Abstract
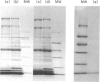
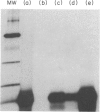
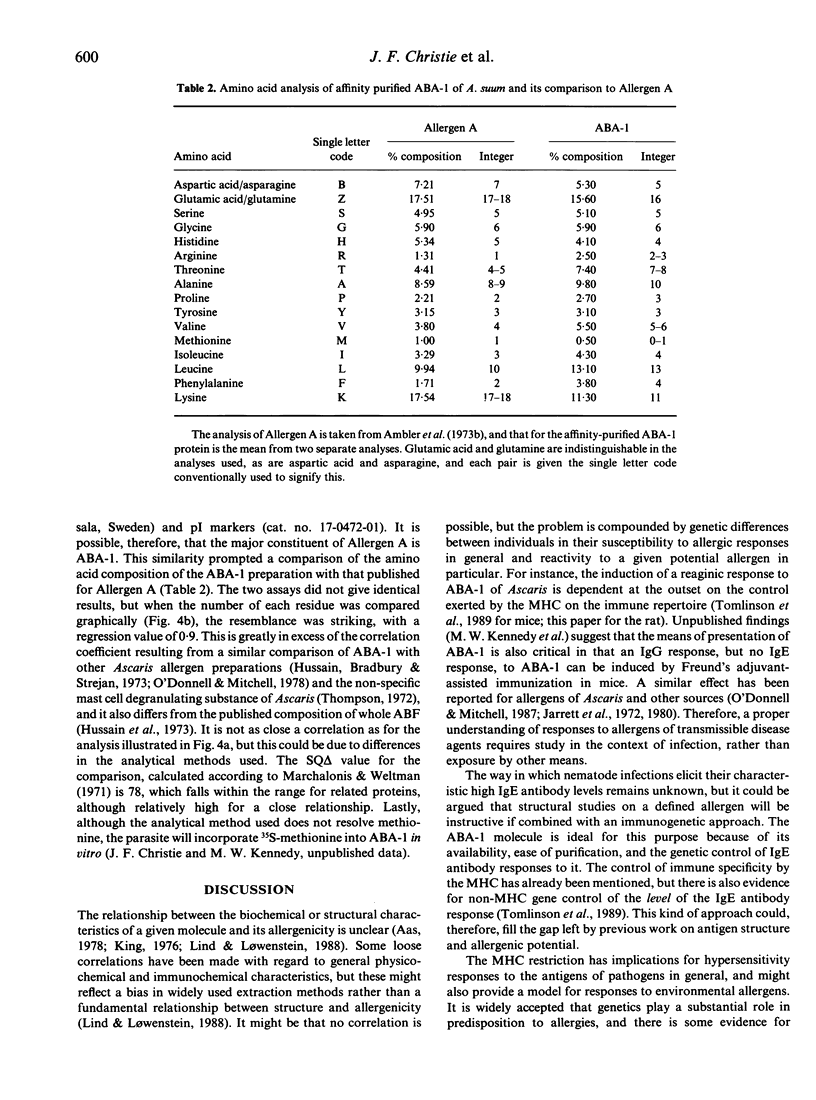
A protein allergen of the parasitic nematode Ascaris has been purified to homogeneity by immunoaffinity chromatography. It is the most abundant protein species in the parasite's body fluid and has been named ABA-1. The allergen's molecular weight (MW) has been previously estimated at 14,000, but this sizing is currently under re-evaluation. The immunological activity of the protein was intact after purification, as attested by immunoprecipitation and passive cutaneous anaphylaxis. The IgE response to ABA-1 was under major histocompatibility complex (MHC) restriction in the rat, in which only RT1u strains were found to respond following infection with the parasite. The tissue-invasive and intestinal stages of both Ascaris lumbricoides (of humans) and Ascaris suum (of pigs) have an antigen of similar MW to ABA-1 in their secretions or among their somatic antigens. These are antigenically indistinguishable; they were found to have similar amino acid compositions, and their N-terminal amino acid sequences were identical to 41 residues. Finally, the apparent MW, amino acid composition and isoelectric point of ABA-1 all argue for close similarity to the previously described Allergen A of the parasite.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler J., Croft A. R., Doe J. E., Gemmell D. K., Miller J. N., Orr T. S. Biological techniques for studying the allergenic components of nematodes. II. The characterisation of the allergen released by Ascaris suum maintaind in saline. J Immunol Methods. 1973 Apr;2(3):315–323. doi: 10.1016/0022-1759(73)90058-6. [DOI] [PubMed] [Google Scholar]
- Ambler J., Miller J. N., Johnson P., Orr T. S. Characterisation of an allergen extracted from Ascaris suum. Determination of the molecular weight, isoelectric point, amino acid and carbohydrate content of the native allergen. Immunochemistry. 1973 Dec;10(12):815–820. doi: 10.1016/0019-2791(73)90185-7. [DOI] [PubMed] [Google Scholar]
- Ambler J., Miller J. N., Orr T. S. Some properties of Ascaris suum allergen A. Int Arch Allergy Appl Immunol. 1974;46(3):427–437. doi: 10.1159/000231146. [DOI] [PubMed] [Google Scholar]
- Barnett B. C., Graham C. M., Burt D. S., Skehel J. J., Thomas D. B. The immune response of BALB/c mice to influenza hemagglutinin: commonality of the B cell and T cell repertoires and their relevance to antigenic drift. Eur J Immunol. 1989 Mar;19(3):515–521. doi: 10.1002/eji.1830190316. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Jarrett E. E. Hypersensitivity reactions in small intestine. I Thymus dependence of experimental 'partial villous atrophy'. Gut. 1975 Feb;16(2):114–117. doi: 10.1136/gut.16.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspon L. W., White J., Shields R. L., Fügner A., Gold W. M. Purification of Ascaris suum antigen: its allergenic activity in vitro and in vivo. J Allergy Clin Immunol. 1986 Mar;77(3):443–451. doi: 10.1016/0091-6749(86)90178-8. [DOI] [PubMed] [Google Scholar]
- Haswell-Elkins M. R., Kennedy M. W., Maizels R. M., Elkins D. B., Anderson R. M. The antibody recognition profiles of humans naturally infected with Ascaris lumbricoides. Parasite Immunol. 1989 Nov;11(6):615–627. doi: 10.1111/j.1365-3024.1989.tb00925.x. [DOI] [PubMed] [Google Scholar]
- Hussain R., Bradbury S. M., Strejan G. Hypersensitivity to Ascaris antigens. 8. Characterization of a highly purified allergen. J Immunol. 1973 Jul;111(1):260–268. [PubMed] [Google Scholar]
- Jarrett E. E., Hall E., Karlsson T., Bennich H. Adjuvants in the induction and enhancement of rat IgE responses. Clin Exp Immunol. 1980 Jan;39(1):183–189. [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Henderson D., Riley P., White R. G. The effect of various adjuvant regimens and of nematode infection on the reaginic antibody response to egg-albumin in the rat. Int Arch Allergy Appl Immunol. 1972;42(6):775–781. doi: 10.1159/000230657. [DOI] [PubMed] [Google Scholar]
- Jarrett E. E., Miller H. R. Production and activities of IgE in helminth infection. Prog Allergy. 1982;31:178–233. [PubMed] [Google Scholar]
- Kennedy M. W. Genetic control of the immune repertoire in nematode infections. Parasitol Today. 1989 Oct;5(10):316–324. doi: 10.1016/0169-4758(89)90122-1. [DOI] [PubMed] [Google Scholar]
- Kennedy M. W., Gordon A. M., Tomlinson L. A., Qureshi F. Genetic (major histocompatibility complex?) control of the antibody repertoire to the secreted antigens of Ascaris. Parasite Immunol. 1987 Mar;9(2):269–273. doi: 10.1111/j.1365-3024.1987.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Kennedy M. W., Qureshi F., Fraser E. M., Haswell-Elkins M. R., Elkins D. B., Smith H. V. Antigenic relationships between the surface-exposed, secreted and somatic materials of the nematode parasites Ascaris lumbricoides, Ascaris suum, and Toxocara canis. Clin Exp Immunol. 1989 Mar;75(3):493–500. [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. W., Qureshi F., Haswell-Elkins M., Elkins D. B. Homology and heterology between the secreted antigens of the parasitic larval stages of Ascaris lumbricoides and Ascaris suum. Clin Exp Immunol. 1987 Jan;67(1):20–30. [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. W., Qureshi F. Stage-specific secreted antigens of the parasitic larval stages of the nematode Ascaris. Immunology. 1986 Jul;58(3):515–522. [PMC free article] [PubMed] [Google Scholar]
- King T. P. Chemical and biological properties of some atopic allergens. Adv Immunol. 1976;23:77–105. doi: 10.1016/s0065-2776(08)60319-3. [DOI] [PubMed] [Google Scholar]
- Marsh D. G., Hsu S. H., Roebber M., Ehrlich-Kautzky E., Freidhoff L. R., Meyers D. A., Pollard M. K., Bias W. B. HLA-Dw2: a genetic marker for human immune response to short ragweed pollen allergen Ra5. I. Response resulting primarily from natural antigenic exposure. J Exp Med. 1982 May 1;155(5):1439–1451. doi: 10.1084/jem.155.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- O'Donnell I. J., Mitchell G. F. An investigation of the allergens of Ascaris lumbricoides using a radioallergosorbent test (RAST) and sera of naturally infected humans: comparison with an allergen for mice identified by a passive cutaneous anaphylaxis test. Aust J Biol Sci. 1978 Oct;31(5):459–487. doi: 10.1071/bi9780459. [DOI] [PubMed] [Google Scholar]
- O'Hehir R. E., Eckels D. D., Frew A. J., Kay A. B., Lamb J. R. MHC class II restriction specificity of cloned human T lymphocytes reactive with Dermatophagoides farinae (house dust mite). Immunology. 1988 Aug;64(4):627–631. [PMC free article] [PubMed] [Google Scholar]
- Odunjo E. O. Helminthic anaphylactic syndrome (HAS) in children. Pathol Microbiol (Basel) 1970;35(1):220–223. doi: 10.1159/000162233. [DOI] [PubMed] [Google Scholar]
- Thompson A. R. Isolation and characterisation of a mast cell degranulating substance from Ascaris suum. Biochim Biophys Acta. 1972 Jan 28;261(1):245–257. doi: 10.1016/0304-4165(72)90335-2. [DOI] [PubMed] [Google Scholar]
- Tomlinson L. A., Christie J. F., Fraser E. M., McLaughlin D., McIntosh A. E., Kennedy M. W. MHC restriction of the antibody repertoire to secretory antigens, and a major allergen, of the nematode parasite Ascaris. J Immunol. 1989 Oct 1;143(7):2349–2356. [PubMed] [Google Scholar]