Abstract
The α4 laminin subunit is a component of the basement membrane of blood vessels where it codistributes with the integrins αvβ3, α3β1, and α6β1. An antibody against the G domain (residues 919-1207; G919–1207) of the α4 laminin subunit inhibits angiogenesis in a mouse–human chimeric model, indicating the functional importance of this domain. Additional support for the latter derives from the ability of recombinant G919–1207 to support endothelial cell adhesion. In particular, endothelial cell adhesion to G919–1207 is half-maximal at 1.4 nM, whereas residues 919-1018 and 1016–1207 of the G domain are poor cellular ligands. Function blocking antibodies against integrins αvβ3 and β1 and a combination of antibodies against α3 and α6 integrin subunits inhibit endothelial cell attachment to G919–1207. Moreover, both αvβ3 and α3β1 integrin bind with high affinity to G919–1207. Together, our studies demonstrate that the G domain of laminin α4 chain is a specific, high affinity ligand for the αvβ3 and α3β1 integrin heterodimers and that these integrins, together with α6β1, function cooperatively to mediate endothelial cell–α4 laminin interaction and hence blood vessel development. We propose a model based on these data that reconcile apparent discrepancies in the recent literature with regard to the role of the αvβ3 integrin in angiogenesis.
Keywords: matrix, matrix receptor, blood vessels
Laminins, heterotrimeric molecules composed of α, β, and γ subunits, are major components of basement membranes found in a variety of different tissue types. There are at least 14 laminin isoforms that regulate a variety of cellular functions including adhesion, migration, proliferation, cell survival, and differentiation (1–3). Although certain laminin isoforms, namely laminin 10 (α5, β1, γ1), show widespread tissue distribution, the expression of other laminin isoforms is tissue specific and tightly regulated during development (3). For example, laminin 2 (α2, β1, γ1) is expressed in the basement membrane of skeletal muscle and is believed to play an important role in basement membrane assembly and clustering of the acetylcholine receptor in the neuromuscular junction (4, 5). Laminin 5 (α3, β3, γ2) is a constituent of the basement membrane of epithelial tissue where it regulates stable adhesion of epithelium to the connective tissue (3, 6, 7). Laminins 8 (α4, β1, γ1) and 9 (α4, β2, γ1) are expressed by endothelial and smooth muscle cells, but their functions in vivo remain unclear (1, 8).
Compared with α1, α2, and α5, the α4 subunit present in laminins 8 and 9 contains a truncated N terminus (8–10). In this regard, it is similar to the α3 subunit present in laminins 5, 6, and 7. Like all other known α subunits, the α4 laminin subunit possesses a large C-terminal G domain, consisting of five structurally and functionally distinct regions (G1–G5; refs. 1 and 10–13). Expression of the α4 laminin subunit is restricted to certain tissues. It is found in vascular endothelial basement membranes of brain, muscle, and bone marrow and the perineurium of peripheral nerves, heart, developing skeletal muscle, and developing kidney (8, 9, 13–15). Indeed, the expression of α4 laminin protein has been used as a marker of the vascularity of certain types of tumors (8, 16).
Recent data indicate that the integrin heterodimers α3β1 and α6β1 may function as cell-surface adhesion receptors for α4-containing laminins (17). Studies from our lab have implicated the αvβ3 integrin in endothelial cell adhesion to laminins containing an α4 subunit. Specifically, the α4 laminin subunit and αvβ3 integrin codistribute in focal contact structures in endothelial cells (18). Moreover, antibodies against the αvβ3 integrin inhibit endothelial cell adhesion to a G domain fragment of the α4 laminin subunit (18). However, these studies fail to address which integrins directly bind the α4 laminin subunit. Thus, the goal of our experiments was to determine integrin partners of the α4 laminin subunit and assess functions for the α4 laminin subunit in endothelial cells in vivo and in vitro. Here, we show that both the α3β1 and the αvβ3 integrin can bind the G domain of laminin α4 subunit with high affinity. Moreover, we detail complex integrin interactions with the α4 laminin and provide evidence that the α4 laminin subunit is involved in blood vessel development in an in vivo model.
Materials and Methods
Cell Culture.
Immortalized human bone marrow endothelial cells (TrHBMEC) were kindly provided by Babette Weksler (Cornell Medical School, New York) and Denise Paulin (Universite Paris VII and Institute Pasteur, Paris) (19). These were derived by immortalizing human bone marrow endothelial cells with a construct encoding the large T antigen of SV40 under the control of a truncated human vimentin gene promoter (19). The transformed cell line retained all of the characteristics of the untransformed cell line including expression of cell-surface markers such as von Willebrand factor, P-selectin, CD31, CD34, CD44, and intercellular adhesion molecule 2 (19). TrHBMEC were maintained in DMEM containing 2 mM L-glutamine, 10% FBS, and 1× RPMI vitamins. Human umbilical vascular endothelial cells (HUVEC) were a kind gift of William Schnapper (Northwestern University). Cells were maintained in endothelial cell growth medium containing 20% FBS and 1× supplement mix (Promo Cell, Heidelberg).
Antibodies.
Mouse monoclonal antibodies against the αvβ3 integrin heterodimer (LM609), α3 integrin subunit (P1B5), β1-integrin subunit (6S6), α3β1 integrin heterodimer (MKID2), and a β3 integrin rabbit anti-serum (AB1932) were obtained from Chemicon. The rat monoclonal α6 integrin antibody (GoH3) was purchased from Beckman Coulter. 2A3, a function-blocking antibody against the G domain of α4 laminin was described (18). A monoclonal antibody against human collagen type IV was obtained from Sigma. An antiserum against von Willebrand factor was purchased from Neomarkers (Fremont, CA).
Matrix Proteins and Integrins.
Human fibronectin and Matrigel were purchased from BD Biosciences (Bedford, MA), whereas laminin-1 was obtained from GIBCO/BRL. They were used according to each manufacturer's instructions. Laminin 5 was derived from conditioned medium of cultured epithelial cells (20). Recombinant α4 laminin, consisting of a portion of the G1 and G2 subdomains (residues 919–1207; G919–1207), was isolated from bacterial extracts as described (18). The α4 laminin G1 (residues 919–1018; G919–1018) and G2 (residues 1016–1207; G1016–1207) fragments were produced in bacteria as follows. In brief, cDNA, generated by RT-PCR from mRNA isolated from TrHBMEC, was used as template for PCR using α4 laminin subunit specific forward and reverse primers. Amplified product, digested with appropriate restriction enzymes, was ligated into the pET32a protein expression vector (Novagen) in frame with sequences encoding a 6 × His tag. Reading frame and sequence was verified by automated sequencing (Biotechnology Facility, Northwestern University). Vectors were transfected into the Escherichia coli strain BL21. The cells were induced to express laminin α4 fusion proteins by addition of 1 mM isopropyl β-D-thiogalactoside (Fisher), and fragments were purified by column chromatography (Novagen). The purity of recombinant polypeptides was assessed by visualizing protein samples by SDS/PAGE as well as by Western blotting using a His probe, following transfer of protein to nitrocellulose (Pierce). Soluble αvβ3 and α3β1 integrin heterodimers were purchased from Chemicon. Their purity was routinely assessed by SDS/PAGE before use.
Cell Adhesion Assay.
Approximately 1 × 105 TrHBMEC or HUVEC were plated onto uncoated or protein-coated wells of a 96-well plate (Sarstedt) and blocked with 1% BSA in PBS for 1 h at 37°C. After 1 h at 37°C, the wells were washed extensively with PBS to remove nonadhering cells, and then adherent cells were fixed in 3.7% formaldehyde in PBS for 15 min. Fixed cells were incubated at room temperature with crystal violet for 15 min and then solubilized with 1% SDS. Absorbance at 570 nm was measured with a Vmax plate reader (Molecular Devices). Values in the concentration-response curves were normalized to maximum cell attachment. The effective concentration (EC50) is defined as the concentration of ligand that produces half-maximal cell attachment.
In certain studies, integrin antibodies and control, isotype-matched immunoglobulins were added to cell suspensions for 30 min at room temperature before the cells were plated onto substrate. In function-blocking antibody studies, values were normalized to control (100%).
ELISAs.
Wells of 96-well nontissue culture-treated plates were coated with protein at varying concentrations for 18 h at 4°C. Each well was rinsed three times and blocked with 1% BSA in PBS for 1 h at 37°C. Soluble integrin heterodimers were diluted in binding buffer (25 mM Tris buffer/150 nM NaCl/1 mM MgCl2/0.5 mM MnCl2/0.05% BSA, pH 7.5) and added to each well for a final concentration of 5 ng/μl. After incubating for 90 min at 37°C, wells were rinsed three times in binding buffer, and appropriate mouse monoclonal anti-integrin antibody was added for 1 h at 37°C. Wells were then rinsed three times in PBS, and alkaline phosphatase-conjugated goat anti-mouse antibody was added to the wells for an additional 1 h at 37°C. Wells were rinsed three times in PBS, and 200 μl of substrate [p-nitrophenyl phosphate (PNPP, Sigma) diluted in ELISA buffer to a final concentration of 1 mg/ml] was added per well. Absorbance at 405 nm was measured with a Vmax plate reader (Molecular Devices). Nonspecific binding was determined by the addition of 10 mM EDTA to binding buffer. Specific binding was obtained by subtracting nonspecific binding from total binding (total binding − nonspecific binding). In saturation binding studies, the dissociation constant (Kd) corresponds to the concentration of ligand that produces half-maximal specific binding. In competition binding studies, the inhibitory concentration (IC50) is defined as the concentration of competitor that blocks 50% of specific binding. All curves were fitted with nonlinear regression by using GRAPHPAD PRISM (v. 3.00).
Immunofluorescence Microscopy.
Human renal carcinoma tissue was frozen in Tissue-Tek O.C.T. compound (Miles), and consecutive frozen sections of 6-μm thickness were prepared by using a Tissue-Tek Cryostat at −20°C. Sections were placed on slides, extracted in acetone at −20°C for 5 min, and then air-dried. Matrigel implants were fixed in 10% buffered formalin, embedded in paraffin, and sectioned. Sections were deparaffinized, and antigens were retrieved in 10 mM citric acid (pH 6.0) by microwaving twice for 7 min. Tissue sections were incubated with primary antibodies, diluted in PBS at 37°C in a humid chamber for at least 1 h, washed three times in PBS, and then incubated with the appropriate mix of fluorochrome-conjugated secondary antibodies for an additional 1 h at 37°C. Stained specimens were viewed by using a Zeiss LSM510 laser-scanning confocal microscope or Zeiss Axioskop microscope.
SDS/PAGE and Western Blotting.
Matrix proteins and integrins were separated on 7.5–12% SDS-polyacrylamide gels following standard procedures (21). Gels were stained or separated proteins were transferred to nitrocellulose, which was subsequently processed for Western blotting as previously described (21–23).
In Vivo Angiogenesis Assay.
Approximately 1 × 106 human dermal microvascular endothelial cells were mixed with 0.5 ml of Matrigel on ice in the presence of either antibody 2A3 or control IgM, and the mixture was implanted into the ventral midline thoracic tissue of a mouse following procedures outlined in ref. 24. At 7 days, the implants were removed, fixed and processed for immunofluorescence microscopy as above. Separate ×10 fields per tissue were taken, and the number of annular structures was counted and averaged.
Results
The α4 Laminin and Angiogenesis.
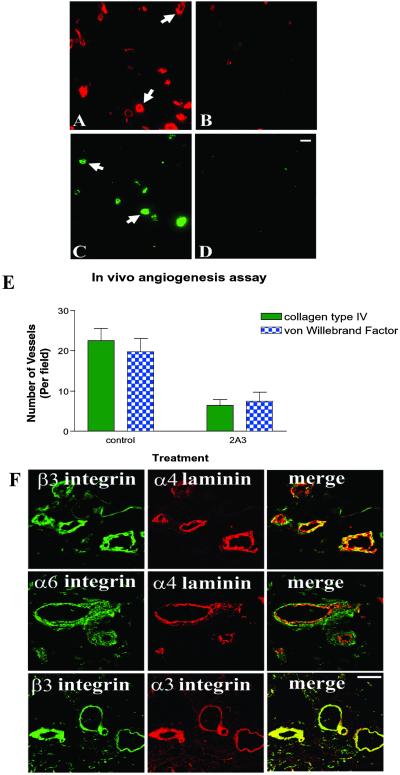
Our previous work has implicated a role for the α4 laminin subunit in endothelial cell branching morphogenesis (18). Such morphogenesis is a component of blood vessel development (25). Thus, we assessed whether the α4 laminin subunit is involved in blood vessel formation assembly in vivo by using a mouse–human chimeric model in which human dermal microvascular endothelial cells are injected into severe combined immunodeficient mice in Matrigel (24). After 7 days, the human cells assembled into blood vessels that can be identified and quantified by using a marker of basement membrane assembly, namely an antibody probe specific for human collagen type IV (Fig. 1 A and B) and an antiserum specific for von Willebrand factor, an endothelial cell marker (Fig. 1 C and D). In our studies, we evaluated blood vessel development in this model system under conditions where an antibody (2A3) against the α4 laminin subunit or an isotype-matched control IgM was added to the cell–Matrigel mix before injection into the severe combined immunodeficient mice. As can be seen in Fig. 1 A–D, there is a significant decrease in both collagen IV antibody and von Willebrand factor staining in the 2A3 antibody-treated samples compared with samples treated with control IgM. Quantification of these results is shown in Fig. 1E. These data provide direct evidence that the α4 laminin subunit, in particular the 2A3 epitope, is involved in angiogenesis. Because the 2A3 epitope lies within the G domain of the α4 laminin, we next studied the cell-surface interactions of this functionally important domain (18). We initiated our studies by first determining which integrins codistributed with the α4 laminin subunit in the basement membrane of blood vessels.
Fig 1.
Antibody 2A3 against α4 laminin inhibits angiogenesis in vivo. Human endothelial cells were mixed with 0.5 ml of Matrigel in the presence of 125 μg/ml control IgM (A and C) or 125 μg/ml antibody 2A3 (B and D). The cell-matrix mix was then injected in the ventral midline thoracic tissue of severe combined immunodeficient mice. After 7 days, implants were removed, fixed, and prepared for immunofluorescence microscopy. Samples were stained with either anti-human type IV collagen antibody (A and B) or an antiserum against von Willebrand factor (C and D). Type IV collagen and von Willebrand factor staining appears in an annular and linear organization in A and C (arrows). (E) The number of vascular structures observed in the specimens shown in A–D were quantified. Results represent mean ± SD of five separate fields. (F) Laminin α4 subunit and integrin subunit localization in blood vessels. Cryostat sections of human renal carcinoma tissue were prepared for double-label immunofluorescence and viewed by laser-scanning confocal microscopy. The tissue sections were incubated with a polyclonal rabbit antiserum against the integrin β3 in combination with either antibody 2A3 against the α4 laminin subunit or antibody P1B5 against α3 integrin as indicated. Sections were also processed by using antibody GoH3 against α6 integrin in combination with the α4 laminin subunit as shown. Merged versions of the red and green images are presented (Lower Right). Yellow color indicates overlap in staining. (Bar = 20 μm in D; bar = 50 μm in F.)
Endothelial Cell Integrins and the α4 Laminin Subunit.
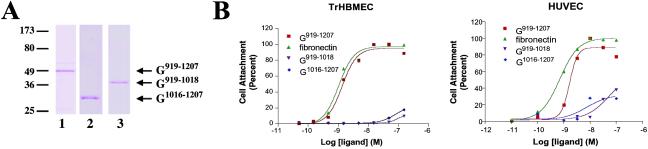
In cryosections of renal carcinoma tissue that possess an extensive vasculature, 2A3 antibodies generated an intense stain along the basement membrane zone of blood vessels, a site rich in α3, α6, and β3 subunit-containing integrins (Fig. 1F). Indeed, the codistribution of the α4 laminin subunit with α3 and α6 integrin along the site of endothelial cell–basement membrane zone interaction is consistent with data indicating that cells interact with laminins 8 and 9 via α3β1 and α6β1 integrin (17). However, we showed that endothelial cells adhere to a fragment of the α4 laminin subunit consisting of a portion of its G1 and G2 subdomains (G919–1207) in an αvβ3 integrin-dependent manner (18). To resolve the issue of integrins involved in endothelial cell attachment to the G domain of the α4 laminin, we undertook endothelial cell adhesion assays by using a number of α4 G domain fragments prepared from bacterial lysates (Fig. 2A). To do so, we made use of bone marrow endothelial cells because these cells have been implicated in pathologically induced angiogenesis (26). Because primary bone marrow endothelial cells are not available in sufficient quantities for our studies, we used an immortalized bone marrow endothelial cell line (TrHBMEC) that shows many, if not all, of the characteristics of primary cells (19). For comparison, we also analyzed matrix adhesion of normal endothelial cells (HUVEC).
Fig 2.
(A) Gel profiles of the recombinant proteins used in these studies. Proteins purified from bacterial cell extracts were processed for SDS/PAGE. Lanes 1–3 show G919–1207, G919–1018, and G1016–1207, respectively. Molecular weight standards are indicated (Left). (B) Endothelial cells (TrHBMEC or HUVEC as indicated) adhere to the G domain of the α4 laminin subunit. Endothelial cells were added to the wells of a 96-well plate coated with varying concentrations of G919–1207, G919–1018, G1016–1207, or human fibronectin as indicated. Cells were allowed to attach at 37°C for 1 h. Nonadherent cells were washed off the wells, and the remaining cells were fixed and stained with crystal violet. Absorbance was read at 570 nm. The curves are representative of three separate experiments.
TrHBMEC or HUVEC were added to wells precoated with varying concentrations of G domain fragments comprising residues 919-1207 (G919–1207), containing the epitope of antibody 2A3, residues 919-1018 (G919–1018) within the G1 subdomain, and residues 1016–1207 (G1016–1207) within the G2 subdomain (Fig. 2). Both TrHBMEC and HUVEC attached to residues G919–1207 in a concentration-dependent manner, with cell binding being half-maximal (EC50) at 1.4 and 1.5 nM, respectively (Fig. 2B). Endothelial cell attachment to fibronectin produced a similar concentration–response curve with an EC50 of 1.0 nM (TrHBMEC) and 0.8 nM (HUVEC). In contrast, both G919–1018 and G1016–1207 fragments were poor ligands for cell attachment, and cell attachment failed to reach half-maximal even at 100 nM ligand concentration (Fig. 2B).
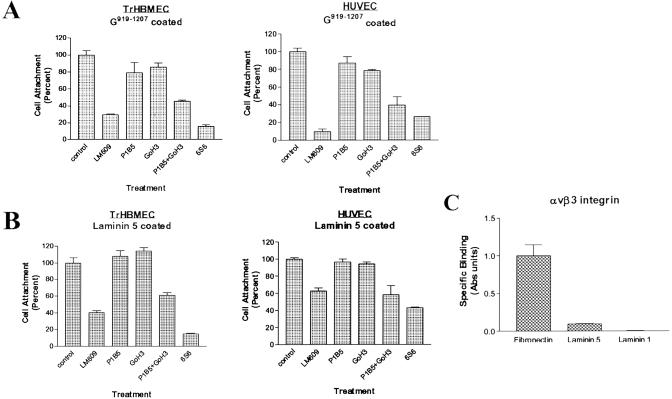
We next investigated integrin involvement in endothelial cell adhesion to G919–1207. TrHBMEC or HUVEC in suspension were treated with various function-blocking integrin antibodies before addition to wells coated with 100 nM G919–1207. Both TrHBMEC and HUVEC showed maximal binding to wells coated with this concentration of protein (Fig. 2B). Antibody LM609, which perturbs the function of the αvβ3 integrin, inhibited TrHBMEC adhesion to G919–1207 by ≈70%, whereas 6S6, an integrin β1 function-blocking antibody, inhibited cell adhesion by ≈84% compared with control IgG-treated cells (Fig. 3A). Studies with HUVEC produced similar results (Fig. 3A). LM609 and 6S6 inhibited cell adhesion by 80% and 70%, respectively. The latter result is contrary to our previous report where we showed that a different β1 integrin antibody (P4C10) failed to inhibit endothelial cell adhesion to the α4 laminin G domain (18). We have repeated our studies numerous times and consistently observe inhibition in adhesion of endothelial cells to G919–1207 with 6S6 but little, if any, inhibition when endothelial cells are treated with P4C10. We cannot explain this anomaly. To resolve the potential role of β1 containing integrins in endothelial cell attachment to the α4 laminin subunit, we examined the effects of antibodies that functionally inhibited α6 and α3 integrin, namely GoH3 and P1B5, on adhesion of endothelial cells to G919–1207. The α3 integrin antibody, P1B5, when used in combination with GoH3, the α6 integrin antibody, inhibited both TrHBMEC and HUVEC adhesion by >54% (Fig. 3A). It should be noted that these same antibodies when used individually have minimal impact on either TrHBMEC or HUVEC cell adhesion to G919–1207, suggesting that the function of α3 and α6 subunit-containing integrins is in some way coupled in endothelial cells (Fig. 3A). Together, these data indicate an involvement of both α6β1 and α3β1 in adhesion to the G domain of the α4 laminin subunit, a finding consistent with a previous report (17). However, these data also indicate that the αvβ3 integrin subunit plays a role in endothelial cell–α4 laminin subunit interaction.
Fig 3.
(A) Cell attachment of TrHBMEC or HUVEC to G919–1207 involves the αvβ3 and α3β1 and α6β1 integrin heterodimers. Cells were pretreated with control IgG or function-blocking antibodies against αvβ3 integrin (LM609), α3 integrin (P1B5), α6 integrin (GoH3), or a combination of P1B5 and GoH3 or β1 integrin (6S6) for 30 min at 37°C before adding cells to wells coated with 100 nM G919–1207 protein. LM609 was used at 25 μg/ml, whereas all other antibodies and control IgG were used at 50 μg/ml. Cell attachment was evaluated as in Fig. 2. (B) TrHBMEC and HUVEC adhesion to laminin 5. Endothelial cells were pretreated with the same function-blocking antibodies as in A for 30 min at 37°C. The attachment of the cells to wells coated with 5 μg/ml laminin 5 was evaluated after 1 h as above. (C) Integrin αvβ3 binds directly to fibronectin but not to laminins 1 and 5. Wells of 96-well plates were coated with equal concentrations of extracellular matrix proteins (5 ng/μl). αvβ3 integrin (5 ng/μl) was then added to the wells and allowed to bind for 1 h at 37°C. Integrin binding was evaluated by ELISA using an antibody against αvβ3, followed by a secondary antibody conjugated to alkaline phosphatase. Absorbance was measured at 405 nm. Values in bar graphs are expressed as means ± SD of three trials.
One intriguing aspect of the above results is that αvβ3 integrin fails to “compensate” in mediating binding to G919–1207 when the α3β1 and α6β1 integrin heterodimers are functionally inhibited and vice versa (Fig. 3A). One possible explanation for our results is that in endothelial cells the activity of one integrin may modulate ligand binding of another via a process that is termed transmodulation (27, 28). To test this possibility, we assayed TrHBMEC and HUVEC adhesion to laminin 5, a ligand for α3β1 but not for αvβ3, in the presence of antibodies that functionally perturb either the αvβ3 or α3β1 integrin heterodimer (Fig. 3 B and C). As would be expected, TrHBMEC and HUVEC adhesion to laminin 5 was inhibited by 40% and 45%, respectively, when treated with a combination of antibodies against the α3 integrin subunit and α6 integrin subunits and by more than 80% and 60%, respectively, by antibody 6S6, a function-blocking antibody against β1 integrin (Fig. 3B). However, in addition, TrHBMEC and HUVEC adhesion was also inhibited by 60% and 40%, respectively, when cells were treated with antibody LM609 against the αvβ3 integrin (Fig. 3B). This indicates that functional perturbation of the αvβ3 integrin has a transmodulating, in this case inhibitory, impact on α3β1 integrin–laminin 5 interaction.
Characterization of Direct Integrin Interaction with the G Domain of the α4 Laminin Subunit.
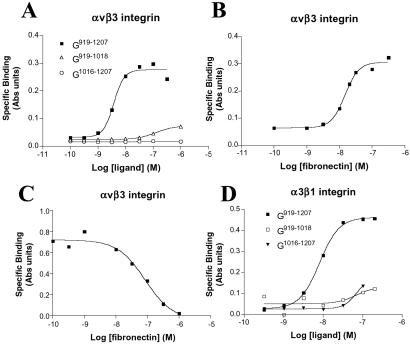
The above data indicate a role for both the αvβ3 and α3β1 and α6β1 integrin heterodimers in mediating endothelial cell adhesion to laminin α4. However, they do not show whether integrins bind directly to the α4 laminin. Hence, to study this issue, we conducted solid-phase saturation binding experiments by using a cell-free system, by using purified integrin and laminin proteins. To date, we have restricted our analyses to αvβ3 and α3β1 integrin binding to ligand because we have been unable to obtain appropriately pure α6β1 integrin to use in our assays. Nevertheless, soluble αvβ3 bound G919–1207 in a concentration-dependent fashion, and binding is saturable (Fig. 4A). The observed dissociation constant (Kd) was 4.0 nM. This is comparable to the dissociation constant when αvβ3 integrin bound fibronectin, a known ligand for this integrin heterodimer (Kd, 15 nM; Fig. 4B; ref. 29). Moreover, fibronectin was able to compete with G919–1207 for binding to αvβ3 in a concentration-dependent fashion with a measured IC50 of 84 nM, suggesting that fibronectin and G919–1207 bound to a similar or nearby site on the αvβ3 integrin molecule (Fig. 4C). α3β1 integrin also bound to G919–1207 with a Kd of 7.3 nM (Fig. 4D). No significant binding of α3β1 or αvβ3 to G919–1207 was detected in the presence of 10 mM EDTA (data not shown). Furthermore, αvβ3 and α3β1 integrin bound poorly to both G919–1018 and G1016–1207 and laminin-1 (Figs. 3C and 4 A and D; α3β1 integrin binding data not shown in Fig. 3C).
Fig 4.
(A and B) Integrin αvβ3 binds directly to the α4 G domain and fibronectin with high affinity. Wells of a 96-well plate were coated with varying concentrations of G919–1207, G919–1018, and G1016–1207 (A) or fibronectin (B). αvβ3 integrin (5 ng/μl) was then added to the wells and allowed to bind for 1 h at 37°C. Integrin binding was evaluated by ELISA using an antibody against αvβ3, followed by a secondary antibody conjugated to alkaline phosphatase. (C) Competition binding curve. Soluble αvβ3 (5 ng/μl) was added to wells coated with G919–1207 in the presence of increasing concentrations of fibronectin at 37°C. Integrin binding was assayed as in A and B after 1 h. (D) α3β1 integrin was added to wells coated with varying concentrations of G919–1207, G919–1018, and G1016–1207 and allowed to bind for 1 h at 37°C. Integrin binding was evaluated by ELISA using MKID2, an antibody against the α3β1 integrin heterodimer, followed by alkaline phosphatase-conjugated secondary antibody. In all studies, absorbance was measured at 405 nm. Each of the graphs is representative of at least three separate experiments.
Discussion
In this study, we have characterized a complex integrin-binding domain within the α4 laminin subunit. During the course of our studies, we have shown that two different endothelial cell types and purified integrins adhere to a fragment of the α4 G domain that contains a portion of the G1 and G2 subdomains. Individually, neither the G1 nor the G2 subdomain possesses an obvious binding site for endothelial cells or its integrin heterodimers. Rather, cells and integrins show interaction with a region that seems to span both subdomains. The functional importance of the α4 laminin G domain is emphasized by our in vitro and in vivo studies. In the former, we have shown that antibodies against the same G1/G2 domain fragment block branching morphogenesis of endothelial cells maintained on artificial basement membrane proteins (18). In the latter, we show that the same antibody inhibits blood vessel development in vivo. The idea that the G domain of the α4 laminin subunit plays an important role in blood vessel formation as well as function extends recent studies in which the phenotype of mice lacking the α4 laminin subunit has been analyzed (30). These mice possess leaky blood vessels.
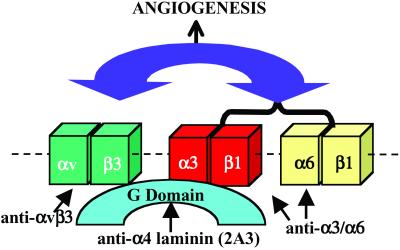
One intriguing aspect of this work is that various integrin heterodimers seem to act in concert in endothelial cell adhesion to the G domain of the laminin α4 subunit. By using cell adhesion assays, we have presented evidence that function-perturbing antibodies against the αvβ3 integrin, β1 integrin subunit or a mix of antibodies against the α3 and α6 integrin subunit inhibit endothelial cell adhesion to the α4 laminin G domain. This result led us to assess which integrins are able to bind directly to the α4 G domain. Our data reveal that both αvβ3 and α3β1 integrin bind the α4 G domain with high affinity. This finding perplexed us. If both are capable of direct interaction with ligand, one might assume that when one is functionally inhibited then the other should be able to compensate and mediate cell-ligand binding alone. However, this is not the case. The most obvious explanation for this finding is that integrin heterodimers show a complex functional interaction when endothelial cells adhere to the α4 laminin subunit G domain such that when the αvβ3 integrin is blocked by antibody, then there is a concomitant block in ligand binding of the α3β1 and α6β1 heterodimers. Likewise, blocking both α3β1 and α6β1 has a negative impact on the ability of αvβ3 integrin heterodimer to bind its ligand. This model of integrin cooperativity is shown diagrammatically (Fig. 5). Moreover, we have provided some experimental evidence for our model because endothelial adhesion to laminin-5, a ligand for the α3β1 integrin but not the αvβ3 integrin, is inhibited by antibody LM609 directed against the αvβ3 integrin heterodimer.
Fig 5.
Diagram showing a scheme in which there is crosstalk among integrins in endothelial cell adhesion to the G domain of the α4 laminin subunit. In the model, we show that both the αvβ3 and α3β1 integrins interact directly with the G domain. Endothelial cell adhesion to the G domain can be inhibited by G domain antibodies (2A3), by antibodies against the αvβ3 integrin, by antibodies against the β1 integrin, or by a combination of antibodies against the α3 and α6 integrin subunits. In the model, when αvβ3 integrin function is inhibited, this also perturbs the function of α3β1 and α6β1 integrin heterodimers (bracketed) and vice versa. In the diagram, integrin interplay is indicated by a double-headed arrow. Moreover, angiogenesis is blocked when integrin function is perturbed. In the absence of αvβ3 integrin, the α3β1 and α6β1 integrin heterodimers are capable of supporting angiogenesis but may do so in a relatively unregulated manner.
For a number of years, the αvβ3 integrin heterodimer has been believed to play an important role in angiogenesis (29, 31, 32). This notion has recently been questioned because a number of groups have shown that mice lacking either the αvβ3 integrin or both αvβ3 and αvβ5 integrins develop normally (33–35). Moreover, mice lacking both αvβ3 and αvβ5 integrins develop more extensive tumors with a rich vascular supply than their normal litter mate controls (33). This was a great surprise, particularly when one considers the large numbers of papers detailing inhibition of vascular development under conditions where the function of αvβ3 integrin is perturbed by specific antagonists (36–38). Our data seem to reconcile these contradictory results. We show this in model form (Fig. 5). In our model, the αvβ3, α3β1, and α6β1 integrins are “activated” by interaction with α4 laminin ligand. A downstream consequence of such interaction is angiogenesis. Furthermore, as we have discussed above, under normal conditions, αvβ3 integrin modulates the function of α3β1 and α6β1 integrins and vice versa. In the model, the antibody LM609 inhibits the function of the αvβ3 integrin and, in turn, indirectly perturbs activity of α3β1 and α6β1 integrins. In the complete absence of αvβ3 integrin, the α3β1 and α6β1 integrins trigger pathways that are necessary for angiogenesis. Moreover, one could also envisage that in the absence of αvβ3 integrin, the activity of the α3β1 and α6β1 integrins may even be enhanced, leading to the observed increase in tumorigenesis in knockout animals (33).
In summary, we have characterized an intricate crosstalk among the integrin heterodimers involved in endothelial cell–α4 laminin interaction. It is now our goal to assess whether function-blocking antibodies against the α4 laminin subunit, such as antibody 2A3, or peptides that compete with endogenous α4 subunit containing laminin heterotrimers for integrin binding, may have therapeutic use as angiogenesis inhibitors.
Acknowledgments
We thank Dr. Michael Pins for the tumor tissue used in our analyses. This work was supported by National Institutes of Health Grants RO1 HL67016 and PO1 DE12328 and American Heart Association Grants 0151136Z (to J.C.R.J.) and PO1 AR44012 (to G.S.H.). A.M.G. was supported by a Minority Postdoctoral Supplement to PO1 DE12328.
Abbreviations
TrHBMEC, immortalized human bone marrow endothelial cells
HUVEC, human umbilical vascular endothelial cells
References
- 1.Aumailley M. & Smyth, N. (1998) J. Anat. 193, 1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timpl R. & Brown, J. C. (1994) Matrix Biol. 14, 275-281. [DOI] [PubMed] [Google Scholar]
- 3.Tunggal P., Smyth, N., Paulsson, M. & Ott, M.-C. (2000) Microsc. Res. Tech. 51, 214-227. [DOI] [PubMed] [Google Scholar]
- 4.Smirnov S., McDearmon, E., Shaohua, L., Ervasti, J., Tryggvason, K. & Yurchenco, P. D. (2002) J. Biol. Chem. 277, 18928-18937. [DOI] [PubMed] [Google Scholar]
- 5.Patton B. L. (2000) Microsc. Res. Tech. 51, 247-261. [DOI] [PubMed] [Google Scholar]
- 6.Baker S. E., Hopkinson, S. B., Fitchmun, M., Andreason, G. L., Frasier, F., Plopper, G., Quaranta, V. & Jones, J. C. R. (1996) J. Cell Sci. 109, 2509-2520. [DOI] [PubMed] [Google Scholar]
- 7.McGowan K. & Marinkovich, M. P. (2000) Microsc. Res. Tech. 51, 262-279. [DOI] [PubMed] [Google Scholar]
- 8.Niimi T., Kumagai, C., Okano, M. & Kitagawa, Y. (1997) Matrix Biol. 16, 223-230. [DOI] [PubMed] [Google Scholar]
- 9.Richards A., Al-Imara, L. & Pope, F. M. (1996) Eur. J. Biochem. 238, 813-821. [DOI] [PubMed] [Google Scholar]
- 10.Frieser M., Nöckel, H., Pausch, F., Röder, C., Hahn, A., Deutzmann, R. & Sorokin, L. M. (1997) Eur. J Biochem. 246, 727-735. [DOI] [PubMed] [Google Scholar]
- 11.Talts J. F., Sasaki, T., Miosge, N., Gohring, W., Mann, K., Mayne, R. & Timpl, R. (2000) J. Biol. Chem. 275, 35192-35199. [DOI] [PubMed] [Google Scholar]
- 12.Iivanainen A., Sainio, H. & Tryggvason, K. (1995) FEBS Lett. 365, 183-188. [DOI] [PubMed] [Google Scholar]
- 13.Miner J. H., Patton, B. L., Lentz, S. I., Gilbert, D. J., Snider, W. D., Jenkins, N. A., Copeland, N. G. & Sanes, J. R. (1997) J. Cell Biol. 137, 685-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J. & Mayne, R. (1996) Matrix Biol. 15, 433-437. [DOI] [PubMed] [Google Scholar]
- 15.Iivanainen A., Kortesmaa, J., Sahlberg, C., Morita, T., Bergmann, U., Thesleff, I. & Tryggvason, K. (1997) J. Biol. Chem. 272, 27862-27868. [DOI] [PubMed] [Google Scholar]
- 16.Tokida Y., Aratani, Y., Morita, A. & Kitagawa, Y. (1990) J. Biol. Chem. 265, 18123-18129. [PubMed] [Google Scholar]
- 17.Fujiwara H., Kikkawa, Y., Sanzen, N. & Sekiguchi, K. (2001) J. Biol. Chem. 276, 17550-17558. [DOI] [PubMed] [Google Scholar]
- 18.Gonzales M., Weksler, B., Tsuruta, D., Golman, R. D., Yoon, K. J., Hopkinson, S. B., Flitney, F. W. & Jones, J. C. R. (2001) Mol. Biol. Cell 12, 85-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schweitzer K. M., Vicart, P., Delouis, C., Paulin, D., Drager, D., Langenhuijsen, M. M. & Weksler, B. (1997) Lab. Invest. 76, 25-36. [PubMed] [Google Scholar]
- 20.Baker S. E., DiPasquale, A., Stock, E. L., Plopper, G., Quaranta, V., Fitchmun, M. & Jones, J. C. R. (1996) Exp. Cell Res. 228, 262-270. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U. K. (1970) Nature 277, 680-685. [DOI] [PubMed] [Google Scholar]
- 22.Harlow E. & Lane, D., (1988) Antibodies: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 92–121.
- 23.Klatte D. H., Kurpakus, M. A., Grelling, K. A. & Jones, J. C. R. (1989) J. Cell Biol. 109, 3377-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J., Nagavarapu, U., Relloma, K., Sjaastad, M. D., Moss, W. C., Passaniti, A. & Herron, G. S. (2001) Nat. Biotech. 19, 219-224. [DOI] [PubMed] [Google Scholar]
- 25.Stromblad S. & Cheresh, D. A. (1996) Trends Cell Biol. 6, 462-468. [DOI] [PubMed] [Google Scholar]
- 26.Lyden D., Hattori, K., Dias, S., Costa, C., Blaikie, P., Butros, L., Chadburn, A., Heissig, B., Marks, W., Witte, L., et al. (2001) Nat. Med. 7, 1194-1201. [DOI] [PubMed] [Google Scholar]
- 27.Blystone S. D., Graham, I. L., Lindberg, F. P. & Brown, E. J. (1994) J. Cell Biol. 127, 1129-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodivala-Dilke K. M., DiPersio, C. M., Kreidberg, J. A. & Hynes, R. O. (1998) J. Cell Biol. 142, 1357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eliceiri B. P. & Cheresh, D. A. (1999) J. Clin. Invest. 103, 1227-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thyboll J., Kortesmaa, J., Cao, R., Soininen, R., Wang, L., Iivanainen, A., Sorokin, L., Rinsling, M., Cao, Y. & Tryggvason, K. (2002) Mol. Cell. Biol. 22, 1194-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks P. C., Clark, R. A. & Cheresh, D. A. (1994) Science 264, 569-571. [DOI] [PubMed] [Google Scholar]
- 32.Ruoslahti E. & Engvall, E. (1997) J. Clin. Invest. 100, S53-S56. [PubMed] [Google Scholar]
- 33.Reynolds L. E., Wyder, L., Lively, J. C., Taverna, D., Robinson, S. D., Huang, X., Sheppard, D., Hynes, R. O. & Hodivala-Dilke, K. M. (2002) Nat. Med. 8, 27-34. [DOI] [PubMed] [Google Scholar]
- 34.Bader B. L., Rayburn, H., Crowley, D. & Hynes, R. O. (1998) Cell 95, 507-519. [DOI] [PubMed] [Google Scholar]
- 35.Hodivala-Dilke K. M., McHugh, K. P., Tsakiris, D. A., Rayburn, H., Crowley, D., Ullman-Cullere, M., Ross, P. F., Coller, B. S., Teitelbaum, S. & Hynes, R. O. (1999) J. Clin. Invest. 103, 229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brooks P. C., Montgomery, A. M. P., Rosenfeld, M., Reisfeld, R. A., Hu, T., Klier, G. & Cheresh, D. A. (1994) Cell 79, 1157-1164. [DOI] [PubMed] [Google Scholar]
- 37.Drake C. J., Cheresh, D. A. & Little, C. D. (1995) J. Cell Sci. 108, 2655-2661. [DOI] [PubMed] [Google Scholar]
- 38.Brooks P. C., Stromblad, S., Klemke, R., Visscher, D., Sarkar, F. H. & Cheresh, D. A. (1995) J. Clin. Invest. 96, 1815-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]