubated as 50-μl reactions for 4 h. Reactions were purified immediately by using the QIAquick PCR purification kit (Qiagen, Valencia, CA) to isolate single-stranded DNA larger than primer, which was subsequently concentrated and redissolved in water. The sequencing primer was 5′-dCCC TAA CCC TAA CCC TAA CC. Products were sequenced by Sanger methods at Biotech Core (Palo Alto, CA).
Telomere-Repeat Amplification Protocol (TRAP) Assay.
TRAP-assay reactions were performed in two steps, with separate elongation and amplification reactions. Each 25-μl reaction contained 12.5 pmol of telomerase substrate primer (5′-dAGC ATC CGT CGA GCA GAG TT-3′) and 1 mM each of dATP, dGTP, and dTTP in pol β or Klenow buffer. Circle, if included, was present at 100 nM; pol β was used at 0.2 units/μl, Klenow (exo−) polymerase was used at 0.01 units/μl, or 1 μl of a 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) extract from HeLa cells (National Cell Culture Center) was used as the telomerase sample. Elongation reactions were run at 30°C for 10 min and terminated by heating at 90°C for 1 min, and the products were ethanol-precipitated then resuspended in 15 μl of water for the amplification reaction. TRAP reactions (25-μl) were run as described (13, 14). Five microliters of native loading buffer was added to each sample, and the products were separated by native PAGE on a 10% 0.5× Tris-borate-EDTA (TBE) nondenaturing gel. Labeled products were visualized by exposure to autoradiography films.
Inhibition Studies.
Primer d(TTAGGG)4 was incubated with TMPyP4 (Aldrich) in the presence of pol β buffer and dNTPs at 37°C for 30 min. HT54 nanocircle and pol β then were added, bringing the final concentrations to 100 nM primer/100 nM circle/1× pol β buffer/0.16 units/ml pol β/1 mM each of dATP, dGTP, dCTP, and dTTP. Reactions proceeded for 10 min at 37°C and were stopped by the addition of an equal volume of PAGE loading buffer followed by heating at 90°C for 90 sec. Samples then were stored at −70°C. Reaction products were run on 10% denaturing PAGE gels at 30 W for 2.5 h. Gels were scanned by using a Molecular Dynamics Storm 860 PhosphorImager, and reaction products were quantified by using IQMAC 1.2 software. All reactions were repeated five times; averages are shown with standard deviations as error bars.
Atomic Force Microscopy (AFM).
Telomere extension reactions were performed essentially as described above except with 1.3 units/μl Sequenase/20 nM HT54 circle/200 nM 18-mer primer. Circle and primer were annealed by heating to 95°C for 3 min and then cooling to 38°C over a period of 15 min. Reaction was begun by adding Sequenase and dNTPs and continued for 4 h at 38°C. Samples were stored overnight at 4°C or longer at −10°C. For AFM imaging, samples were diluted 10-fold with 40 mM Hepes (pH 7.4)/10 mM MgCl2; 5-μl aliquots on freshly cleaved mica were incubated for 3 min at ambient temperature, rinsed with a few milliliters of MilliQ-purified water, and dried in a stream of compressed dry N2. Tapping-mode AFM imaging in air was performed with a silicon Nanosensor cantilever, resonant frequency ≈290–340 kHz, in a MultiMode atomic force microscope (Digital Instruments, Santa Barbara, CA).
Telomere Extension on Metaphase Chromosomes.
Metaphase spreads on glass slides were prepared from human 293 human embryonic kidney cells according to standard protocols. Slides were denatured in 70% formamide/2× SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7.0) for 2 min at 72°C followed by dehydration in 70, 85, and 95% aqueous ethanol solutions. A 25-μl mixture containing 0.2 mM dATP, 0.2 mM dGTP, 0.02 mM dTTP, 0.05 mM fluorescein-12-dUTP (Molecular Probes), 0.5 μM HT54 nanocircle, 5 units of Taq polymerase, and Thermopol buffer (New England Biolabs) was added to one slide. A similar mixture containing no circle was added to the control slide. Slides were incubated at 68°C for 20 min followed by 1 h at 72°C. After incubation, they were washed in 4× SSC/0.5% Tween-20 for 10 min at 65°C followed by dehydration in 70, 85, and 95% ethanol serially. Counterstaining was performed with a 90% glycerol solution containing 4,6-diamino-2-phenylindole (0.1 μg/ml, Sigma) and diazabicyclo[2.2.2]octane (20 mg/ml, Sigma). Digital images were acquired with a SPOT charge-coupled device camera mounted on a Nikon E800 epifluorescence microscope equipped with appropriate filters. For pol β extensions, pretreatment conditions (cell preparation, denaturation, and dehydration) were identical to those for the Taq reaction. Initially, 5 μl of a 12 μM solution of HT54 circle was added to the slide and heated at 95°C for 5 min. After cooling to room temperature, 10 μl of nucleotide mix (0.5 mM dATP and dGTP/50 μM dTTP/130 μM fluorescein-12-dUTP) and 5 μl of polymerase solution [10 units of pol β in the buffer recommended by the manufacturer (Chimerx)] were added. The slide was sealed and incubated for 1.5 h at 37°C. Subsequent steps (washing) were identical to those for the Taq extensions. The scrambled circle control (HT54SCR) had the sequence 5′-CAC TCC ACT CAC AAC ATC CAC ACC TCA CAC TAC AAC TCC AAC ACA CTC ACT CCT-3′.
Results and Discussion
Extension of Telomeric Primers.
This approach to mimicry of telomerase requires very small single-stranded DNA circles encoding telomere repeats. Such DNA circles of repetitive sequence are difficult or impossible to construct by standard methods. We initially prepared a chimeric 54-nt circular single-stranded DNA (YT54) encoding two Saccharomyces cerevisiae telomeric hexamers followed by seven human telomeric hexamers (Fig. 1b). This chimeric template sequence was chosen in part because of the problems posed by cyclization of a perfect repeating sequence with standard circle-closing ligation methods. To avoid this we included yeast sequences to allow a splint to bind uniquely at the ends, enabling closure by T4 DNA ligase. As controls we also prepared a sequence-scrambled 54-mer template circle (YS54) and a linear 54-mer of the yeast/human template sequence (YL54).
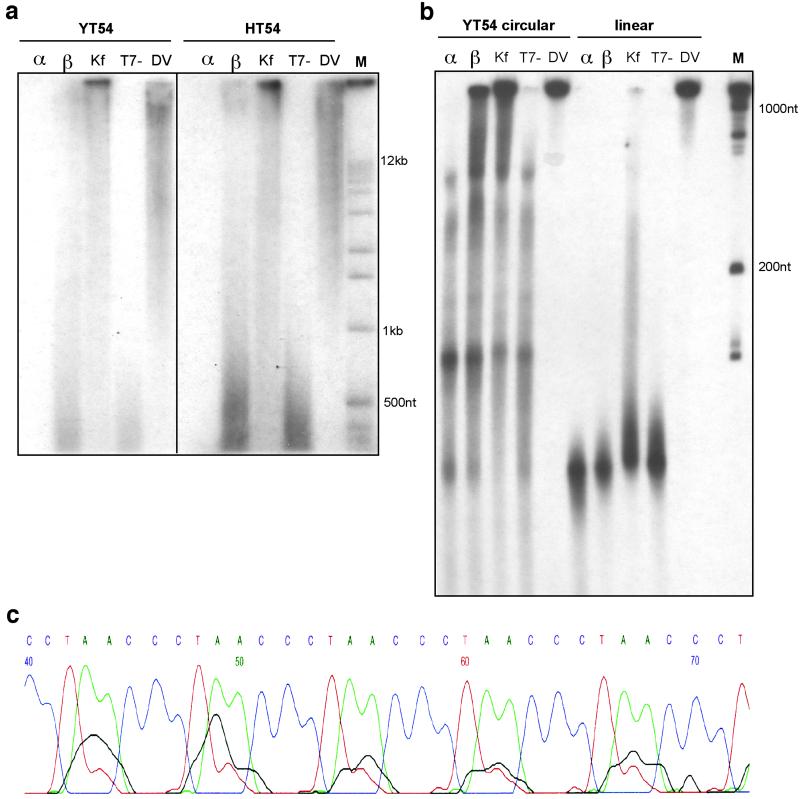
Standard end-labeled telomeric primers 18 nt in length then were reacted with these templates in the presence of common DNA polymerases and nucleoside triphosphates (Fig. 2). With the YT54 nanocircle, results showed rapid elongation of the primers to lengths ranging from a few hundred to >12 kb after 3 h (Fig. 2a). This is comparable in length to human telomeres, which are commonly 12 kb in length in primary human cells (10, 15). A sequence-scrambled circular control DNA, YS54, showed little or no elongation (data not shown), presumably because of a lack of complementarity to the primer, whereas the linear version of YT54, YL54, generally showed only products with lengths shorter than 100 nt (Fig. 2b). One interesting exception was the thermophilic polymerase, which produced relatively long products with the linear variant. Most enzymes tested showed telomerase-mimicking activity in the presence of the YT54 nanocircle, with thermophilic DV, KF polymerase, and human pol β giving the longest products. It is noteworthy that partially purified human telomerase is able to extend telomeres by only a few dozen repeats after similar times (ref. 13 and data not shown); thus rolling-circle templates can especially active in this context.
Fig 2.
Extension of telomeric primers in vitro with common DNA polymerases. (a) Agarose gel electrophoretic analysis of primer extension with YT54 and HT54 circles. α, pol α; β, pol β; Kf, KF of DNA pol I; T7-, T7 DNA pol (Sequenase 2.0). (b) PAGE gel analysis showing extension lengths relative to a 100-nt ladder with comparison to linear control. (c) Segment of sequencing trace showing results of Sanger sequencing of telomeric repeats on HT54 nanocircle template.
We then prepared a new DNA nanocircle (HT54) encoding nine uninterrupted human telomeric repeats by application of orthogonal protecting group chemistry that forces the splint to bind only to the ends (12). Rolling-replication experiments in vitro with this nanocircle proceeded as efficiently as with the YT54 chimera (Fig. 2a). Sanger sequencing of the elongated products using the HT54 nanocircle template and human pol β confirmed the presence of the expected human repeating sequences encoded by the rolling template (Fig. 2c).
Mimicry of Positive and Negative Telomerase Activities.
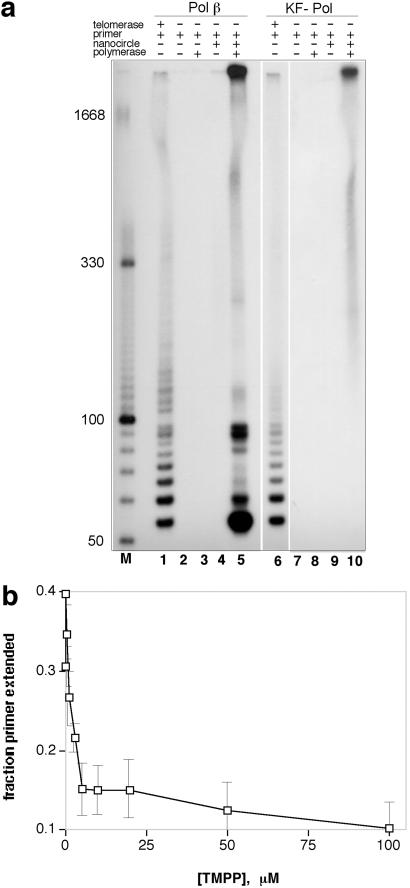
The standard method for measuring telomerase activity is the TRAP assay, which amplifies and labels repeating sequences by using a PCR after extension by telomerase (13, 14). We tested whether combinations of nanocircles and DNA polymerases could mimic human telomerase successfully in a TRAP assay. Experiments confirmed that the HT54 nanocircle in combination with pol β or the Escherichia coli KF− polymerase does in fact give a positive TRAP-labeling result (Fig. 3a, lanes 5 and 10). Controls lacking enzyme or circle gave negative results (lanes 3 and 4). Comparisons of the circle-mediated signal (lane 5) with the standard TRAP signal from HeLa extract (lane 1) show both similarities and differences. The circle+polymerase lane for pol β (lane 5) shows mostly the same short repeat bands observed with HeLa extract; however, a few are relatively much more abundant. Although the origins of these more intense bands are not confirmed yet, we hypothesize that some (particularly those ≈90 bp in length) may result from extension on small amounts of linear DNAs that are common in synthetic circular DNA preparations. The KF− experiment (lane 10) shows only very long extended products; this is the expected outcome of a TRAP assay if extension is very efficient. For both polymerase+circle experiments there is a high abundance of very long DNAs, which are low in abundance with HeLa extract.
Fig 3.
Mimicry of human telomerase activity and telomerase inhibition. (a) TRAP assay showing positive telomerase signals for human pol β with HT54 nanocircle (lane 5) with comparison to authentic telomerase result (HeLa extract, lane 1). Lanes 6–10 show results for E. coli KF (exo−) polymerase. (b) Inhibition of telomere extension by the cationic porphyrin TMPyP4 (TMPP), mimicking the known inhibition of telomerase extension by this compound. Experiments were done with pol β and HT54 circle.
To investigate further the extent of the mimicry of telomerase ribonucleoprotein by polymerase–nanocircle complexes, we then examined whether a known telomerase inhibitor might also inhibit our telomerase-mimicking system. DNA tetraplex-binding molecules are hypothesized to inhibit telomerase elongation by stabilizing the folded telomeric 3′ end, preventing binding of the ribonucleoprotein to the primer (16). The tetracationic porphyrin derivative TMPyP4 has been studied as such an inhibitor (17). Our experiments revealed (Fig. 3b) that TMPyP4 also inhibits elongation of 30-mer telomere primers by DNA polymerases (e.g., pol β) with nanocircle HT54. The IC50 was measured at ≈3 μM, similar to that reported for TMPyP4 with human telomerase (16). Significantly, the porphyrin inhibits elongation more effectively when added before polymerase elongation than after it has begun (data not shown), which supports the idea that stabilization of the primer tetraplex by TMPyP4 inhibits binding of the nanocircle, analogous to its mode of inhibition with telomerase itself. It also suggests that rolling synthesis by pol β is processive, because dissociation of the polymerase and circle might be expected to result in folding of the newly synthesized telomere into the stabilized tetraplex.
Imaging Artificial Telomeres.
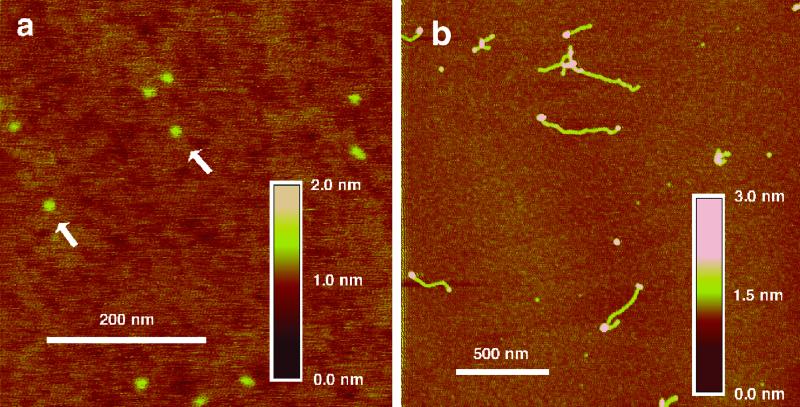
The simplicity of this approach to synthesis of artificial telomeres without telomerase (18) suggests its use in study of telomere structure in general. In this light we attempted to image the produced synthetic telomeric single strands by AFM (19). The results with exonuclease-deficient T7 DNA polymerase (Sequenase 2.0) in combination with the HT54 nanocircle clearly show (Fig. 4) apparent telomeric DNAs up to ≈0.5 μm in length (corresponding to ≈1,500 nt), and polymerase enzymes, imaged as roughly spherical features, are found to be associated with the ends. The nanocircles are smaller in diameter than the polymerases and resolved in the images when polymerase is absent (Fig. 4a). Also observed are aggregated structures, which might arise from intermolecular DNA–DNA interactions such as G tetraplexes (20). No long products were observed in the absence of polymerase or nanocircle.
Fig 4.
Imaging of newly synthesized telomeric single-stranded DNAs and associated polymerases by AFM. DNA nanocircles without polymerase are shown in a, and extended strands and polymerases are shown in b. The arrows in a indicate nanocircles. Length and height scale bars are given.
Extension of Telomeres on Metaphase Chromosomes.
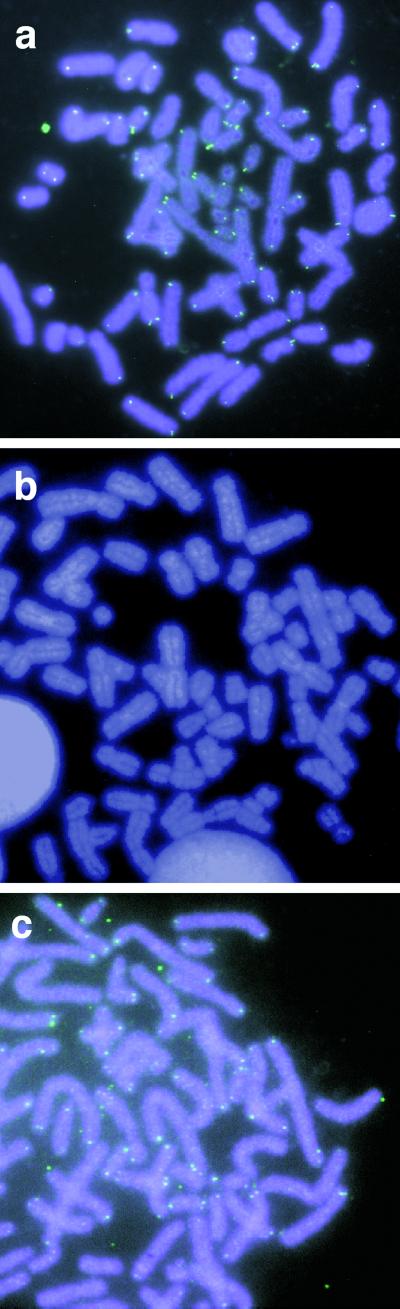
We then tested whether a DNA polymerase would be able to extend telomeres on human chromosomes in fixed cells. Metaphase spreads of cells on microscope slides were prepared from human 293 human embryonic kidney cells. These spreads were incubated with the HT54 nanocircle, nucleoside triphosphates, and the thermophilic Taq polymerase. New sequences were visualized by the uptake of fluorescein-labeled dUTP in the extension reaction. Results showed (Fig. 5a) that new, apparently telomeric sequences are clearly visible as green signals at chromosome ends. Controls lacking nanocircle gave no signal (Fig. 5b), and we found that dCTP was not needed to generate this signal, consistent with the expected (TTAGGG)n sequence. Experiments with a different control, in which a circle of scrambled sequence was used (HT54SCR), showed no signals (data not shown), consistent with the need for complementarity to the existing telomere end. Experiments with a second polymerase, human pol β, also showed new telomeric signals (Fig. 5c), thus establishing that (i) high temperatures are not necessary for elongation and (ii) polymerases of eukaryotic origin can function in this mechanism. Interestingly, for the majority of chromosomes we observe fewer than four signals per chromosome (Fig. 5 a and c), whereas four are observed by standard telomere fluorescence in situ hybridization methods (21). This may be due to either variations in end accessibility to the circle/polymerase complex as an artifact of the nuclear preparation methods or the possibility that chromatid telomere ends are inherently differently accessible. Overall, the results establish that human chromosome ends are able to act as primers for telomere extension by nanocircles, at least in fixed nuclear preparations. This suggests the possibility of telomere engineering in artificial chromosome constructs by this strategy.
Fig 5.
Synthesis of artificial telomeres on human chromosomes. Images show 4,6-diamino-2-phenylindole-counterstained metaphase chromosomes from 293 human embryonic kidney cells. (a) Fluorescein putative telomere signals in the presence of HT54 nanocircle, DNA polymerase, and three nucleotides (dATP, dGTP, and fluorescein-dUTP). (b) Lack of fluorescein signal when nanocircle is omitted. (c) Newly extended telomere signals resulting from extension by human pol β + HT54 circle.
The data show that the combination of a DNA polymerase and a nanocircle template effectively mimics the natural ribonucleoprotein composed of the human protein (hTERT) and the human telomerase RNA template. This approach offers an efficient and simple method for production of long telomeric repeats and may have utility in the study of the unusual secondary and tertiary structures formed by telomeres and their associated proteins (22–25). Chimeric nanocircles may make it possible also to encode chimeric telomeres, which otherwise are not possible to make by altering the human telomerase RNA template sequence. This may be useful in the investigation of mechanisms of apoptosis induced by telomere mutations (26). Last, the results suggest the future possibility of using nanocircles to engineer telomeres in cells without the need for expression of hTERT, by supplying circular templates for polymerases already present (11).
Rolling Circles in Alternative Pathways.
Finally, in certain human cells that lack telomerase, telomeres can be maintained by an alternative pathway (ALT) that results in greatly elongated telomeric tracts (27, 28). Evidence has been reported in ALT cells for extrachromosomal telomeric DNA that may be present in circular form (29). Such observations have led to the proposal that a rolling-circle mechanism might provide an alternative biological pathway for elongation and maintenance of telomere-repeat length (30). In addition, recent experiments in Kluyveromyces lactis have shown that double-stranded circular constructs containing some telomeric sequence can lead to telomere elongation by a mixture of mechanisms including integration at telomere ends and elongation by rolling and recombination (31). The present experiments add support to the above hypotheses and observations and establish that, at least in vitro, a simple rolling-circle mechanism is indeed feasible and can be highly efficient. Moreover, the single-stranded nanocircle design presents a minimal and readily accessible molecular template for achieving this elongation.
Acknowledgments
We thank Drs. S. Artandi and V. Lundblad for helpful comments. U.M.L. acknowledges a postdoctoral fellowship from the Swedish Research Council. H.G.H. acknowledges support from the National Science Foundation.
Abbreviations
pol α, DNA polymerase α
pol β, DNA polymerase β
KF, Klenow fragment
DV, Deep Vent DNA polymerase
TRAP, telomere-repeat amplification protocol
AFM, atomic force microscopy
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Greider C. W. (1998) Proc. Natl. Acad. Sci. USA 95, 90-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cech T. R. (2000) Angew. Chem. Int. Ed. Engl. 39, 34-43. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn E. H. (2001) Cell 106, 661-673. [DOI] [PubMed] [Google Scholar]
- 4.Fire A. & Xu, S. Q. (1995) Proc. Natl. Acad. Sci. USA 92, 4641-4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu D., Daubendiek, S. L., Zillmann, M. A., Ryan, K. & Kool, E. T. (1996) J. Am. Chem. Soc. 118, 1587-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lizardi P. M., Huang, X., Zhu, Z., Bray-Ward, P., Thomas, D. C. & Ward, D. C. (1998) Nat. Genet. 19, 225-232. [DOI] [PubMed] [Google Scholar]
- 7.Baner J., Nilsson, M., Mendel-Hartvig, M. & Landegren, U. (1998) Nucleic Acids Res. 26, 5073-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweitzer B., Wiltshire, S., Lambert, J., O'Malley, S., Kukanskis, K., Zhu, Z., Kingsmore, S. F., Lizardi, P. M. & Ward, D. C. (2000) Proc. Natl. Acad. Sci. USA 97, 10113-10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allsopp R. C., Vaziri, H., Patterson, C., Goldstein, S., Younglai, E. V., Futcher, A. B., Greider, C. W. & Harley, C. B. (1992) Proc. Natl. Acad. Sci. USA 89, 10114-10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayflick L. (2000) Br. J. Cancer 83, 841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohmichi T., Maki, A. & Kool, E. T. (2002) Proc. Natl. Acad. Sci. USA 99, 54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindström U. M. & Kool, E. T. (2002) Nucleic Acids Res. 30, E101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szatmari I. & Aradi, J. (2001) Nucleic Acids Res. 29, E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim N. W., Piatyszek, M. A., Prowse, K. R., Harley, C. B., West, M. D., Ho, P. L. C., Coviello, G. M., Wright, W. E., Weinrich, S. L. & Shay, J. W. (1994) Science 266, 2011-2015. [DOI] [PubMed] [Google Scholar]
- 15.Lindsey J., McGill, N. I., Lindsey, L. A., Green, D. K. & Cooke, H. J. (1991) Mutat. Res. 256, 45-48. [DOI] [PubMed] [Google Scholar]
- 16.Sun D. & Hurley, L. H. (2001) Methods Enzymol. 340, 573-592. [DOI] [PubMed] [Google Scholar]
- 17.Shi D. F., Wheelhouse, R. T., Sun, D. Y. & Hurley, L. H. (2001) J. Med. Chem. 44, 4509-4523. [DOI] [PubMed] [Google Scholar]
- 18.Guiducci C., Anglana, M., Wang, A. & Bacchetti, S. (2001) Exp. Cell Res. 265, 304-311. [DOI] [PubMed] [Google Scholar]
- 19.Hansma H. G. (2001) Annu. Rev. Phys. Chem. 52, 71-92. [DOI] [PubMed] [Google Scholar]
- 20.Marsh T. C., Vesenka, J. & Henderson, E. (1995) Nucleic Acids Res. 23, 696-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyne J., Ratliff, R. L. & Moyzis, R. K. (1989) Proc. Natl. Acad. Sci. USA 86, 7049-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zahler A. M., Williamson, J. R., Cech, T. R. & Prescott, D. M. (1991) Nature 350, 718-720. [DOI] [PubMed] [Google Scholar]
- 23.Griffith J. D., Comeau, L., Rosenfield, S., Stansel, R. M., Bianchi, A., Moss, H. & de Lange, T. (1999) Cell 97, 503-514. [DOI] [PubMed] [Google Scholar]
- 24.Evans S. K. & Lundblad, V. (2000) J. Cell Sci. 113, 3357-3364. [DOI] [PubMed] [Google Scholar]
- 25.de Lange T. (2002) Oncogene 21, 532-540. [DOI] [PubMed] [Google Scholar]
- 26.Kim M. M., Rivera, M. A., Botchkina, I. L., Shalaby, R., Thor, A. D. & Blackburn, E. H. (2001) Proc. Natl. Acad. Sci. USA 98, 7982-7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryan T. M., Marusic, L., Bacchetti, S., Namba, M. & Reddel, R. R. (1997) Hum. Mol. Genet. 6, 921-926. [DOI] [PubMed] [Google Scholar]
- 28.Lundblad V. (2002) Oncogene 21, 522-531. [DOI] [PubMed] [Google Scholar]
- 29.Regev A., Cohen, S., Cohen, E., Bar-Am, I. & Lavi, S. (1998) Oncogene 17, 3455-3461. [DOI] [PubMed] [Google Scholar]
- 30.McEachern M. J., Krauskopf, A. & Blackburn, E. H. (2000) Annu. Rev. Genet. 34, 331-358. [DOI] [PubMed] [Google Scholar]
- 31.Natarajan S. & McEachern, M. J. (2002) Mol. Cell. Biol. 22, 4512-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]