Abstract
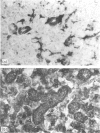
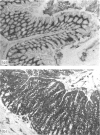
The normal and interferon-gamma induced expression of class II MHC antigens was investigated immunohistologically in the digestive system of LEW rats. In the normal state class II molecules were present in interstitial dendritic cells, B lymphocytes and epithelial cells. Epithelial class II expression was restricted to enterocytes in certain portions of the small intestine and to some duct epithelia in salivary glands. After continuous intravenous infusion of interferon-gamma (IFN-gamma) for 3 days, class II MHC antigens were induced in large vessel endothelium and in the surface epithelia of the tongue, oesophagus and proventricle. In the gastric glands class II molecules appeared in mucous neck cells and in parietal cells, while adjacent mucous surface cells and chief cells did not acquire class II reactivity. All enterocytes, including the previously negative colonic epithelium, were induced to express class II antigens. In the salivary glands class II antigens appeared in all duct epithelia. Serous acinar cells were induced in the parotids, but in the submandibular glands and in the pancreas the serous gland epithelium stayed negative. Our study thus shows that the effects of IFN-gamma on class II MHC antigen expression in vivo depend on the differentiation pathway of the individual cell. The normal distribution in rats suggest that class II MHC antigens may play a role in peptide transport across specialized epithelia. It remains to be determined whether such a function is enhanced after IFN treatment.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buus S., Sette A., Grey H. M. The interaction between protein-derived immunogenic peptides and Ia. Immunol Rev. 1987 Aug;98:115–141. doi: 10.1111/j.1600-065x.1987.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Fontana A., Frei K., Bodmer S., Hofer E. Immune-mediated encephalitis: on the role of antigen-presenting cells in brain tissue. Immunol Rev. 1987 Dec;100:185–201. doi: 10.1111/j.1600-065X.1987.tb00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulis A. K. Class II major histocompatibility complex and organ specific autoimmunity in man. J Pathol. 1986 Sep;150(1):5–11. doi: 10.1002/path.1711500103. [DOI] [PubMed] [Google Scholar]
- Fuggle S. V., Errasti P., Daar A. S., Fabre J. W., Ting A., Morris P. J. Localization of major histocompatibility complex (HLA-ABC and DR) antigens in 46 kidneys. Differences in HLA-DR staining of tubules among kidneys. Transplantation. 1983 Apr;35(4):385–390. doi: 10.1097/00007890-198304000-00024. [DOI] [PubMed] [Google Scholar]
- Fukumoto T., McMaster W. R., Williams A. F. Mouse monoclonal antibodies against rat major histocompatibility antigens. Two Ia antigens and expression of Ia and class I antigens in rat thymus. Eur J Immunol. 1982 Mar;12(3):237–243. doi: 10.1002/eji.1830120313. [DOI] [PubMed] [Google Scholar]
- Gaspari A. A., Jenkins M. K., Katz S. I. Class II MHC-bearing keratinocytes induce antigen-specific unresponsiveness in hapten-specific Th1 clones. J Immunol. 1988 Oct 1;141(7):2216–2220. [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Antigen presentation by interferon-gamma-treated endothelial cells and fibroblasts: differential ability to function as antigen-presenting cells despite comparable Ia expression. J Immunol. 1985 Dec;135(6):3750–3762. [PubMed] [Google Scholar]
- Groenewegen G., Buurman W. A., van der Linden C. J. Lymphokine dependence of in vivo expression of MHC class II antigens by endothelium. Nature. 1985 Jul 25;316(6026):361–363. doi: 10.1038/316361a0. [DOI] [PubMed] [Google Scholar]
- Guillet J. G., Lai M. Z., Briner T. J., Smith J. A., Gefter M. L. Interaction of peptide antigens and class II major histocompatibility complex antigens. Nature. 1986 Nov 20;324(6094):260–262. doi: 10.1038/324260a0. [DOI] [PubMed] [Google Scholar]
- Hart D. N., Fabre J. W. Endogenously produced Ia antigens within cells of convoluted tubules of rat kidney. J Immunol. 1981 Jun;126(6):2109–2113. [PubMed] [Google Scholar]
- Hart D. N., Fuggle S. V., Williams K. A., Fabre J. W., Ting A., Morris P. J. Localization of HLA-ABC and DR antigens in human kidney. Transplantation. 1981 Jun;31(6):428–433. doi: 10.1097/00007890-198106000-00005. [DOI] [PubMed] [Google Scholar]
- JACOBY F., LEESON C. R. The postnatal development of the rat submaxillary gland. J Anat. 1959 Apr;93(2):201–216. [PMC free article] [PubMed] [Google Scholar]
- Kurzrock R., Rosenblum M. G., Sherwin S. A., Rios A., Talpaz M., Quesada J. R., Gutterman J. U. Pharmacokinetics, single-dose tolerance, and biological activity of recombinant gamma-interferon in cancer patients. Cancer Res. 1985 Jun;45(6):2866–2872. [PubMed] [Google Scholar]
- Mayer L., Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987 Nov 1;166(5):1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer G., Pugh C. W., Barclay A. N. The distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol. 1983 Feb;13(2):112–122. doi: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- Mayrhofer G., Schon-Hegrad M. A. Ia antigens in rat kidney, with special reference to their expression in tubular epithelium. J Exp Med. 1983 Jun 1;157(6):2097–2109. doi: 10.1084/jem.157.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster W. R. A monoclonal antibody which detects a polymorphic Ia antigenic determinant reacts with purified beta polypeptide chain. Immunogenetics. 1981;13(4):347–350. doi: 10.1007/BF00364500. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Momburg F., Koch N., Möller P., Moldenhauer G., Butcher G. W., Hämmerling G. J. Differential expression of Ia and Ia-associated invariant chain in mouse tissues after in vivo treatment with IFN-gamma. J Immunol. 1986 Feb 1;136(3):940–948. [PubMed] [Google Scholar]
- Newcombe J., Cuzner M. L. Monoclonal antibody 14E identifies the oligodendrocyte cell body in normal adult human and rat white matter. J Neuroimmunol. 1988 Aug;19(1-2):11–20. doi: 10.1016/0165-5728(88)90031-8. [DOI] [PubMed] [Google Scholar]
- Pujol-Borrell R., Todd I., Doshi M., Bottazzo G. F., Sutton R., Gray D., Adolf G. R., Feldmann M. HLA class II induction in human islet cells by interferon-gamma plus tumour necrosis factor or lymphotoxin. Nature. 1987 Mar 19;326(6110):304–306. doi: 10.1038/326304a0. [DOI] [PubMed] [Google Scholar]
- Scott H., Solheim B. G., Brandtzaeg P., Thorsby E. HLA-DR-like antigens in the epithelium of the human small intestine. Scand J Immunol. 1980;12(1):77–82. doi: 10.1111/j.1365-3083.1980.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Skoskiewicz M. J., Colvin R. B., Schneeberger E. E., Russell P. S. Widespread and selective induction of major histocompatibility complex-determined antigens in vivo by gamma interferon. J Exp Med. 1985 Nov 1;162(5):1645–1664. doi: 10.1084/jem.162.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiniger B., Falk P., Van der Meide P. H. Interferon-gamma in vivo. Induction and loss of class II MHC antigens and immature myelomonocytic cells in rat organs. Eur J Immunol. 1988 May;18(5):661–669. doi: 10.1002/eji.1830180502. [DOI] [PubMed] [Google Scholar]
- Steiniger B., Klempnauer J., Wonigeit K. Altered distribution of class I and class II MHC antigens during acute pancreas allograft rejection in the rat. Transplantation. 1985 Sep;40(3):234–239. doi: 10.1097/00007890-198509000-00002. [DOI] [PubMed] [Google Scholar]
- Steiniger B., Klempnauer J., Wonigeit K. Phenotype and histological distribution of interstitial dendritic cells in the rat pancreas, liver, heart, and kidney. Transplantation. 1984 Aug;38(2):169–174. doi: 10.1097/00007890-198408000-00016. [DOI] [PubMed] [Google Scholar]
- Tamarin A., Sreebny L. M. The rat submaxillary salivary gland. A correlative study by light and electron microscopy. J Morphol. 1965 Nov;117(3):295–352. doi: 10.1002/jmor.1051170303. [DOI] [PubMed] [Google Scholar]
- Todd I., Pujol-Borrell R., Hammond L. J., Bottazzo G. F., Feldmann M. Interferon-gamma induces HLA-DR expression by thyroid epithelium. Clin Exp Immunol. 1985 Aug;61(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. Comment on the finding of Ia expression in nonlymphoid cells. Lab Invest. 1986 Aug;55(2):123–125. [PubMed] [Google Scholar]
- Wong G. H., Clark-Lewis I., Harris A. W., Schrader J. W. Effect of cloned interferon-gamma on expression of H-2 and Ia antigens on cell lines of hemopoietic, lymphoid, epithelial, fibroblastic and neuronal origin. Eur J Immunol. 1984 Jan;14(1):52–56. doi: 10.1002/eji.1830140110. [DOI] [PubMed] [Google Scholar]
- van der Meide P. H., Dubbeld M., Vijverberg K., Kos T., Schellekens H. The purification and characterization of rat gamma interferon by use of two monoclonal antibodies. J Gen Virol. 1986 Jun;67(Pt 6):1059–1071. doi: 10.1099/0022-1317-67-6-1059. [DOI] [PubMed] [Google Scholar]