Abstract
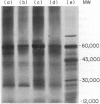
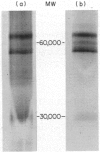
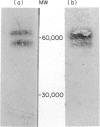
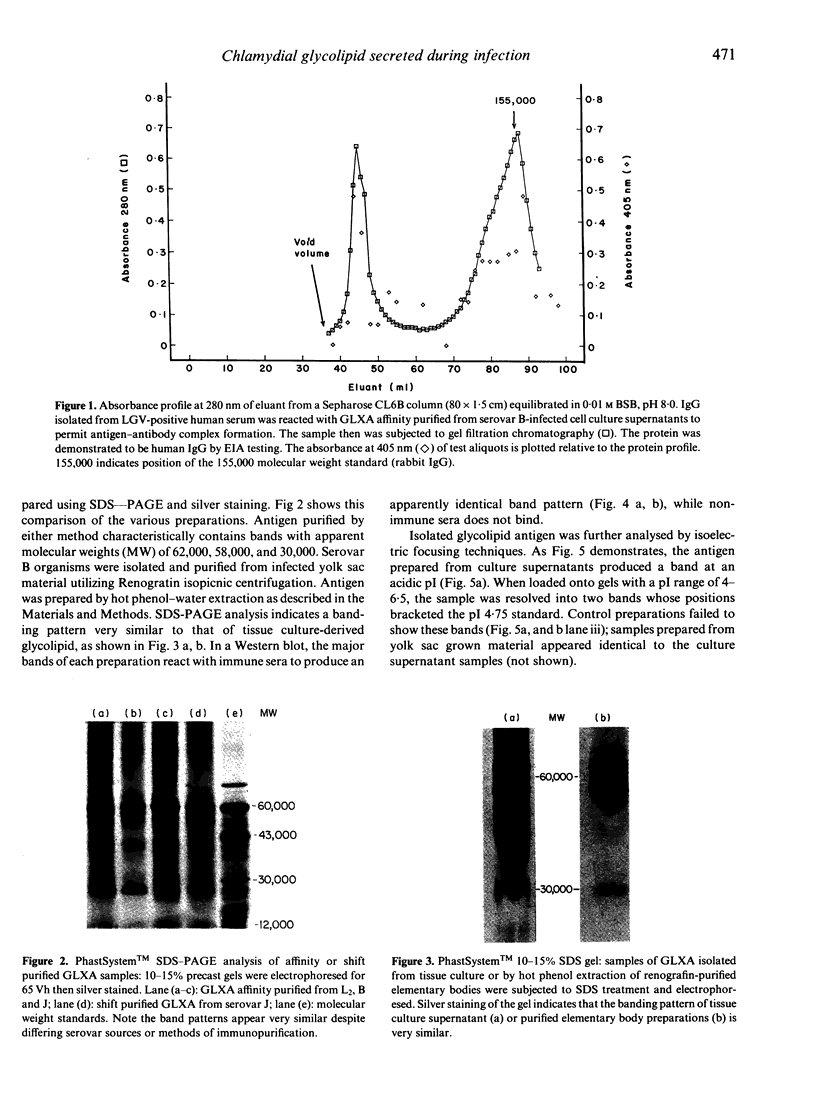
Chlamydia trachomatis serovars release a glycolipid antigen (GLXA) into the culture supernatant during the infective cycle. This antigen is recognized by IgG isolated from humans with a natural chlamydial infection; GLXA may be a major antigenic determinant of chlamydia. It can be immunopurified by molecular shift or affinity chromatography. Silver staining of SDS-PAGE gels demonstrates a pattern of bands that is essentially the same for preparations isolated by either method. GLXA can also be extracted from mature elementary bodies (EB). These preparations show the same pattern of silver staining bands, and the major bands are immunoreactive as shown by Western blot analysis. Isoelectric focusing studies demonstrate that purified GLXA has an acidic pI.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell H. D., Hitchcock P. J. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect Immun. 1984 May;44(2):306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T. Identification and properties of chlamydial polypeptides that bind eucaryotic cell surface components. J Bacteriol. 1986 Jan;165(1):13–20. doi: 10.1128/jb.165.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourihan J. T., Rota T. R., MacDonald A. B. Isolation and purification of a type-specific antigen from Chlamydia trachomatis propagated in cell culture utilizing molecular shift chromatography. J Immunol. 1980 May;124(5):2399–2404. [PubMed] [Google Scholar]
- MacDonald A. B. Antigens of Chlamydia trachomatis. Rev Infect Dis. 1985 Nov-Dec;7(6):731–736. doi: 10.1093/clinids/7.6.731. [DOI] [PubMed] [Google Scholar]
- Newhall W. J., 5th Biosynthesis and disulfide cross-linking of outer membrane components during the growth cycle of Chlamydia trachomatis. Infect Immun. 1987 Jan;55(1):162–168. doi: 10.1128/iai.55.1.162-168.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D. L., Todd W. J., Macdonald A. B. Cell-mediated immune responses in owl monkeys (Aotus trivirgatus) with trachoma to soluble antigens of Chlamydia trachomatis. Clin Exp Immunol. 1978 Jul;33(1):57–64. [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Stuart E. S., Tirrell S. M., MacDonald A. B. Characterization of an antigen secreted by Chlamydia-infected cell culture. Immunology. 1987 Aug;61(4):527–533. [PMC free article] [PubMed] [Google Scholar]
- Watkins N. G., Hadlow W. J., Moos A. B., Caldwell H. D. Ocular delayed hypersensitivity: a pathogenetic mechanism of chlamydial-conjunctivitis in guinea pigs. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7480–7484. doi: 10.1073/pnas.83.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenman W. M., Meuser R. U. Chlamydia trachomatis elementary bodies possess proteins which bind to eucaryotic cell membranes. J Bacteriol. 1986 Feb;165(2):602–607. doi: 10.1128/jb.165.2.602-607.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]