Abstract
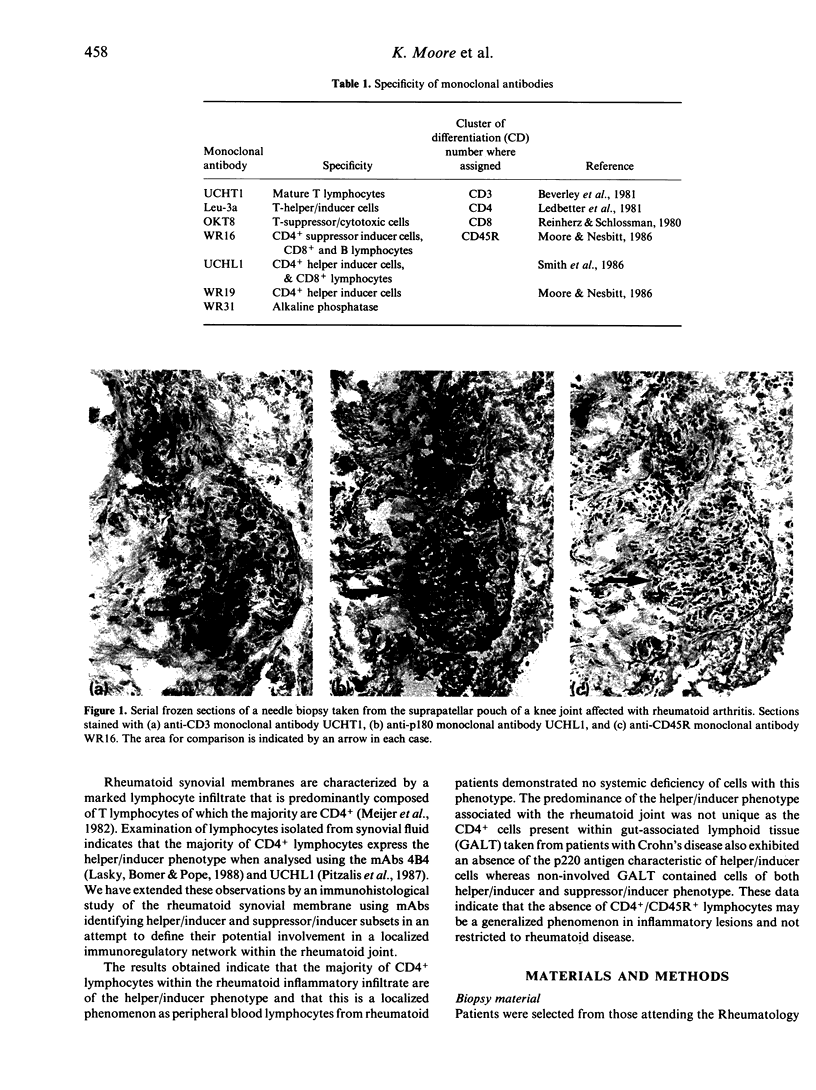
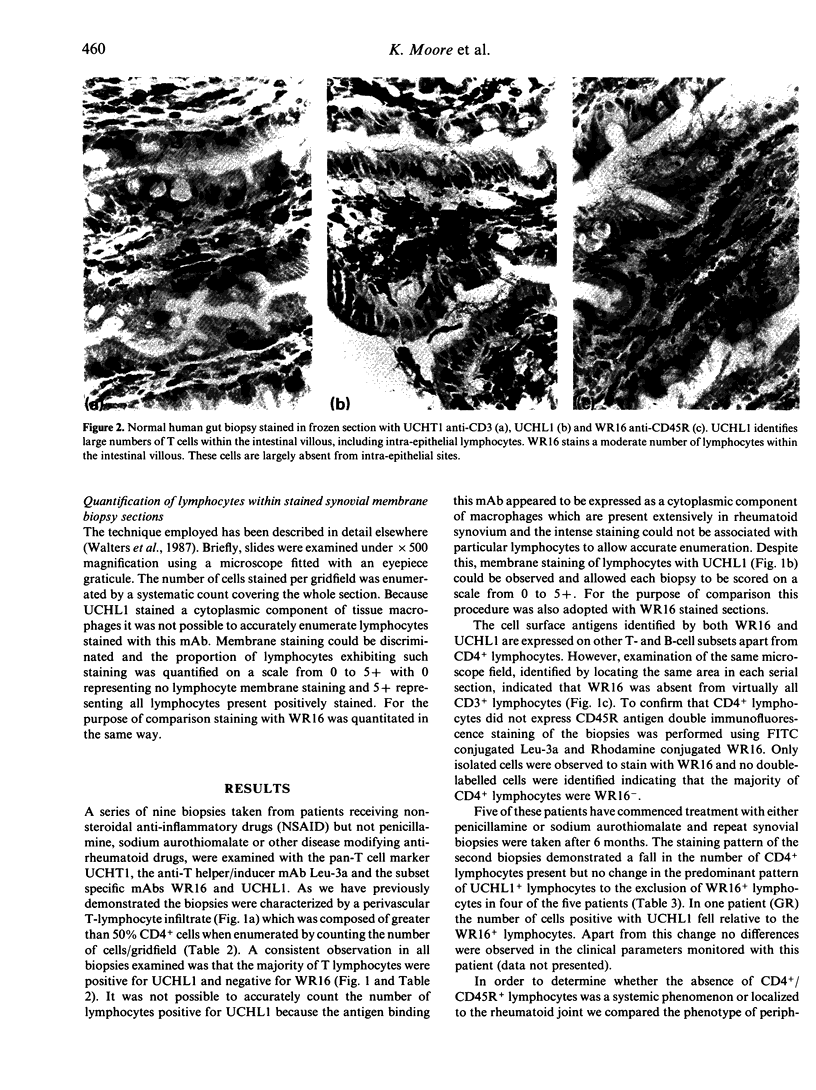
The monoclonal antibodies (mAbs) WR16, UCHL1 and WR19 identify subsets of CD4+ lymphocytes that have been functionally characterized as suppressor inducer cells or helper inducer cells. These were applied as components of a panel of lymphocyte-specific mAbs for the phenotypic analysis of lymphocyte populations within biopsies taken from rheumatoid synovial membrane and normal and inflamed gut. The phenotype of peripheral blood lymphocytes from patients with rheumatoid arthritis were also compared to normal controls. The rheumatoid synovium was characterized immunohistologically by a lymphocytic infiltrate composed predominantly of CD4+ lymphocytes and a CD4:CD8 ratio of 2.4. The CD4+ population was composed of UCHL1+ cells to the exclusion of WR16+ cells. This finding was confirmed by double immunofluorescence staining using directly conjugated Leu-3a and WR16. The UCHL1+/WR16-/CD4+ phenotype was maintained in the synovial biopsies regardless of whether the patient had commenced treatment with disease modifying drugs. The absence of WR16+ cells within the rheumatoid synovium was shown to be a localized phenomenon as there was a slight elevation of circulating WR16+ lymphocytes in the peripheral blood of rheumatoids whilst the levels of UCHL1+ and WR19+ lymphocytes remained unchanged. As no appropriate normal control tissue is available for comparison to the rheumatoid synovium we also examined the lymphocytes present within Crohn's disease-involved bowel biopsies and compared them to normal gut tissue lymphocytes using WR16 and UCHL1 mAbs. The CD3+ lymphocytes present within normal tissue comprised a mixture of WR16+ and UCHL1+ cells. In contrast the CD3+ lymphocytes within Crohn's involved tissue were exclusively UCHL1+ as previously observed in the rheumatoid synovium. These data indicate that the CD4+ lymphocyte infiltrate present within inflammatory lesions of presumed distinct aetiology exhibit a localized selective loss of cells with the CD45R+/CD4+ suppressor inducer phenotype. This may be a consequence of the selective extravasation of CD4+ helper induced cells or more likely, in view of the previously documented loss of the p220 molecule identified by CD45R mAbs upon T-cell activation, the result of CD4+ T-cell activation at sites of inflammation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander G. J., Nouri-Aria K. T., Eddleston A. L., Williams R. Contrasting relations between suppressor-cell function and suppressor-cell number in chronic liver disease. Lancet. 1983 Jun 11;1(8337):1291–1293. doi: 10.1016/s0140-6736(83)92410-8. [DOI] [PubMed] [Google Scholar]
- Braziel R. M., Sussman E., Jaffe E. S., Neckers L. M., Cossman J. Induction of immunoglobulin secretion in follicular non-Hodgkin's lymphomas: role of immunoregulatory T cells. Blood. 1985 Jul;66(1):128–134. [PubMed] [Google Scholar]
- Lasky H. P., Bauer K., Pope R. M. Increased helper inducer and decreased suppressor inducer phenotypes in the rheumatoid joint. Arthritis Rheum. 1988 Jan;31(1):52–59. doi: 10.1002/art.1780310108. [DOI] [PubMed] [Google Scholar]
- Meijer C. J., Lafeber G. J., Cnossen J., Damsteeg M. G., Cats A. T lymphocyte subpopulations in rheumatoid arthritis. J Rheumatol. 1982 Jan-Feb;9(1):18–24. [PubMed] [Google Scholar]
- Moore K., Nesbitt A. M. Functional heterogeneity of CD4+ T lymphocytes: two subpopulations with counteracting immunoregulatory functions identified with the monoclonal antibodies WR16 and WR19. Immunology. 1987 Jun;61(2):159–165. [PMC free article] [PubMed] [Google Scholar]
- Moore K., Nesbitt A. M. Identification and isolation of OKT4+ suppressor cells with the monoclonal antibody WR16. Immunology. 1986 Aug;58(4):659–664. [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Brown H. M., Schlossman S. F. The cellular basis for the induction of antigen-specific T8 suppressor cells. Eur J Immunol. 1986 Feb;16(2):198–204. doi: 10.1002/eji.1830160216. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Borel Y., Mantzouranis E., Steinberg A. D., Schlossman S. F. Autoantibody to an immunoregulatory inducer population in patients with juvenile rheumatoid arthritis. J Clin Invest. 1981 Mar;67(3):753–761. doi: 10.1172/JCI110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Borel Y., Schlossman S. F. Direct demonstration of the human suppressor inducer subset by anti-T cell antibodies. J Immunol. 1983 Jan;130(1):157–161. [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Murphy J., Panayi G. Abnormal distribution of the helper-inducer and suppressor-inducer T-lymphocyte subsets in the rheumatoid joint. Clin Immunol Immunopathol. 1987 Nov;45(2):252–258. doi: 10.1016/0090-1229(87)90040-7. [DOI] [PubMed] [Google Scholar]
- Rose L. M., Ginsberg A. H., Rothstein T. L., Ledbetter J. A., Clark E. A. Selective loss of a subset of T helper cells in active multiple sclerosis. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7389–7393. doi: 10.1073/pnas.82.21.7389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Walters M. T., Smith J. L., Moore K., Evans P. R., Cawley M. I. An investigation of the action of disease modifying antirheumatic drugs on the rheumatoid synovial membrane: reduction in T lymphocyte subpopulations and HLA-DP and DQ antigen expression after gold or penicillamine therapy. Ann Rheum Dis. 1987 Jan;46(1):7–16. doi: 10.1136/ard.46.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]






