Abstract
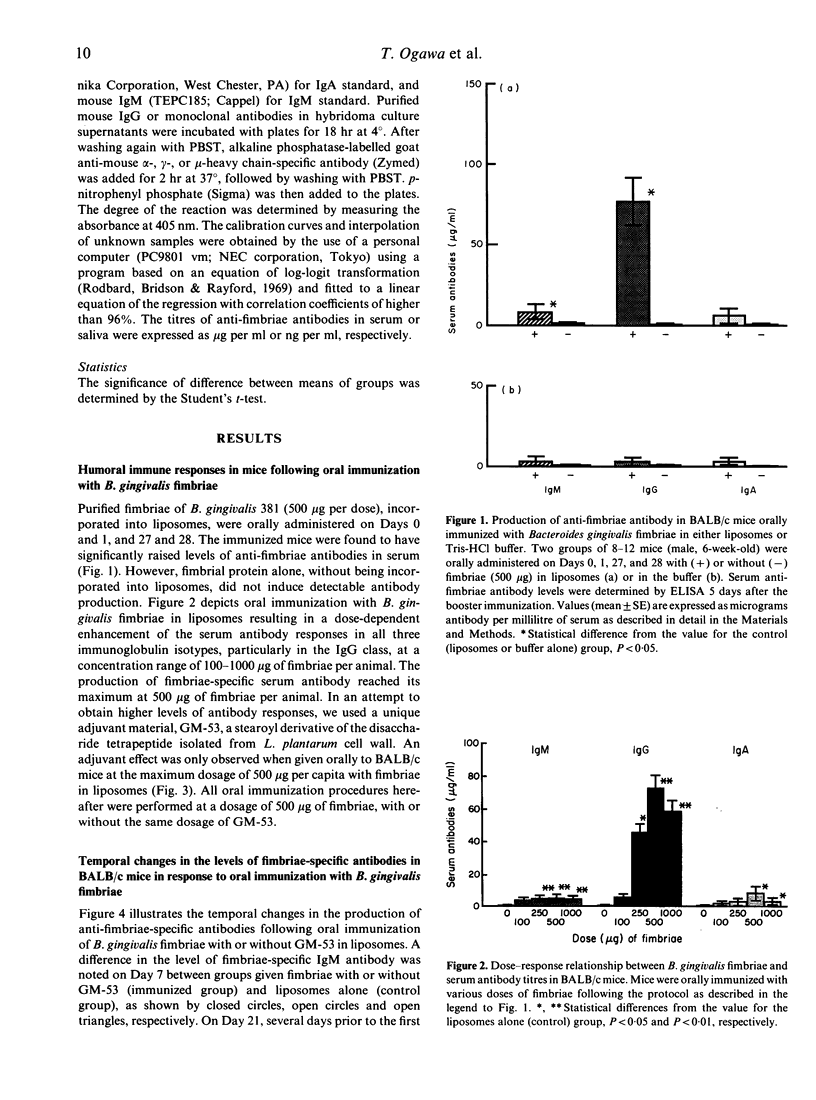
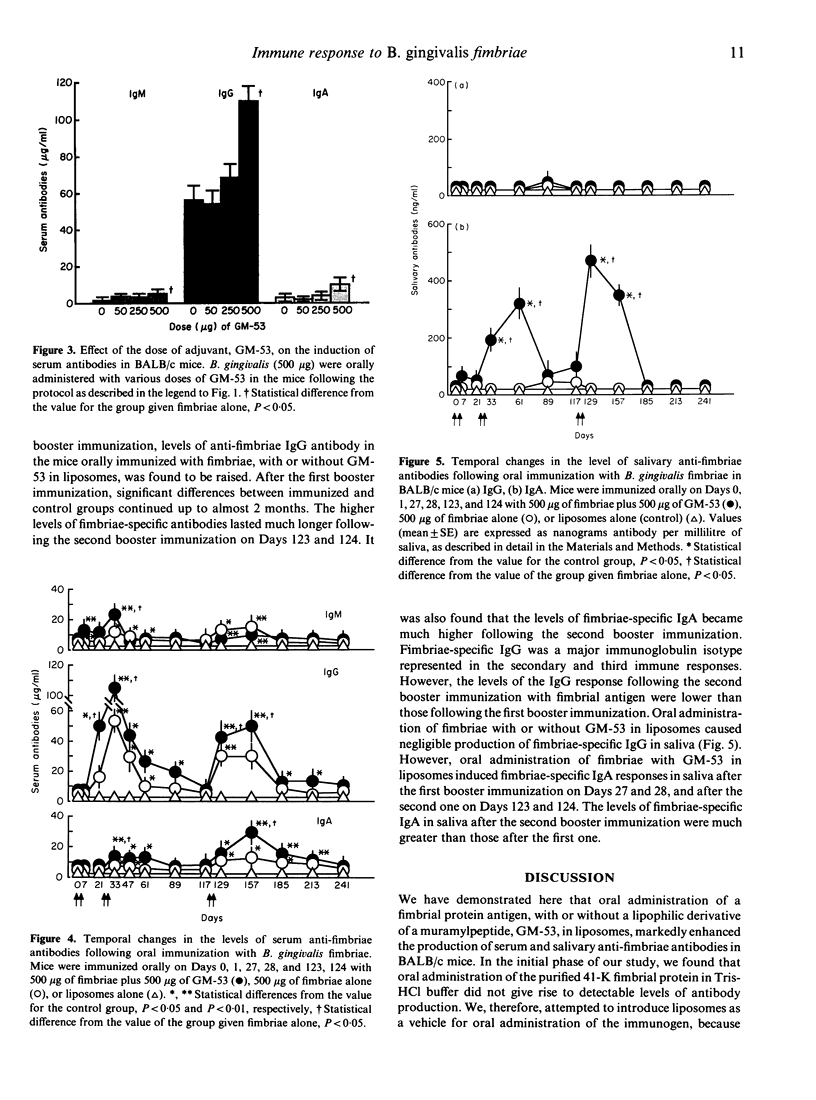
Bacteroides gingivalis fimbrial antigen incorporated into liposomes, but not in Tris-HCl buffer, significantly raised the levels of anti-fimbriae antibodies in serum, particularly of the IgG class, after oral primary and booster immunizations in BALB/c mice. An approximately linear relationship was observed between the dose of fimbrial antigen and the level of fimbriae-specific antibodies produced; antibody production reached its maximum at an immunization dosage of 500 micrograms of fimbriae per mouse. Fimbriae-specific antibody production was enhanced by use of a semi-synthetic adjuvant, a stearoyl derivative of sodium beta-N-acetylglucosaminyl-(1----4)-N-acetylmuramyl-L-alanyl-D-isoglutaminyl-(L) - stearoyl-(D)-meso-diamino-pimelic acid-(D)-amide-D-alanine (GM)-53) in liposomes. High anti-fimbriae antibody levels in serum and saliva were maintained for several months in the mice that had received two orally administered boosters of fimbrial antigen with GM-53 in liposomes. Salivary anti-fimbriae antibody levels, particularly of the IgA class, were markedly raised.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Hasty D. L., Simpson W. A., Beachey E. H. Antiadhesive properties of a quaternary structure-specific hybridoma antibody against type 1 fimbriae of Escherichia coli. J Exp Med. 1983 Oct 1;158(4):1114–1128. doi: 10.1084/jem.158.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. G., Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974 Nov 15;252(5480):252–252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- Chen S. S., Keenan R. M. Effect of phosphatidylcholine liposomes on the mitogen-stimulated lymphocyte activation. Biochem Biophys Res Commun. 1977 Dec 7;79(3):852–858. doi: 10.1016/0006-291x(77)91189-5. [DOI] [PubMed] [Google Scholar]
- Gmür R., Hrodek K., Saxer U. P., Guggenheim B. Double-blind analysis of the relation between adult periodontitis and systemic host response to suspected periodontal pathogens. Infect Immun. 1986 Jun;52(3):768–776. doi: 10.1128/iai.52.3.768-776.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerina N. G., Kessler T. W., Guerina V. J., Neutra M. R., Clegg H. W., Langermann S., Scannapieco F. A., Goldmann D. A. The role of pili and capsule in the pathogenesis of neonatal infection with Escherichia coli K1. J Infect Dis. 1983 Sep;148(3):395–405. doi: 10.1093/infdis/148.3.395. [DOI] [PubMed] [Google Scholar]
- Heath T. D., Edwards D. C., Ryman B. E. The adjuvant properties of liposomes. Biochem Soc Trans. 1976;4(1):129–133. doi: 10.1042/bst0040129. [DOI] [PubMed] [Google Scholar]
- Inoue K. Permeability properties of liposomes prepared from dipalmitoyllecithin, dimyristoyllecithin, egg lecithin, rat liver lecithin and beef brain sphingomyelin. Biochim Biophys Acta. 1974 Mar 29;339(3):390–402. doi: 10.1016/0005-2736(74)90166-7. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E., Fusco P. C., Brinton C. C., Moon H. W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978 Aug;21(2):392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayappa H. G., Goodnow R. A., Geary S. J. Role of Escherichia coli type 1 pilus in colonization of porcine ileum and its protective nature as a vaccine antigen in controlling colibacillosis. Infect Immun. 1985 May;48(2):350–354. doi: 10.1128/iai.48.2.350-354.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Kern S. E., Bauer D. H., Scott P. J., Porter P. Direct demonstration in intestinal secretions of an IgA memory response to orally administered Shigella flexneri antigens. J Immunol. 1982 Jan;128(1):475–479. [PubMed] [Google Scholar]
- Kramers M. T., Patrick J., Bottomley J. M., Quinn P. J., Chapman D. Studies of liposome interactions with rat thymocytes. Eur J Biochem. 1980 Sep;110(2):579–585. doi: 10.1111/j.1432-1033.1980.tb04901.x. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T. Enhancement and suppression of the Peyer's patch immune response by systemic priming. Clin Exp Immunol. 1982 Aug;49(2):441–448. [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T. Immunosuppression caused by antigen feeding. I. Evidence for the activation of a feedback suppressor pathway in the spleens of antigen-fed mice. Eur J Immunol. 1982 Sep;12(9):767–773. doi: 10.1002/eji.1830120912. [DOI] [PubMed] [Google Scholar]
- Mattingly J. A., Waksman B. H. Immunologic suppression after oral administration of antigen. I. Specific suppressor cells formed in rat Peyer's patches after oral administration of sheep erythrocytes and their systemic migration. J Immunol. 1978 Nov;121(5):1878–1883. [PubMed] [Google Scholar]
- McChesney D., Tramont E. C., Boslego J. W., Ciak J., Sadoff J., Brinton C. C. Genital antibody response to a parenteral gonococcal pilus vaccine. Infect Immun. 1982 Jun;36(3):1006–1012. doi: 10.1128/iai.36.3.1006-1012.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Mouton C., Hammond P. G., Slots J., Genco R. J. Serum antibodies to oral Bacteroides asaccharolyticus (Bacteroides gingivalis): relationship to age and periondontal disease. Infect Immun. 1981 Jan;31(1):182–192. doi: 10.1128/iai.31.1.182-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Ogawa T., Kotani S., Shimauchi H. Enhancement of serum antibody production in mice by oral administration of lipophilic derivatives of muramylpeptides and bacterial lipopolysaccharides with bovine serum albumin. Methods Find Exp Clin Pharmacol. 1986 Jan;8(1):19–26. [PubMed] [Google Scholar]
- Ogawa T., McGhee M. L., Moldoveanu Z., Hamada S., Mestecky J., McGhee J. R., Kiyono H. Bacteroides-specific IgG and IgA subclass antibody-secreting cells isolated from chronically inflamed gingival tissues. Clin Exp Immunol. 1989 Apr;76(1):103–110. [PMC free article] [PubMed] [Google Scholar]
- Richman L. K., Graeff A. S., Yarchoan R., Strober W. Simultaneous induction of antigen-specific IgA helper T cells and IgG suppressor T cells in the murine Peyer's patch after protein feeding. J Immunol. 1981 Jun;126(6):2079–2083. [PubMed] [Google Scholar]
- Rodbard D., Bridson W., Rayford P. L. Rapid calculation of radioimmunoassay results. J Lab Clin Med. 1969 Nov;74(5):770–781. [PubMed] [Google Scholar]
- Silverblatt F. J., Cohen L. S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979 Jul;64(1):333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Tew J. G., Marshall D. R., Burmeister J. A., Ranney R. R. Relationship between gingival crevicular fluid and serum antibody titers in young adults with generalized and localized periodontitis. Infect Immun. 1985 Sep;49(3):487–493. doi: 10.1128/iai.49.3.487-493.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R., Silverblatt F. J. Antibacterial mechanisms of antibody to mannose-sensitive pili of Escherichia coli. J Infect Dis. 1983 May;147(5):882–889. doi: 10.1093/infdis/147.5.882. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Liposomes in immunology: further evidence for the adjuvant activity of liposomes. Immunol Commun. 1978;7(6):635–644. doi: 10.3109/08820137809068724. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Liposomes in immunology: impairment of the adjuvant effect of liposomes by incorporation of the adjuvant lysolecithin and the role of macrophages. Immunol Commun. 1979;8(4):381–396. doi: 10.3109/08820137909050052. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Liposomes in immunology: the immune response against antigen-containing liposomes. Immunol Commun. 1977;6(5):489–498. doi: 10.3109/08820137709094148. [DOI] [PubMed] [Google Scholar]


