Abstract
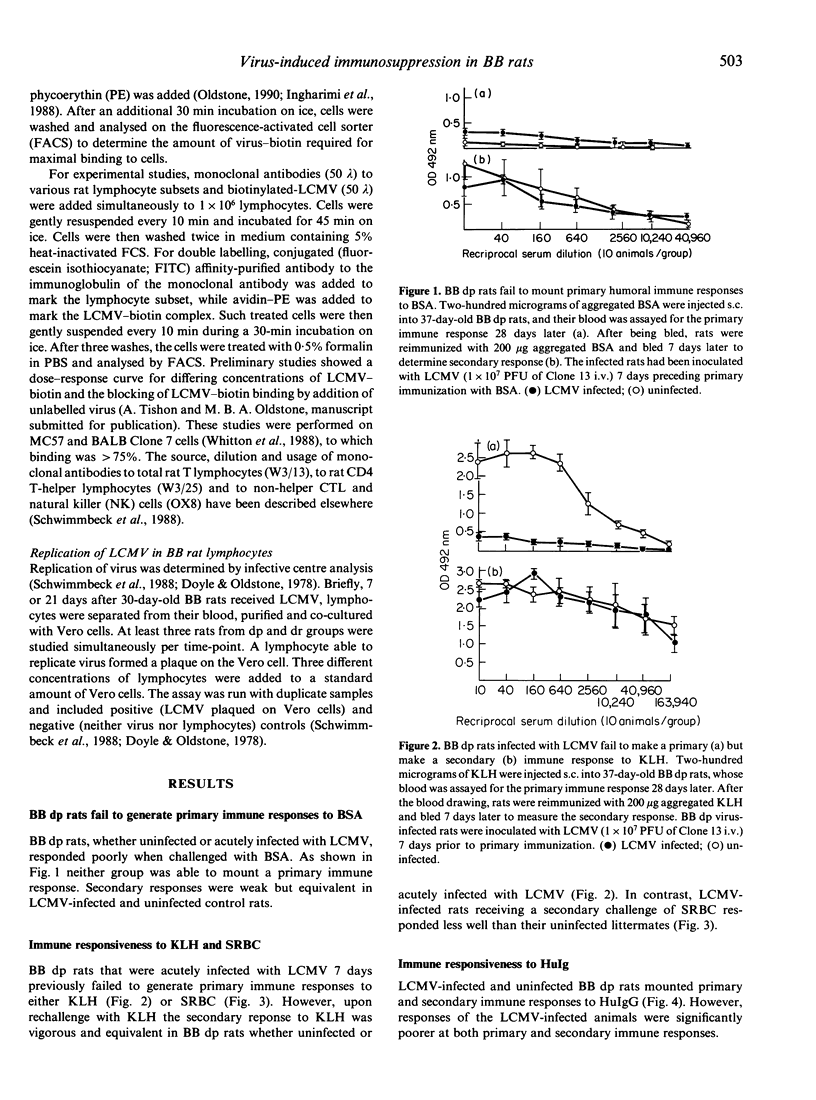
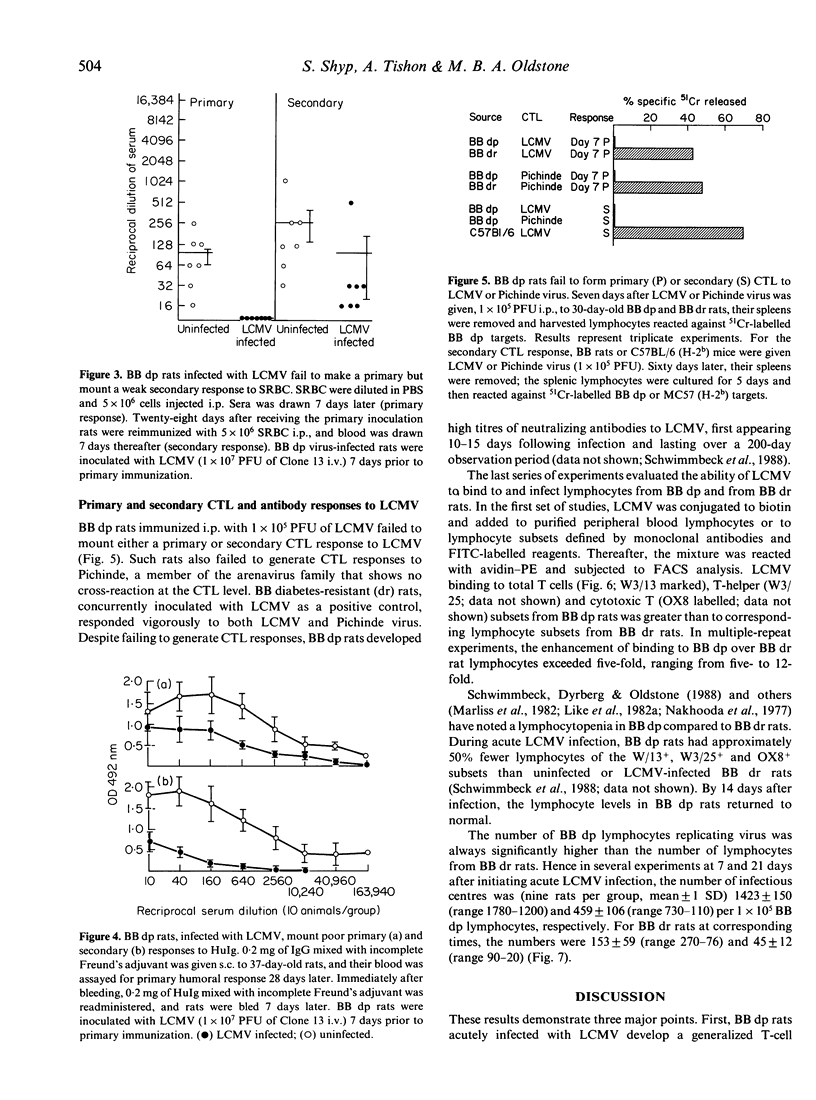
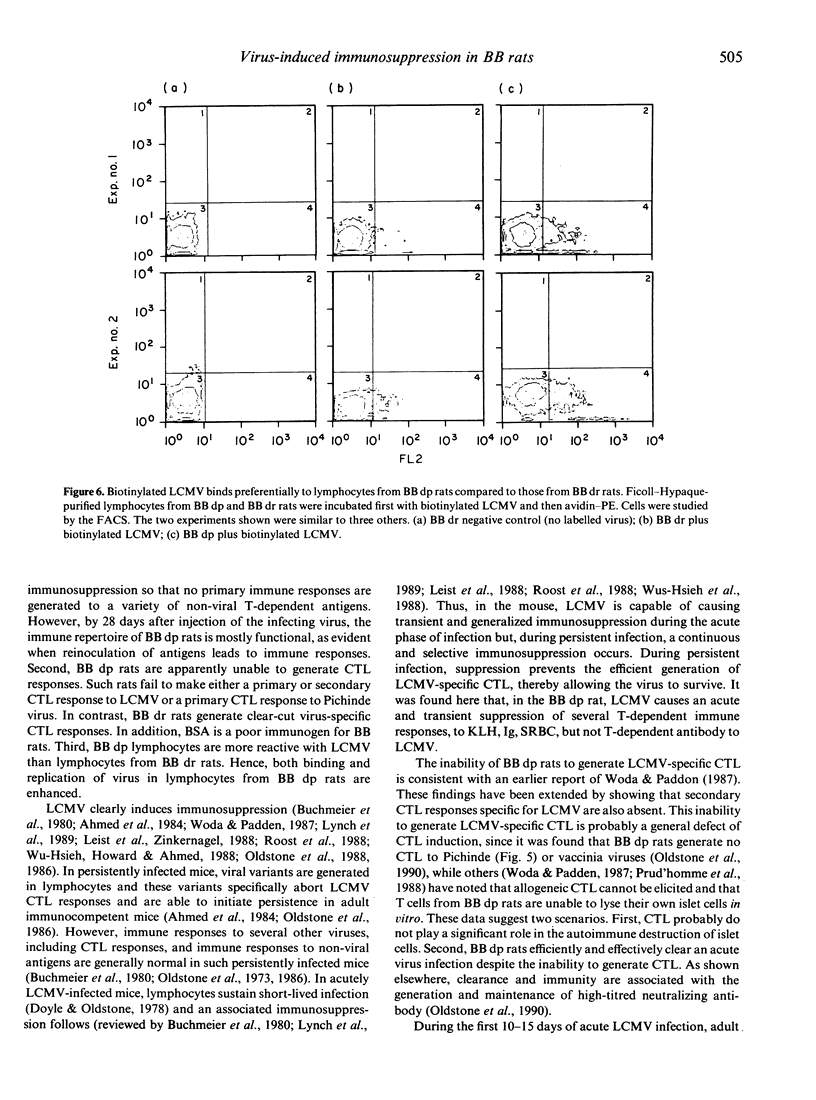
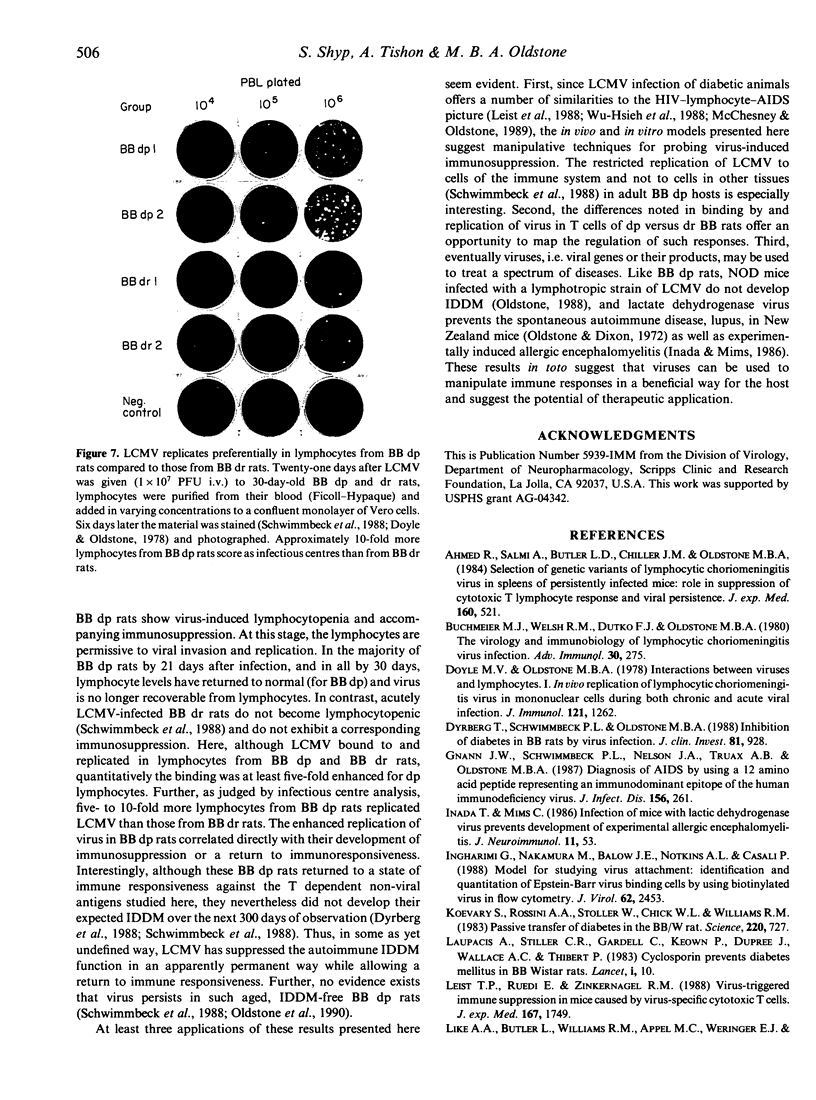
Lymphocytic choriomeningitis virus (LCMV) infection prevents the usual insulin-dependent diabetes mellitus of aged BB rats (Dyrberg, Schwimmbeck & Oldstone, 1988; Schwimmbeck, Dyrberg & Oldstone, 1988). In this study earlier observations are extended by noting that LCMV infection substantially alters the immune responsesof BB diabetic-prone (dp) rats. The control, uninfected rats make vigorous primary and secondary antibody responses when challenged with keyhole limpet haemocyanin (KLH), human immunoglobulin (HuIg) or sheep red blood cells (SRBC). Such rats fail to mount a primary response to bovine serum albumin (BSA) but do produce a moderate secondary response. They mount good antibody responses to LCMV but fail to generate either primary or secondary LCMV-specific cytotoxic T-lymphocyte (CTL) responses or CTL responses to Pichinde virus. In contrast, BB dp rats acutely infected with LCMV generate no primary immune responses to SRBC, KLH or BSA and only meager responses when challenged with HuIg. They mount secondary responses to KLH, HuIg and BSA that approximate those of their uninfected litter mates, but have a comparatively lower response to SRBC. LCMV binds to and infects lymphocytes of the BB dp rat. Binding is enhanced over that observed with lymphocytes from BB diabetic-resistant (dr) rats, which are able to generate CTL immune responses to LCMV and Pichinde viruses. Hence, lymphocytes from BB dp rats are uniquely susceptible to binding and replication of LCMV. During the acute stage of LCMV infection, a general primary T-cell immunosuppression occurs with respect to a variety of viral and non-viral antigens. Over time, responsiveness to T-cell dependent antigens returns except for the ability to generate CTL responses to LCMV or the autoimmune response(s) required to cause insulin-dependent diabetes mellitus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Salmi A., Butler L. D., Chiller J. M., Oldstone M. B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984 Aug 1;160(2):521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaroch P., Bordenave G. Enhancement in Igha mouse strains of the "natural" suppressive activity of normal T splenocytes against the expression of Igh-1b allotype. I. Molecular aspects of the chronic suppression obtained. Eur J Immunol. 1988 Jan;18(1):51–58. doi: 10.1002/eji.1830180109. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Doyle M. V., Oldstone M. B. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J Immunol. 1978 Oct;121(4):1262–1269. [PubMed] [Google Scholar]
- Dyrberg T., Schwimmbeck P. L., Oldstone M. B. Inhibition of diabetes in BB rats by virus infection. J Clin Invest. 1988 Mar;81(3):928–931. doi: 10.1172/JCI113405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnann J. W., Jr, Schwimmbeck P. L., Nelson J. A., Truax A. B., Oldstone M. B. Diagnosis of AIDS by using a 12-amino acid peptide representing an immunodominant epitope of the human immunodeficiency virus. J Infect Dis. 1987 Aug;156(2):261–267. doi: 10.1093/infdis/156.2.261. [DOI] [PubMed] [Google Scholar]
- Inada T., Mims C. A. Infection of mice with lactic dehydrogenase virus prevents development of experimental allergic encephalomyelitis. J Neuroimmunol. 1986 Mar;11(1):53–56. doi: 10.1016/0165-5728(86)90074-3. [DOI] [PubMed] [Google Scholar]
- Inghirami G., Nakamura M., Balow J. E., Notkins A. L., Casali P. Model for studying virus attachment: identification and quantitation of Epstein-Barr virus-binding cells by using biotinylated virus in flow cytometry. J Virol. 1988 Jul;62(7):2453–2463. doi: 10.1128/jvi.62.7.2453-2463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koevary S., Rossini A., Stoller W., Chick W., Williams R. M. Passive transfer of diabetes in the BB/W rat. Science. 1983 May 13;220(4598):727–728. doi: 10.1126/science.6836309. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Rüedi E., Zinkernagel R. M. Virus-triggered immune suppression in mice caused by virus-specific cytotoxic T cells. J Exp Med. 1988 May 1;167(5):1749–1754. doi: 10.1084/jem.167.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Like A. A., Kislauskis E., Williams R. R., Rossini A. A. Neonatal thymectomy prevents spontaneous diabetes mellitus in the BB/W rat. Science. 1982 May 7;216(4546):644–646. doi: 10.1126/science.7041259. [DOI] [PubMed] [Google Scholar]
- Lynch F., Ceredig R., Hartley D., Doherty P. C. Persistence of the irradiated host component in thymocyte populations from bone marrow radiation chimeras infected with lymphocytic choriomeningitis virus. Cell Immunol. 1989 Feb;118(2):482–490. doi: 10.1016/0008-8749(89)90395-x. [DOI] [PubMed] [Google Scholar]
- Marliss E. B., Nakhooda A. F., Poussier P., Sima A. A. The diabetic syndrome of the 'BB' Wistar rat: possible relevance to type 1 (insulin-dependent) diabetes in man. Diabetologia. 1982 Apr;22(4):225–232. doi: 10.1007/BF00281296. [DOI] [PubMed] [Google Scholar]
- McChesney M. B., Oldstone M. B. Virus-induced immunosuppression: infections with measles virus and human immunodeficiency virus. Adv Immunol. 1989;45:335–380. doi: 10.1016/s0065-2776(08)60696-3. [DOI] [PubMed] [Google Scholar]
- Nakhooda A. F., Like A. A., Chappel C. I., Murray F. T., Marliss E. B. The spontaneously diabetic Wistar rat. Metabolic and morphologic studies. Diabetes. 1977 Feb;26(2):100–112. doi: 10.2337/diab.26.2.100. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Inhibition of antibodies to nuclear antigen and to DNA in New Zealand mice infected with lactate dehydrogenase virus. Science. 1972 Feb 18;175(4023):784–786. doi: 10.1126/science.175.4023.784. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science. 1988 Jan 29;239(4839):500–502. doi: 10.1126/science.3277269. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Salvato M., Tishon A., Lewicki H. Virus-lymphocyte interactions. III. Biologic parameters of a virus variant that fails to generate CTL and establishes persistent infection in immunocompetent hosts. Virology. 1988 Jun;164(2):507–516. doi: 10.1016/0042-6822(88)90565-x. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Chiller J. M., Weigle W. O., Dixon F. J. Effect of chronic viral infection on the immune system. I. Comparison of the immune responsiveness of mice chronically infected with LCM virus with that of noninfected mice. J Immunol. 1973 May;110(5):1268–1278. [PubMed] [Google Scholar]
- Popescu M., Löhler J., Lehmann-Grube F. Infectious lymphocytes in lymphocytic choriomeningitis virus carrier mice. J Gen Virol. 1979 Mar;42(3):481–492. doi: 10.1099/0022-1317-42-3-481. [DOI] [PubMed] [Google Scholar]
- Prud'homme G. J., Lapchak P. H., Parfrey N. A., Colle E., Guttmann R. D. Autoimmunity-prone BB rats lack functional cytotoxic T cells. Cell Immunol. 1988 Jun;114(1):198–208. doi: 10.1016/0008-8749(88)90266-3. [DOI] [PubMed] [Google Scholar]
- Schwimmbeck P. L., Dyrberg T., Oldstone M. B. Abrogation of diabetes in BB rats by acute virus infection. Association of viral-lymphocyte interactions. J Immunol. 1988 May 15;140(10):3394–3400. [PubMed] [Google Scholar]
- Whitton J. L., Southern P. J., Oldstone M. B. Analyses of the cytotoxic T lymphocyte responses to glycoprotein and nucleoprotein components of lymphocytic choriomeningitis virus. Virology. 1988 Feb;162(2):321–327. doi: 10.1016/0042-6822(88)90471-0. [DOI] [PubMed] [Google Scholar]
- Woda B. A., Padden C. BioBreeding/Worcester (BB/Wor) rats are deficient in the generation of functional cytotoxic T cells. J Immunol. 1987 Sep 1;139(5):1514–1517. [PubMed] [Google Scholar]
- Wu-Hsieh B., Howard D. H., Ahmed R. Virus-induced immunosuppression: a murine model of susceptibility to opportunistic infection. J Infect Dis. 1988 Jul;158(1):232–235. doi: 10.1093/infdis/158.1.232. [DOI] [PubMed] [Google Scholar]



