Abstract
Bilirubin, an abundant pigment that causes jaundice, has long lacked any clear physiologic role. It arises from enzymatic reduction by biliverdin reductase of biliverdin, a product of heme oxygenase activity. Bilirubin is a potent antioxidant that we show can protect cells from a 10,000-fold excess of H2O2. We report that bilirubin is a major physiologic antioxidant cytoprotectant. Thus, cellular depletion of bilirubin by RNA interference markedly augments tissue levels of reactive oxygen species and causes apoptotic cell death. Depletion of glutathione, generally regarded as a physiologic antioxidant cytoprotectant, elicits lesser increases in reactive oxygen species and cell death. The potent physiologic antioxidant actions of bilirubin reflect an amplification cycle whereby bilirubin, acting as an antioxidant, is itself oxidized to biliverdin and then recycled by biliverdin reductase back to bilirubin. This redox cycle may constitute the principal physiologic function of bilirubin.
Bilirubin is a lipophilic linear tetrapyrrole, abundant in blood plasma, which occurs uniquely in mammals. It is the final product of heme catabolism, as heme oxygenase (HO) cleaves the heme ring to form the water-soluble biliverdin, which is reduced by biliverdin reductase (BVR) to bilirubin. Because bilirubin is toxic and insoluble, it must be glucuronidated before being excreted in the bile. Excessive elevations of bilirubin lead to substantial deposits in the brain with the resultant kernicterus causing major brain damage (1). Given that biliverdin is water-soluble and readily excreted, why should mammals have evolved the energetically expensive, potentially toxic, and apparently unnecessary capacity to reduce biliverdin? One hypothesis holds that bilirubin can cross the placenta and thus transfer from the fetal to maternal circulation more readily than biliverdin (2). However, the fetus expresses a distinct biliverdin reductase, BVRB, with different isomer specificity. Thus, the predominant bilirubin isomer in the early fetus is bilirubin IXβ, whereas the adult, which expresses BVRA, produces bilirubin IXα exclusively (3–5). Accordingly, the adult BVRA could not have evolved for fetal needs.
Stocker et al. (6, 7) noted that bilirubin possesses strong antioxidant potential against peroxyl radicals. At physiologic oxygen tension, bilirubin surpassed α-tocopherol as the most potent protector against lipid peroxidation. Moreover, bilirubin serum concentrations are high enough to provide a substantial portion of the total antioxidant capacity of serum (8, 9). However, the low concentration of bilirubin in tissues, ≈20–50 nM [<0.1% the levels of established antioxidants such as glutathione (GSH)], argues that bilirubin is not physiologically relevant to intracellular oxidative stress. Nonetheless, we obtained evidence that bilirubin is a physiologic antioxidant neuroprotectant (10). Thus, mice with genomic deletion of the HO2 locus are more susceptible to stroke damage and the excitotoxicity of seizures (11, 12). This puzzling effect could be explained by our observation that bilirubin very potently protects brain cultures from H2O2 neurotoxicity. As little as 10 nM bilirubin protects against almost 10,000-fold higher concentrations of H2O2, indicating that bilirubin cannot be acting simply as a stoichiometric antioxidant against H2O2. To account for this dramatic action, we hypothesize that bilirubin acts in a catalytic fashion whereby bilirubin oxidized to biliverdin is rapidly reduced back to bilirubin, a process that could readily afford 10,000-fold amplification (13). Here we establish that a redox cycle based on BVRA activity provides physiologic cytoprotection as BVRA depletion exacerbates the formation of reactive oxygen species (ROS) and augments cell death.
Methods
All chemicals were obtained from Sigma unless otherwise indicated.
Cell Culture and Viability Measurements.
HeLa cells (American Type Culture Collection) were cultured in DMEM with 10% FBS, penicillin and streptomycin, and glutamine. For viability measurements, cells were plated at 25,000 cells per well into a 96-well plate. After the indicated treatment, the media was replaced with fresh media containing 0.5 mg/ml methylthiazoletetrazolium (MTT). Then, the cells were cultured for 1 h before removal of the media and dissolution of the reduced dye in DMSO. The viability was determined by measuring the absorbance at 570 nm.
HPLC.
Aliquots of the reactions were analyzed by HPLC as described (14). Briefly, the reaction was run on a 4-μm C18 3.9 × 150 mm column (Waters) equilibrated in solvent A [0.1 M ammonium phosphate (pH 4.0) in 50% methanol]. A 15-min gradient was used to increase solvent B (100% methanol) from 0% to 100%.
In Vitro Reactions.
All reactions were assembled on ice in dim light. Stock solutions of bilirubin IXα and biliverdin IXα (Porphyrin Products, Logan, UT) and 2,2′-azobis(2-amidinopropane) hydrochloride (AAPH) were made fresh in the dark immediately before use. The reactions were started by addition of AAPH, mixed, and placed at 37°C in the spectrophotometer. In Fig. 1b, the final 1-ml reaction contained 10 μM bilirubin IXα, 50 mM AAPH, 0.5 mM albumin, 150 mM NaCl, and 50 mM phosphate buffer (pH 8.7). In Fig. 1d, the final 1-ml reaction contained 20 μM bilirubin, 50 mM AAPH, 0.5 mM albumin, 10 μM NADPH, 50 mM glucose-6-phosphate, 1 unit/ml glucose-6-phosphate dehydrogenase, 150 mM NaCl, and 50 mM phosphate buffer (pH 8.7). The reaction was monitored at 37°C in a spectrophotometer by measuring the absorbance at 453 and 650 nm.
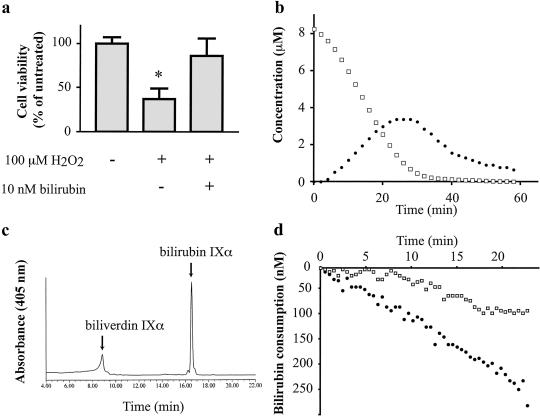
Fig 1.
Bilirubin is a suprastoichiometric cytoprotectant against oxidative stress (a) and BVRA can redox cycle the tetrapyrrole in vitro (b–d). (a) Viability of cultured HeLa cells was measured by MTT assay 8 h after the indicated treatment. (b) Bilirubin (Porphyrin Products) is oxidized to biliverdin by peroxyl radicals. The reaction was monitored by A453 for bilirubin (□) or A650 for biliverdin (•). (c) Bilirubin IXα is oxidized specifically to biliverdin IXα. An aliquot of the reaction shown in b was taken at 10 min and analyzed by HPLC as described (14). (d) BVRA regenerates bilirubin, slowing the consumption of bilirubin by ROS. The reaction was monitored at A453 with (□) or without (•) 1 μg/ml recombinant BVRA. *, P < 0.005, using an unpaired Student's t test.
RNA Interference.
RNA interference (RNAi) of the BVRA transcript was performed as described (15). Briefly, small interfering RNA duplexes were targeted to the human BVRA transcript (GAGGUGGAGGUCGCCUAUAUU). Mock RNAi was targeted to unrelated genes, whose sequences were not found in the human EST database (UACCCCAUGGCAUUGUCAUUU and UGUGGACGUGCCGCUGGUCUU). RNA oligonucleotides were synthesized by Dharmacon and transfected into HeLa cells by using Oligofectamine according to the manufacturer's instructions. For transfection of primary neuronal cultures, small interfering RNA duplexes targeted to the rat BVRA transcript (UACCCCAUGGCAUUGUCAUUU) were cotransfected with a red fluorescent protein (RFP) reporter plasmid. Rat neurons from embryonic day 18 were cultured as described and transfected after 3 days in vitro (16). Five micrograms of plasmid and 400 pmol of small interfering RNA duplex were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Under these conditions, the RNAi blocks the expression of cotransfected BVRA in a sequence-specific manner.
Production of Antibody and Western Blotting.
The appropriate human BVRA cDNA was amplified by PCR and ligated into pGEX vector containing a GST tag. Five milligrams of bacterially expressed BVRA protein was purified using a GSH-agarose column, and the tag was removed using a Thrombin Cleavage Capture Kit (Novagen). The antibody was raised in a chicken and epitope-purified by Affinity Bioreagents. Cells were lysed in PBS with 1% Triton, 2 mM EDTA, and a protease inhibitor mixture. Fifty micrograms of the lysate were run on a 4–12% polyacrylamide SDS gel and transferred to a nitrocellulose membrane. The chicken anti-hBVRA antibody was used at 1:2,000 dilution. The anti-poly(ADP-ribose) polymerase (PARP) antibody (Alexis) was used at a 1:1,000 dilution. The anti-HO-1 antibody was used at a 1:10,000 dilution.
Enzyme Assays.
Biliverdin reductase activity was assayed in 1-ml reactions containing 50 mM Tris (pH 8.7), 100 μM NADPH, 10 μM biliverdin IXα, and 50 μg of cell lysate. The reaction was started by addition of lysate, mixed, and placed in at 37°C in the spectrophotometer. The rate of reaction was determined by monitoring the change in absorbance at 453 nm over time. Caspase activity in cell lysates was measured using the EnzChek Caspase-3 Assay Kit #1 (Molecular Probes).
ROS Levels.
ROS levels were measured using a carboxy-dichlorodihydrofluorescein diacetate (H2DCF; Molecular Probes). H2DCF is a nonfluorescent compound that is loaded into cells as a cell-permeant ester. On cleavage by intracellular esterases, it can be oxidized to a fluorescent product by reactive oxygen species. For the HeLa cultures, cells were loaded with 5 μM H2DCF for 10 min at 37°C. The cultures were rinsed three times with PBS, trypsinized, and resuspended in Opti-Mem (Invitrogen). The rate of oxidation of the dye for 10,000 cells was measured in a spectrometer (excitation, 487 nm; emission, 510 nm) at 37°C. For neuronal cultures, neurons were grown on coverslips and transfected as described above. They were loaded with H2DCF as described above. After rinsing, they were imaged by fluorescent microscopy. Transfected neurons were identified using the RFP, and the average H2DCF fluorescence was quantitated with openlab imaging software (Improvision). Twenty-five individual neurons were identified in each condition.
Microarray and Northern Analysis.
Forty hours after RNAi transfection, total RNA was isolated using the RNeasy Mini kit (Qiagen) and submitted to the Johns Hopkins Microarray Core Facility. Two separate transfections of each condition were analyzed on the Affymetrix HG U95A chip. Northern blots were performed using 10 μg of total RNA. Probes were generated by amplifying ≈500-bp fragments of the respective gene by PCR.
Results
In HeLa cells, 10 nM bilirubin IXα completely reverses cell death elicited by a 10,000-fold excess (100 μM) concentration of H2O2 (Fig. 1a), resembling our earlier observations in brain cultures (10). To assess whether BVRA can augment antioxidant actions of bilirubin in a model system, we show that AAPH, a free radical initiator, degrades bilirubin with the concomitant generation of biliverdin, similar to observations of others (ref. 7; Fig. 1 b and c). For BVRA to recycle the biliverdin generated by ROS, the biliverdin isomer would have to be the IXα. We confirm that this is the case by HPLC. Finally, we reconstitute the entire cycle in vitro. When BVRA and a source of NADPH are included in the reaction, the rate of bilirubin consumption is greatly reduced (Fig. 1d).
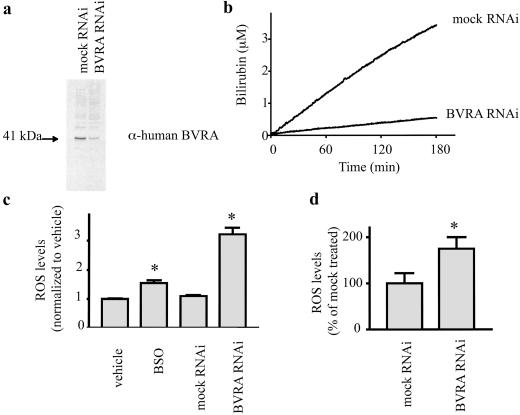
We used RNAi to deplete HeLa cells of BVRA, leading to 85–90% reduction in BVRA protein and catalytic activity (Fig. 2 a and b). If BVRA is a physiologic antioxidant, its depletion should influence ROS levels. Indeed, HeLa cells deficient in BVRA manifest a tripling of ROS levels (Fig. 2c), measured by H2DCF, which is oxidized by ROS to a fluorescent product. We wanted to compare BVRA's antioxidant role to that of a well accepted intracellular antioxidant system. GSH is considered a major, and possibly the principal, soluble intracellular antioxidant (17). The rate-limiting step in GSH synthesis, γ-glutamyl cysteine synthase, is inhibited by buthionine sulfoxime (BSO). BSO treatment of HeLa cells depletes GSH levels >95% (data not shown) and leads to a 50% increase in ROS concentration, substantially less than the augmentation produced by BVRA depletion (Fig. 2c).
Fig 2.
Depletion of BVRA with RNAi leads to elevated ROS levels in mammalian cells. (a) Expression of the BVRA protein is decreased following transfection with RNAi directed to the BVRA transcript. (b) Biliverdin reductase activity is decreased following treatment with RNAi directed to the BVRA transcript. (c) Intracellular ROS levels are higher in HeLa cells deficient in BVRA. ROS levels were measured using H2DCF. (d) Intracellular ROS levels are higher in neurons transfected with RNAi directed to the BVRA transcript. *, P < 0.005 using an unpaired Student's t test.
To ascertain whether the antioxidant actions of endogenous BVRA are of general importance for multiple cell types, we examined primary cultures of cerebral cortical neurons transfected with RNAi for BVRA and monitored ROS levels in individual neurons (Fig. 2d). BVRA depletion leads to a 70% augmentation of ROS levels.
We investigated the gene expression profile of BVRA-deficient cells by using an oligonucleotide microarray of 12,599 genes. Two principal classes of genes are up-regulated in BVRA-deficient cells (Fig. 3a). First, several antioxidant genes are induced, including NADPH:quinone oxidoreductase, heme oxygenase-1, metallothioneins, nrf2, and thioredoxin reductase. Each of these genes is known to be induced by oxidative stress and to be involved in cytoprotection. The BVRA RNAi also increases the expression of genes involved in heme metabolism: coproporphyrinogen oxidase, ferrochelatase, and heme oxygenase-1. Thus, cells respond to insufficient BVRA by increasing the synthesis of its substrate, biliverdin. We have confirmed the microarray results by Northern analysis, comparing the induction in BVRA-deficient cells to that of GSH-deficient cells (Fig. 3b).
Fig 3.
(a) Microarray analysis of BVRA-deficient cells indicates increased expression of antioxidant genes. Heme synthesis as well as degradation is up-regulated. Of the 12,599 genes represented, 4,368 genes were detected. Of these genes, 645 were significantly increased in the BVRA-deficient condition and 550 were significantly decreased. Many antioxidant genes were increased in the cells deficient in BVRA. The average fold increase in the normalized signal is indicated. (b) To confirm the microarray data, Northern blot analysis was performed for some of the genes, comparing the induction to that of cells depleted of GSH by BSO treatment. HO1 expression was analyzed by Western blot.
To assess whether the antioxidant actions of endogenous BVRA physiologically protect cells, we monitored the viability of HeLa cells 50 h after transfection with RNAi for BVRA or mock RNAi species (Fig. 4a). When exposed to supraphysiologic concentrations of oxygen, HeLa cells compensate, displaying normal viability and healthy growth in culture. Depletion of BVRA reduces cell viability by >50%. To ascertain whether the decrease in cell viability merely reflects lesser cell growth or augmented cell death, we monitored apoptotic cell death by assays of caspase activity and cleavage of PARP (Fig. 4 b and c). BVRA depletion leads to a doubling of caspase activity and a marked augmentation in PARP cleavage, establishing that BVRA physiologically protects against apoptotic cell death.
Fig 4.
Depletion of BVRA leads to apoptosis and increased sensitivity to oxidative stress. (a) Cells depleted of BVRA are less viable 50 h after transfection. (b) Caspase activity is elevated in cells treated with BVRA RNAi (○) compared with two distinct mock RNAis (▾ and ▴). (c) Cell death is apoptotic, as indicated by poly(ADP-ribose) polymerase (PARP) cleavage, 50 h after transfection. (d) Cells deficient in BVRA (○) are more sensitive to an oxidative challenge than mock RNAi-treated cells (▾ and ▴). Thirty hours after transfection, the cells were treated with H2O2 and cell survival was measured. (e) In contrast, GSH depletion with BSO (○) treatment leads to only a small increase in sensitivity, significant only at 200 μM H2O2. *, P < 0.005 using an unpaired Student's t test.
To ascertain whether BVRA physiologically protects against substantial additional oxidative stress, we exposed HeLa cells to H2O2 (Fig. 4 d and e). BVRA depletion markedly increases the sensitivity of the cells to H2O2-elicited cell death. By contrast, GSH depletion provides a slight augmentation of cell death evident only at a single concentration of H2O2 (200 μM).
Discussion
Our main finding is that BVRA physiologically regenerates bilirubin in a catalytic cycle, providing antioxidant cytoprotection (Fig. 5). The amplification afforded by this cycle can readily explain the ability of low nanomolar concentrations of bilirubin to overcome 10,000-fold higher concentrations of oxidants.
Fig 5.
A model for cytoprotection conferred by BVR. HO control the total level of biliverdin/bilirubin. BVRA keeps the linear tetrapyrrole in the reduced form, where it associates with membranes and quenches the propagation of ROS. This confers protection to the membrane components of the cell in much the same way as soluble GSH and GSH reductase (GR) protect the cytoplasmic components. M, methyl; V, vinyl; P, propionate; ER, endoplasmic reticulum; GSH, GSH-reduced; GSSG, GSH-oxidized.
Cells use multiple systems to protect against ROS. For instance, concentrations of free heavy metals, which can catalyze the formation of free radicals, are tightly regulated by chelators such as ferritin and transferrin. Enzymes with antioxidant actions include catalase and superoxide dismutase that together convert superoxide to water. Small molecules, such as ascorbate and α-tocopherol, act as direct antioxidants, quenching the propagation of free radicals. GSH occurs at millimolar concentrations in most tissues and is generally regarded as the principal endogenous intracellular small molecule antioxidant cytoprotectant. Our evaluations of cells depleted of GSH or BVRA indicate that BVRA is of comparable, or greater, importance to GSH in physiologic cytoprotection.
Several parallels can be drawn between the bilirubin redox cycle and the cycling of GSH, another chain-breaking antioxidant (Fig. 5). Obviously, both involve a single chemical that exists in two states: an oxidized form and a reduced form. In both cases, the reduced form predominates under healthy conditions. Both are part of a concerted stress response, including heat shock proteins, that is induced when cells are challenged with a wide range of toxic insults. When either system is disrupted, oxidative stress increases and cells die.
There are also some striking differences between the two systems. GSH concentrations are millimolar, whereas bilirubin levels are well below 100 nM. However, the abundance of BVR activity in all tissues indicates that bilirubin cycles at a much higher rate than GSH.
Patterns of enzymatic regulation also differentiate the two systems. Glutathione recycling involves a peroxidase and a reductase, as well as distinct enzymes for synthesizing the antioxidant. In contrast, biliverdin is oxidized directly to bilirubin without the evident need of a peroxidase, although the existence of such a peroxidase cannot be altogether discounted.
Conceivably, bilirubin and GSH provide physiologic antioxidant activity for distinct types of intracellular molecules. Bilirubin, which is highly lipophilic, is associated intimately with cell membranes, where it might prevent lipid peroxidation and protect membrane proteins. On the other hand, GSH is highly water-soluble and might be expected to protect cytoplasmic constituents.
The limited previous studies that have evaluated antioxidant actions of bilirubin either have been restricted to in vitro experiments measuring the antioxidant potential of bilirubin, or examined protection conferred by exogenous bilirubin (18–22). Our studies are the first to establish a physiologic antioxidant and cytoprotectant role for endogenous bilirubin in mammalian cells. We propose that the cytoprotective role of the BVR cycle is the principal function for the mammalian bilirubin generating system. Why is bilirubin formation restricted to mammals? If its physiologic role relates to cytoprotection, presumably other species have evolved different mechanisms that warrant investigation.
Our findings may have clinical relevance (Table 1). Oxidative stress plays a critical role in the development of vascular disease. Several epidemiological studies have found that bilirubin levels are inversely associated with coronary artery disease and mortality from myocardial infarction. Gilbert's Syndrome is a genetic disorder of bilirubin conjugation leading to a mild unconjugated hyperbilirubinemia (23). The incidence of ischemic heart disease in middle-aged individuals with Gilbert's Syndrome is reduced >5-fold compared with the general population (24). In the general population, high plasma bilirubin levels are correlated with a reduced risk of coronary heart disease (25–27). Premature babies treated with supplemental oxygen suffer retinopathy of prematurity because of increased oxidative stress. In these patients, a higher serum bilirubin level is associated with diminished incidence of retinal damage (28). A 10-year retrospective study of the Belgian population found serum bilirubin levels inversely related to cancer mortality (29).
Table 1.
Clinical protective effects of bilirubin
| Condition | Link to bilirubin | Ref. |
|---|---|---|
| Coronary artery disease (CAD) | Coronary stenosis in pilots correlates with low bilirubin | 25 |
| Framington Offspring study, a prospective cohort study of 5,124 subjects, finds total bilirubin inversely related to cardiovascular disease in men | 27 | |
| In families with high incidence of CAD, low bilirubin levels are an independent risk factor for CAD | 26 | |
| Patients with Gilbert's syndrome, with defective bilirubin conjugation results in a mild asymptomatic hyperbilirubinemia, have a decreased incidence of ischemic heart disease | 24 | |
| Cancer | In a 10-year prospective cohort study of the Belgian population, cancer mortality is inversely associated with bilirubin concentrations | 29 |
| Retinopathy of prematurity | Incidence of retinopathy of prematurity is inversely correlated with serum bilirubin levels | 28 |
Acknowledgments
We thank K. Whitford and A. Ghosh for advice and assistance with neuronal cultures, and F. Murillo for microarray analysis. This work was supported by U.S. Public Health Service Grant DA-00266, Research Scientist Award DA-00074 (to S.H.S.), and National Research Service Award DA05900 (to D.E.B.).
Abbreviations
BVR, biliverdin reductase
GSH, glutathione
H2DCF, carboxy-dichlorodihydrofluorescein diacetate
HO, heme oxygenase
RNAi, RNA interference
ROS, reactive oxygen species
See commentary on page 15837.
References
- 1.Orth J. (1875) Virchows Arch. Pathol. Anat. 63, 447-462. [Google Scholar]
- 2.Schmid R. (1976) Trans. Assoc. Am. Physicians 89, 64-76. [PubMed] [Google Scholar]
- 3.Blumenthal S. G., Stucker, T., Rasmussen, R. D., Ikeda, R. M., Ruebner, B. H., Bergstrom, D. E. & Hanson, F. W. (1980) Biochem. J. 186, 693-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi T. & Nakajima, H. (1995) Eur. J. Biochem. 233, 467-472. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi T., Komoda, Y. & Nakajima, H. (1994) J. Biol. Chem. 269, 24343-24348. [PubMed] [Google Scholar]
- 6.Stocker R., Yamamoto, Y., McDonagh, A. F., Glazer, A. N. & Ames, B. N. (1987) Science 235, 1043-1046. [DOI] [PubMed] [Google Scholar]
- 7.Stocker R., Glazer, A. N. & Ames, B. N. (1987) Proc. Natl. Acad. Sci. USA 84, 5918-5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gopinathan V., Miller, N. J., Milner, A. D. & Rice-Evans, C. A. (1994) FEBS Lett. 349, 197-200. [DOI] [PubMed] [Google Scholar]
- 9.Belanger S., Lavoie, J. C. & Chessex, P. (1997) Biol. Neonate 71, 233-238. [DOI] [PubMed] [Google Scholar]
- 10.Doré S., Takahashi, M., Ferris, C. D., Zakhary, R., Hester, L. D., Guastella, D. & Snyder, S. H. (1999) Proc. Natl. Acad. Sci. USA 96, 2445-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doré S., Sampei, K., Goto, S., Alkayed, N. J., Guastella, D., Blackshaw, S., Gallagher, M., Traystman, R. J., Hurn, P. D., Koehler, R. C. & Snyder, S. H. (1999) Mol. Med. 5, 656-663. [PMC free article] [PubMed] [Google Scholar]
- 12.Doré S., Goto, S., Sampei, K., Blackshaw, S., Hester, L. D., Ingi, T., Sawa, A., Traystman, R. J., Koehler, R. C. & Snyder, S. H. (2000) Neuroscience 99, 587-592. [DOI] [PubMed] [Google Scholar]
- 13.Barañano D. E. & Snyder, S. H. (2001) Proc. Natl. Acad. Sci. USA 98, 10996-11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonkovsky H. L., Wood, S. G., Howell, S. K., Sinclair, P. R., Lincoln, B., Healey, J. F. & Sinclair, J. F. (1986) Anal. Biochem. 155, 56-64. [DOI] [PubMed] [Google Scholar]
- 15.Elbashir S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K. & Tuschl, T. (2001) Nature 411, 494-498. [DOI] [PubMed] [Google Scholar]
- 16.Threadgill R., Bobb, K. & Ghosh, A. (1997) Neuron 19, 625-634. [DOI] [PubMed] [Google Scholar]
- 17.Meister A. (1994) J. Biol. Chem. 269, 9397-9400. [PubMed] [Google Scholar]
- 18.Stocker R. & Peterhans, E. (1989) Biochim. Biophys. Acta 1002, 238-244. [DOI] [PubMed] [Google Scholar]
- 19.Neuzil J. & Stocker, R. (1994) J. Biol. Chem. 269, 16712-16719. [PubMed] [Google Scholar]
- 20.Minetti M., Mallozzi, C., Di Stasi, A. M. & Pietraforte, D. (1998) Arch. Biochem. Biophys. 352, 165-174. [DOI] [PubMed] [Google Scholar]
- 21.Clark J. E., Foresti, R., Sarathchandra, P., Kaur, H., Green, C. J. & Motterlini, R. (2000) Am. J. Physiol. 278, H643-H651. [DOI] [PubMed] [Google Scholar]
- 22.Foresti R., Goatly, H., Green, C. J. & Motterlini, R. (2001) Am. J. Physiol. 281, H1976-H1984. [DOI] [PubMed] [Google Scholar]
- 23.Scriver C. R., (1995) The Metabolic and Molecular Bases of Inherited Disease (McGraw–Hill, New York).
- 24.Vitek L., Jirsa, M., Brodanova, M., Kalab, M., Marecek, Z., Danzig, V., Novotny, L. & Kotal, P. (2002) Atherosclerosis 160, 449-456. [DOI] [PubMed] [Google Scholar]
- 25.Schwertner H. A., Jackson, W. G. & Tolan, G. (1994) Clin. Chem. 40, 18-23. [PubMed] [Google Scholar]
- 26.Hopkins P. N., Wu, L. L., Hunt, S. C., James, B. C., Vincent, G. M. & Williams, R. R. (1996) Arterioscler. Thromb. Vasc. Biol. 16, 250-255. [DOI] [PubMed] [Google Scholar]
- 27.Djousse L., Levy, D., Cupples, L. A., Evans, J. C., D'Agostino, R. B. & Ellison, R. C. (2001) Am. J. Cardiol. 87, 1196-200.; A 4, 7. [DOI] [PubMed] [Google Scholar]
- 28.Heyman E., Ohlsson, A. & Girschek, P. (1989) N. Engl. J. Med. 320, 256. [DOI] [PubMed] [Google Scholar]
- 29.Temme E. H., Zhang, J., Schouten, E. G. & Kesteloot, H. (2001) Cancer Causes Control 12, 887-894. [DOI] [PubMed] [Google Scholar]