Abstract
Oligopeptides of the highly conserved herpes virus glycoprotein B (gB) were expressed from DNA fragments of the EBV gB (BALF4) and HSV-2 gB open reading frames as fusion proteins with the lambda CII protein and beta-galactosidase (GZ), respectively, in Escherichia coli. After immunopurification using anti-gB or anti-GZ affinity columns, the fusion proteins were used in vitro to stimulate human peripheral blood lymphocytes (PBL) or murine lymph node cells that have been primed with EBV, HSV-1, HSV-2, VZV or HCMV (all human herpes viruses) to proliferate. Results obtained in BALB/c mice indicate that different herpes viruses induce different levels of T-cell response to each other and to gB, over a range of type-specific and cross-reactive T-cell epitopes. There is a lack of correlation of immunogenicity and antigenicity in the generation of T-cell responses between some of the viruses. Major T-cell epitopes are located at the C terminal half of the gB molecule. The T-cell response to gB in healthy individuals seropositive for various combinations of the five herpes viruses differed markedly from individual to individual, even when they are seropositive to the same set of herpes viruses. However, two individuals with high proliferative T-cell response to VZV and sharing HLA A2, B7, DR2 and DQw1 are also good responders for cross-reactive gB/fragments and for virus antigen of all the five herpes viruses. Therefore the data obtained demonstrated that the MHC and the immune interaction arising from cross-reactive T-cell response evoked by other herpes viruses may determine the pathogenesis of a herpes virus infection.
Full text
PDF



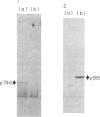
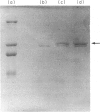
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur R. P., Mason D. T cells that help B cell responses to soluble antigen are distinguishable from those producing interleukin 2 on mitogenic or allogeneic stimulation. J Exp Med. 1986 Apr 1;163(4):774–786. doi: 10.1084/jem.163.4.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Oba D. E., Hutt-Fletcher L. M. Antigenic cross-reactions among herpes simplex virus types 1 and 2, Epstein-Barr virus, and cytomegalovirus. J Virol. 1987 Apr;61(4):1125–1135. doi: 10.1128/jvi.61.4.1125-1135.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney W. P., Rubin R. H., Hoffman R. A., Hansen W. P., Healey K., Hirsch M. S. Analysis of T lymphocyte subsets in cytomegalovirus mononucleosis. J Immunol. 1981 Jun;126(6):2114–2116. [PubMed] [Google Scholar]
- Chan W. L., Lukig M. L., Liew F. Y. Helper T cells induced by an immunopurified herpes simplex virus type I (HSV-I) 115 kilodalton glycoprotein (gB) protect mice against HSV-I infection. J Exp Med. 1985 Oct 1;162(4):1304–1318. doi: 10.1084/jem.162.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. L. Protective immunization of mice with specific HSV-1 glycoproteins. Immunology. 1983 Jun;49(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Luka J., Armstrong M. E., Keller P. M., Ellis R. W., Pearson G. R. Identification of an Epstein-Barr virus glycoprotein which is antigenically homologous to the varicella-zoster virus glycoprotein II and the herpes simplex virus glycoprotein B. Virology. 1987 Apr;157(2):552–555. doi: 10.1016/0042-6822(87)90300-x. [DOI] [PubMed] [Google Scholar]
- Fischer A., Sterkers G., Charron D., Durandy A. HLA class II restriction governing cell cooperation between antigen-specific helper T lymphocytes, B lymphocytes and monocytes for in vitro antibody production to influenza virus. Eur J Immunol. 1985 Jun;15(6):620–626. doi: 10.1002/eji.1830150617. [DOI] [PubMed] [Google Scholar]
- Fox B. S., Carbone F. R., Germain R. N., Paterson Y., Schwartz R. H. Processing of a minimal antigenic peptide alters its interaction with MHC molecules. Nature. 1988 Feb 11;331(6156):538–540. doi: 10.1038/331538a0. [DOI] [PubMed] [Google Scholar]
- Gong M., Ooka T., Matsuo T., Kieff E. Epstein-Barr virus glycoprotein homologous to herpes simplex virus gB. J Virol. 1987 Feb;61(2):499–508. doi: 10.1128/jvi.61.2.499-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet J. G., Lai M. Z., Briner T. J., Buus S., Sette A., Grey H. M., Smith J. A., Gefter M. L. Immunological self, nonself discrimination. Science. 1987 Feb 20;235(4791):865–870. doi: 10.1126/science.2433769. [DOI] [PubMed] [Google Scholar]
- Lindsley M. D., Torpey D. J., 3rd, Rinaldo C. R., Jr HLA-DR-restricted cytotoxicity of cytomegalovirus-infected monocytes mediated by Leu-3-positive T cells. J Immunol. 1986 Apr 15;136(8):3045–3051. [PubMed] [Google Scholar]
- McMichael A. J., Ting A., Zweerink H. J., Askonas B. A. HLA restriction of cell-mediated lysis of influenza virus-infected human cells. Nature. 1977 Dec 8;270(5637):524–526. doi: 10.1038/270524a0. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Rickinson A. B., Pope J. H. Long-term T-cell-mediated immunity to Epstein-Barr virus in man. III. Activation of cytotoxic T cells in virus-infected leukocyte cultures. Int J Cancer. 1979 May 15;23(5):618–625. doi: 10.1002/ijc.2910230506. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Generation of beta-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli. 1984 Jun 28-Jul 4Nature. 309(5971):810–812. doi: 10.1038/309810a0. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Liew F. Y. Immune response to chemically modified flagellin. 3. Enhanced cell-mediated immunity during high and low zone antibody tolerance to flagellin. J Exp Med. 1972 Feb 1;135(2):298–311. doi: 10.1084/jem.135.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., Biggin M. D., Barrell B., Roizman B. Epstein-Barr virus genome may encode a protein showing significant amino acid and predicted secondary structure homology with glycoprotein B of herpes simplex virus 1. J Virol. 1985 Dec;56(3):807–813. doi: 10.1128/jvi.56.3.807-813.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person S., Warner S. C., Bzik D. J., Debroy C., Fox B. A. Expression in bacteria of gB-glycoprotein-coding sequences of Herpes simplex virus type 2. Gene. 1985;35(3):279–287. doi: 10.1016/0378-1119(85)90006-x. [DOI] [PubMed] [Google Scholar]
- Qvigstad E., Moen T., Thorsby E. T-cell clones with similar antigen specificity may be restricted by DR, MT(DC), or SB class II HLA molecules. Immunogenetics. 1984;19(5):455–460. doi: 10.1007/BF00364648. [DOI] [PubMed] [Google Scholar]
- Rhodes J., Ivanyi J., Cozens P. Antigen presentation by human monocytes: effects of modifying major histocompatibility complex class II antigen expression and interleukin 1 production by using recombinant interferons and corticosteroids. Eur J Immunol. 1986 Apr;16(4):370–375. doi: 10.1002/eji.1830160410. [DOI] [PubMed] [Google Scholar]
- Schmid D. S. The human MHC-restricted cellular response to herpes simplex virus type 1 is mediated by CD4+, CD8- T cells and is restricted to the DR region of the MHC complex. J Immunol. 1988 May 15;140(10):3610–3616. [PubMed] [Google Scholar]
- Thorsby E., Berle E., Nousiainen H. HLA-D region molecules restrict proliferative T cell responses to antigen. Immunol Rev. 1982;66:39–56. doi: 10.1111/j.1600-065x.1982.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Winther M. D., Allen G., Bomford R. H., Brown F. Bacterially expressed antigenic peptide from foot-and-mouth disease virus capsid elicits variable immunologic responses in animals. J Immunol. 1986 Mar 1;136(5):1835–1840. [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. II. Bifunctional clones with cytotoxic and virus-induced proliferative activities exhibit herpes simplex virus type 1 and 2 specific or type common reactivities. J Immunol. 1984 Nov;133(5):2736–2742. [PubMed] [Google Scholar]