Abstract
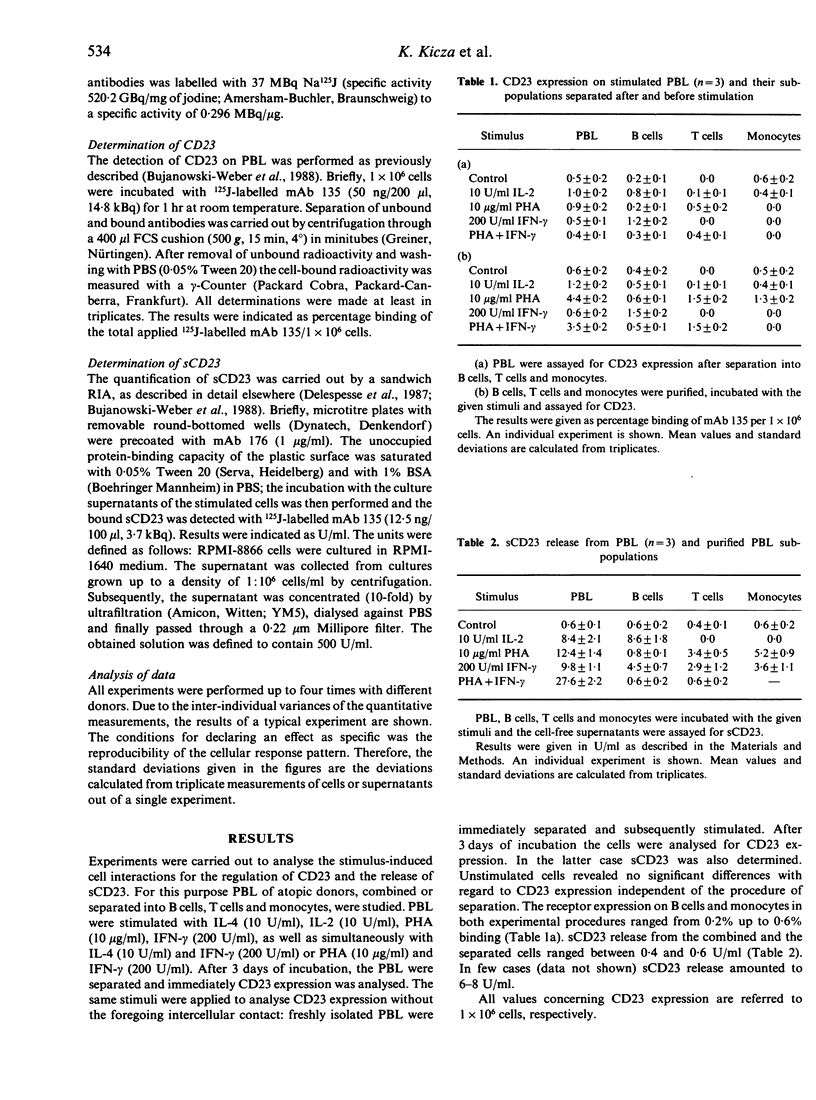
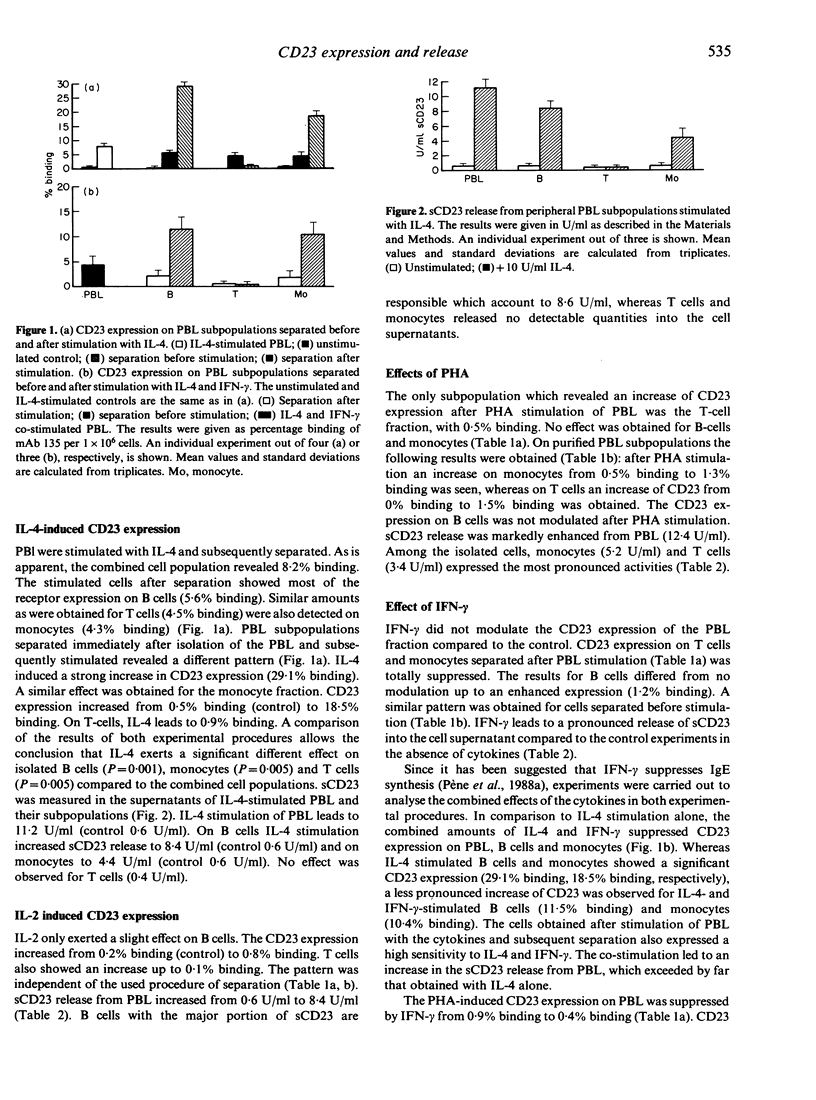
The cell-cell interactions for CD23 expression and soluble (s)CD23 release from peripheral blood lymphocytes (PBL), as well as purified cells (B, T cells, monocytes) of atopic donors, were studied. Cells either stimulated combined and subsequently separated or stimulated after separation were analysed. IL-4, IL-2, phytohaemagglutinin (PHA), interferon-gamma (IFN-gamma) and the combined interaction of IL-4 and IFN-gamma as well as PHA and IFN-gamma were used as stimuli. sCD23 release in the cell supernatant was determined from cells separated before stimulation. CD23 expression induced by IL-4 on cells stimulated and subsequently separated was significantly lower compared with amounts on separated cells which were subsequently stimulated. Major expression of CD23 was obtained on B cells and monocytes. Stimulation with PHA led to an increased expression on T cells compared to the control. When cells were stimulated, combined and separated, the combined stimuli of IL-4 and IFN-gamma showed a reduced CD23 expression for both experimental procedures and an enhanced release of sCD23. The data suggest an important role for cell-cell interactions. These results were supported by experiments in which separated cells were either co-cultured or cultured in Transwells. Co-culture of T cells with B cells and monocytes suggested that T cells are responsible for suppressed CD23 expression. No or only slight enhancement was obtained for sCD23 release. Our data indicate that cell-cell interactions and cytokines regulate CD23 expression, while sCD23 release is apparently solely regulated by soluble mediators (e.g. cytokines).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage R. J., Goff L. K., Beverley P. C. Expression and functional role of CD23 on T cells. Eur J Immunol. 1989 Jan;19(1):31–35. doi: 10.1002/eji.1830190106. [DOI] [PubMed] [Google Scholar]
- Armitage R. J., Goff L. K. Functional interaction between B cell subpopulations defined by CD23 expression. Eur J Immunol. 1988 Nov;18(11):1753–1760. doi: 10.1002/eji.1830181115. [DOI] [PubMed] [Google Scholar]
- Bonnefoy J. Y., Guillot O., Spits H., Blanchard D., Ishizaka K., Banchereau J. The low-affinity receptor for IgE (CD23) on B lymphocytes is spatially associated with HLA-DR antigens. J Exp Med. 1988 Jan 1;167(1):57–72. doi: 10.1084/jem.167.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujanowski-Weber J., Brings B., Knöller I., Pfeil T., König W. Expression of low-affinity receptor for IgE (Fc epsilon RII, CD23) and IgE-BF (soluble CD23) release by lymphoblastoid B-cell line RPMI-8866 and human peripheral lymphocytes of normal and atopic donors. Immunology. 1989 Apr;66(4):505–511. [PMC free article] [PubMed] [Google Scholar]
- Callard R. E., Lau Y. L., Shields J. G., Smith S. H., Cairns J., Flores-Romo L., Gordon J. The marmoset B-lymphoblastoid cell line (B95-8) produces and responds to B-cell growth and differentiation factors: role of shed CD23 (sCD23). Immunology. 1988 Nov;65(3):379–384. [PMC free article] [PubMed] [Google Scholar]
- Capron M., Spiegelberg H. L., Prin L., Bennich H., Butterworth A. E., Pierce R. J., Ouaissi M. A., Capron A. Role of IgE receptors in effector function of human eosinophils. J Immunol. 1984 Jan;132(1):462–468. [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Rousset F., Vanbervliet B., Bonnefoy J. Y., Arai N., Takebe Y., Yokota T., Lee F., Arai K. Human recombinant interleukin 4 induces Fc epsilon receptors (CD23) on normal human B lymphocytes. J Exp Med. 1987 Jun 1;165(6):1459–1467. doi: 10.1084/jem.165.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delespesse G., Sarfati M., Rubio-Trujillo M. In vitro production of IgE-binding factors by human mononuclear cells. Immunology. 1987 Jan;60(1):103–110. [PMC free article] [PubMed] [Google Scholar]
- Galizzi J. P., Cabrillat H., Rousset F., Ménétrier C., de Vries J. E., Banchereau J. IFN-gamma and prostaglandin E2 inhibit IL-4-induced expression of Fc epsilon R2/CD23 on B lymphocytes through different mechanisms without altering binding of IL-4 to its receptor. J Immunol. 1988 Sep 15;141(6):1982–1988. [PubMed] [Google Scholar]
- Gonzalez-Molina A., Spiegelberg H. L. A subpopulation of normal human peripheral B lymphcytes that bind IgE. J Clin Invest. 1977 Apr;59(4):616–624. doi: 10.1172/JCI108679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Cairns J. A., Millsum M. J., Gillis S., Guy G. R. Interleukin 4 and soluble CD23 as progression factors for human B lymphocytes: analysis of their interactions with agonists of the phosphoinositide "dual pathway" of signalling. Eur J Immunol. 1988 Oct;18(10):1561–1565. doi: 10.1002/eji.1830181014. [DOI] [PubMed] [Google Scholar]
- Gordon J., Webb A. J., Walker L., Guy G. R., Rowe M. Evidence for an association between CD23 and the receptor for a low molecular weight B cell growth factor. Eur J Immunol. 1986 Dec;16(12):1627–1630. doi: 10.1002/eji.1830161225. [DOI] [PubMed] [Google Scholar]
- Ishizaka K. IgE-binding factors and regulation of the IgE antibody response. Annu Rev Immunol. 1988;6:513–534. doi: 10.1146/annurev.iy.06.040188.002501. [DOI] [PubMed] [Google Scholar]
- Joseph M., Capron A., Ameisen J. C., Capron M., Vorng H., Pancré V., Kusnierz J. P., Auriault C. The receptor for IgE on blood platelets. Eur J Immunol. 1986 Mar;16(3):306–312. doi: 10.1002/eji.1830160318. [DOI] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Kikutani H., Suemura M., Owaki H., Nakamura H., Sato R., Yamasaki K., Barsumian E. L., Hardy R. R., Kishimoto T. Fc epsilon receptor, a specific differentiation marker transiently expressed on mature B cells before isotype switching. J Exp Med. 1986 Nov 1;164(5):1455–1469. doi: 10.1084/jem.164.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisaki T., Leung D. Y., Jardieu P., Geha R. S., Ishizaka K. Regulatory effects of human IgE-binding factors in the IgE synthesis by human and rat lymphocytes. Eur J Immunol. 1988 Nov;18(11):1663–1670. doi: 10.1002/eji.1830181103. [DOI] [PubMed] [Google Scholar]
- Klinman N. R., Taylor R. B. General methods for the study of cells and serum during the immune response: the response to dinitrophenyl in mice. Clin Exp Immunol. 1969 Apr;4(4):473–487. [PMC free article] [PubMed] [Google Scholar]
- Knöller I., Bujanowski-Weber J., Brings B., König W. Influence of IL-2 and IL-4 on the IgE synthesis and the IgE-binding factor (sCD23) production by human lymphocytes in vitro. Immunology. 1989 Mar;66(3):368–375. [PMC free article] [PubMed] [Google Scholar]
- König W., Pfeiffer P., Rauschen I., Knöller J., Schönfeld W., Theobald K. The role of immunoglobulin E receptors in allergic diseases. Arzneimittelforschung. 1985;35(12A):1953–1957. [PubMed] [Google Scholar]
- Leung D. Y., Frankel R., Wood N., Geha R. S. Potentiation of human immunoglobulin E synthesis by plasma immunoglobulin E binding factors from patients with the hyperimmunoglobulin E syndrome. J Clin Invest. 1986 Mar;77(3):952–957. doi: 10.1172/JCI112395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell L. C., Davis L. S., Lipsky P. E. Promotion of human T lymphocyte proliferation by IL-4. J Immunol. 1989 Mar 1;142(5):1548–1557. [PubMed] [Google Scholar]
- Prinz J. C., Endres N., Rank G., Ring J., Rieber E. P. Expression of Fc epsilon receptors on activated human T lymphocytes. Eur J Immunol. 1987 Jun;17(6):757–761. doi: 10.1002/eji.1830170604. [DOI] [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Paliard X., Banchereau J., Spits H., De Vries J. E. IgE production by normal human B cells induced by alloreactive T cell clones is mediated by IL-4 and suppressed by IFN-gamma. J Immunol. 1988 Aug 15;141(4):1218–1224. [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Wideman J., Bonnefoy J. Y., De Vries J. E. Interleukin 5 enhances interleukin 4-induced IgE production by normal human B cells. The role of soluble CD23 antigen. Eur J Immunol. 1988 Jun;18(6):929–935. doi: 10.1002/eji.1830180615. [DOI] [PubMed] [Google Scholar]
- Rector E., Nakajima T., Rocha C., Duncan D., Lestourgeon D., Mitchell R. S., Fischer J., Sehon A. H., Delespesse G. Detection and characterization of monoclonal antibodies specific to IgE receptors on human lymphocytes by flow cytometry. Immunology. 1985 Jul;55(3):481–488. [PMC free article] [PubMed] [Google Scholar]
- Reynolds D. S., Boom W. H., Abbas A. K. Inhibition of B lymphocyte activation by interferon-gamma. J Immunol. 1987 Aug 1;139(3):767–773. [PubMed] [Google Scholar]
- Sarfati M., Nutman T., Fonteyn C., Delespesse G. Presence of antigenic determinants common to Fc IgE receptors on human macrophages, T and B lymphocytes and IgE-binding factors. Immunology. 1986 Dec;59(4):569–575. [PMC free article] [PubMed] [Google Scholar]
- Shields J. G., Armitage R. J., Jamieson B. N., Beverley P. C., Callard R. E. Increased expression of surface IgM but not IgD or IgG on human B cells in response to IL-4. Immunology. 1989 Feb;66(2):224–227. [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L., O'Connor R. D., Simon R. A., Mathison D. A. Lymphocytes with immunoglobulin E Fc receptors in patients with atopic disorders. J Clin Invest. 1979 Sep;64(3):714–720. doi: 10.1172/JCI109514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendeman S., Thorley-Lawson D. A. The activation antigen BLAST-2, when shed, is an autocrine BCGF for normal and transformed B cells. EMBO J. 1987 Jun;6(6):1637–1642. doi: 10.1002/j.1460-2075.1987.tb02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., Okumura K. Regulation of homocytotropic antibody formation in the rat. II. Effect of X-irradiation. J Immunol. 1971 Apr;106(4):1012–1018. [PubMed] [Google Scholar]
- Thompson L. F., Mellon M. H., Zeiger R. S., Spiegelberg H. L. Characterization with monoclonal antibodies of T lymphocytes bearing Fc receptors for IgE (T epsilon cells) and IgG (T gamma cells) in atopic patients. J Immunol. 1983 Dec;131(6):2772–2776. [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Vercelli D., Jabara H. H., Arai K., Geha R. S. Induction of human IgE synthesis requires interleukin 4 and T/B cell interactions involving the T cell receptor/CD3 complex and MHC class II antigens. J Exp Med. 1989 Apr 1;169(4):1295–1307. doi: 10.1084/jem.169.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Gregory C. D., Rowe M., Rickinson A. B., Wang D., Birkenbach M., Kikutani H., Kishimoto T., Kieff E. Epstein-Barr virus nuclear antigen 2 specifically induces expression of the B-cell activation antigen CD23. Proc Natl Acad Sci U S A. 1987 May;84(10):3452–3456. doi: 10.1073/pnas.84.10.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota A., Kikutani H., Tanaka T., Sato R., Barsumian E. L., Suemura M., Kishimoto T. Two species of human Fc epsilon receptor II (Fc epsilon RII/CD23): tissue-specific and IL-4-specific regulation of gene expression. Cell. 1988 Nov 18;55(4):611–618. doi: 10.1016/0092-8674(88)90219-x. [DOI] [PubMed] [Google Scholar]
- Yukawa K., Kikutani H., Owaki H., Yamasaki K., Yokota A., Nakamura H., Barsumian E. L., Hardy R. R., Suemura M., Kishimoto T. A B cell-specific differentiation antigen, CD23, is a receptor for IgE (Fc epsilon R) on lymphocytes. J Immunol. 1987 Apr 15;138(8):2576–2580. [PubMed] [Google Scholar]


