Abstract
The use of embryonic stem cells for cell-replacement therapy in diseases like diabetes mellitus requires methods to control the development of multipotent cells. We report that treatment of mouse embryonic stem cells with inhibitors of phosphoinositide 3-kinase, an essential intracellular signaling regulator, produced cells that resembled pancreatic β cells in several ways. These cells aggregated in structures similar, but not identical, to pancreatic islets of Langerhans, produced insulin at levels far greater than previously reported, and displayed glucose-dependent insulin release in vitro. Transplantation of these cell aggregates increased circulating insulin levels, reduced weight loss, improved glycemic control, and completely rescued survival in mice with diabetes mellitus. Graft removal resulted in rapid relapse and death. Graft analysis revealed that transplanted insulin-producing cells remained differentiated, enlarged, and did not form detectable tumors. These results provide evidence that embryonic stem cells can serve as the source of insulin-producing replacement tissue in an experimental model of diabetes mellitus. Strategies for producing cells that can replace islet functions described here can be adapted for similar uses with human cells.
Advances in cell-replacement therapy for type 1 diabetes mellitus (1) and a shortage of transplantable islets of Langerhans have focused interest in developing renewable sources of insulin-producing cells appropriate for engraftment. Recent studies suggest that mouse embryonic stem (ES) cells can be manipulated to express and secrete insulin (2–4). Insulin-producing grafts derived from ES cells in these initial reports, however, had a high degree of cellular heterogeneity and proliferation, uncharacterized growth and tumor-forming potential, and low insulin levels compared with pancreatic islets. Here we describe experimental strategies for developing insulin-producing cell clusters (IPCCs) from mouse ES cells that increase circulating insulin levels, improve glucose homeostasis, and rescue survival in an experimental model of diabetes mellitus.
Epithelial cells comprising the endocrine pancreas are derived from endoderm, but display many properties of neurons (reviewed in ref. 5). For example, pancreatic islet cells and most neurons are characteristically postmitotic; however, little is known about the molecular steps governing cell cycle arrest in either cell type (6). In vitro conditions (7, 8) that promote neuronal differentiation of ES cells, including exposure to a mixture of neurotrophic factors (B27 supplement) and nicotinamide, were used to produce IPCCs mainly comprised of proliferating neuronal-like cells intermixed with a smaller fraction of insulin+ cells (2). In vitro treatment of human fetal pancreas with nicotinamide and LY294002, an inhibitor of phosphoinositide 3-kinase (PI3K), increased total endocrine cell number and insulin content while reducing DNA synthesis (9). Treatment of neuroendocrine cell lines with wortmannin, another PI3K inhibitor, prevented neurite outgrowth (10). Thus, we hypothesized that substitution of PI3K inhibitors like LY294002 for B27 during development of insulin+ cells from ES cells would produce IPCCs with greater similarities to pancreatic islets, including a predominant insulin+ cell component, a minor neuronal component, and reduced cellular proliferation.
Materials and Methods
Cell Lines and Culture Conditions.
The JM1 and ROSA mouse ES cell lines are described in refs. 11 and 12. These distinct lines gave comparable results, but the JM1 line consistently produced greater levels of insulin than the ROSA line.
Undifferentiated mouse ES cells (stage 1) were cultured on a feeder layer of irradiated mouse embryonic fibroblasts with medium containing knockout-DMEM, penicillin-streptomycin, 100 μM β-mercaptoethanol, 2 mM l-glutamine, 100 mM nonessential amino acids (GIBCO/BRL), 15% FCS (HyClone), and 1,000 units/ml leukemia inhibitory factor (Chemicon). Medium was changed daily for up to 4 days, and then cells were harvested and placed in fresh gelatin-coated culture plates (Fisher). After 2 days cells were placed in Costar ultra low cluster plates (Corning) and cultured in medium without leukemia inhibitory factor (stage 2). Resultant embryoid bodies were transferred to culture dishes and allowed to adhere, then cultured for 6 days (stage 3) in ITSFn serum-free medium (7). Cells were transferred to plates coated with poly(l-ornithine) (Sigma) and fibronectin (GIBCO/BRL) and cultured for 6 days (stage 4) in N2 medium (8) supplemented with 10 ng/ml bFGF (R & D Systems) and B27 supplement (GIBCO/BRL). During stage 5NB, IPCCs were cultured in N2 medium supplemented with B27 and 10 mM nicotinamide (Sigma). Stage 5NL IPCCs were cultured in N2 medium supplemented with 10 mM nicotinamide and 10 μM LY294002 (Calbiochem) for at least 6 days. Over a range of concentrations we found that 10 μM LY294002 was optimal for inhibiting cell growth and neurite outgrowth in stage 5NL (J.D.C. and S.K.K., unpublished results). Media were changed every other day during stages 4 and 5. A detailed protocol is available on request.
Islet Isolation and in Vitro Studies.
We isolated pancreatic islets by intraductal collagenase perfusion using standard methods. We measured total protein content in sonicated handpicked IPCCs by using standard assays (Bio-Rad). We measured total insulin content from islets and IPCCs, and serum insulin levels from IPCC engrafted mice by using an insulin ELISA kit (ALPCO, Windham, NH). Pancreatic glucagon and insulin, and serum glucagon and insulin were measured by radioimmunoassay (Linco Research Immunoassay, St. Charles, MO). Results from these and all other assays, unless otherwise noted, are reported as average value ± standard error of the mean. We serially measured in vitro insulin release from isolated islets and IPCCs by static batch incubation in 25 mM glucose, as described (13). Buffer overlying groups of 10 handpicked IPCCs was removed at specific times (0–60 min) after addition of glucose, and insulin content measured by ELISA.
Immunohistochemistry and Molecular Biology.
Cells were fixed in 4% paraformaldehyde, embedded in Matrigel (Becton Dickinson), and then embedded in paraffin. We performed immunohistochemistry on 6-μm tissue sections prepared by microtomy (Leica) using standard methods. We used primary antibodies at the following dilutions: guinea pig anti-insulin 1:200 (Linco Research Immunoassay), mouse anti-glucagon 1:500 (Sigma), mouse anti-α-fetoprotein 1:500 (Sigma), mouse anti-MAP2 1:500 (Sigma), mouse anti-β tubIII 1:500 (Sigma), mouse anti-GLUT2 1:200 (ADI, San Antonio, TX), mouse anti-glucokinase 1:200 (C. Newgard, University of Texas Southwestern, Dallas), rabbit anti-C-peptide 1:500 and mouse anti-proinsulin 1:500 (O. Madsen, Hagedorn, Denmark), and rabbit anti-Pdx1 1:500 (C. Wright, Nashville, TN). Confocal immunofluorescence microscopy with an optical slice thickness of 0.6 μm was performed on a Bio-Rad MRC1000. Dithizone staining of IPCCs and isolated pancreatic islets was performed as described (14).
Total RNA was prepared by using a RNeasy kit (Qiagen, Valencia, CA) and RQ1 RNase-free DNase (Promega). For cDNA synthesis, oligo(dT) primers (Invitrogen, Carlsbad, CA) were used to prime reverse transcription reactions and synthesis was carried out by Thermoscript RT (Invitrogen). PCR was performed by using Taq polymerase (Applied Biosystems, Foster City, CA), and an Opti-Prime Optimization kit (Stratagene). In addition to β-actin, GAPDH expression (not shown) was used to normalize input template cDNA to analyze relative gene expression. Primer sequences for insulin, glucagon, and β-actin were from ref. 2. Other primer sequences are available on request.
IPCC Transplantation and Physiologic Tests.
All animal studies were performed in accordance with Stanford University Animal Care and Use Guidelines. Experimental diabetes was induced 7 days before transplant in 9- to 10-week-old male NOD scid mice (The Jackson Laboratory) by i.p. injection of 175 mg/kg streptozotocin (STZ; Sigma). Blood glucose was measured with a glucometer (Bayer, Elkhart, IN), and statistical significance was calculated as described (15). Three weeks after IPCC transplantation, glucose tolerance testing was performed with 1 g of glucose per kg of body weight as described (15). Under general anesthesia, mice were engrafted with 300 handpicked IPCCs or received a sham transplant of saline solution in the left subcapsular renal space. Stage 5NL IPCC diameter was ≈300–400 μm, two to three times larger than pancreatic islets, and transplantation of more IPCCs in a single renal graft site was not feasible. Grafts were removed after 3 weeks by unilateral nephrectomy for analysis. Survival distributions were determined by using the standard product limit method (16). By 3 weeks, we detected tumors in four of four mice transplanted with handpicked stage 5NB IPCCs, and by 6 weeks, all four mice had died. Tumors following transplantation of stage 5NL IPCCs were not observed 8 weeks after engraftment, the maximum period of observation (n = 3).
Results
Morphology, Cell Composition, and Gene Expression of Insulin-Producing Cell Clusters.
To assess IPCC developmental changes resulting from specific modifications of culture conditions, we examined cellular morphology and expression of gene products that govern or define pancreatic cell fates. We exposed undifferentiated ES cells (stage 1) to a sequence of conditions (see Materials and Methods) that culminated in treatment with nicotinamide and LY294002 (stage 5NL). As a control, other ES cells were exposed to a similar sequence of conditions culminating in treatment with nicotinamide and B27 supplement (stage 5NB) as described (2). Stage 5NL IPCCs were smaller (Fig. 1 A–C) and had more contacts between insulin+ cells than stage 5NB IPCCs (Fig. 1 E and F). By morphometry, we found that 95–97% of cells comprising IPCCs express insulin, and 2–3% express glucagon. We did not detect expression of pancreatic polypeptide or somatostatin by immunohistochemistry in stage 5NL IPCCs (data not shown). Thus, the cell composition of IPCCs was similar but not identical to pancreatic islets. Compared with pancreatic β cells, insulin+ stage 5NL cells were smaller and had a reduced cytoplasmic volume (Fig. 1 D and E; see below), consistent with the known ability of LY294002 to reduce cell size (17).
Fig 1.
Similarities of gene expression and mitotic status in pancreatic β cells and insulin-producing cells comprising stage 5NL IPCCs. (A–C) Dithizone staining of isolated pancreatic islets (A), stage 5NL IPCCs (B), and stage 5NB IPCCs (C). Magnification in A–C is equal. Images in D–R were obtained by confocal microscopy and are representative of at least 12 samples for each immunohistochemical probe. Samples were processed identically and in parallel for each immunohistochemical probe. Shown is simultaneous immunofluorescent detection of insulin and glucagon (D–F), α-fetoprotein (α-FP) (G–I), MAP2 (J–L), or β tubIII (M–O). In mature islets, there is localized expression of insulin and glucagon (D) but no detectable expression of α-FP (G) or the neuronal markers MAP2 (J) and β tubIII (M). Stage 5NL IPCCs cellular homogeneity (B, E, H, K, and N) contrasts with cellular heterogeneity of stage 5NB IPCCs (F, I, L, and O). (P–R) Immunofluorescent detection of Ki67, a nuclear marker of proliferating cells. Stage 5NL IPCCs (Q) are predominantly nonproliferating, similar to mature pancreatic islets (P), whereas stage 5NB culture conditions result in significant numbers of proliferating cells (R). Magnification in D–R is equal.
In stage 5NL insulin+ cells and pancreatic β cells, co-expression of glucagon, and other markers like α-fetoprotein, a marker of visceral and definitive endoderm, or microtubule-associated protein 2 (MAP2) and β-tubulin III (β tubIII), neuronal-specific markers, was rare or not detected (Fig. 1 D, E, G, H, J, K, M, and N). Insulin+ cells in stage 5NL IPCCs expressed numerous pancreatic β cell markers, including C-peptide, proinsulin, the transcription factor PDX1, type 2 glucose transporter (GLUT2), and glucokinase (Fig. 2 A–J). The localization of insulin, C-peptide, and other cytoplasmic proteins was difficult to visualize by immunofluorescence in stage 5NL IPCCs because of marked reduction of cytoplasmic volume; however, additional analysis revealed cytoplasmic localization of these markers (see below). Stage 5NL IPCCs had reduced levels of the proinsulin processing enzyme, prohormone convertase 1/3 (data not shown), and accumulated greater levels of proinsulin than pancreatic islets (Fig. 2 C and D). RT-PCR analysis showed that isolated IPCCs expressed Islet-1, nestin, neurogenin-3 (Fig. 2K), and IAPP (data not shown) markers also expressed in isolated pancreatic islets. Immunohistochemical detection of Ki67, a nuclear marker of proliferating cells, indicated that the majority of cells in stage 5NL IPCCs were not proliferating, similar to cells in pancreatic islets (Fig. 1 P and Q). Our analyses (Fig. 2K and data not shown) also revealed the presence of stage 5NL cells lacking insulin that produced carboxypeptidase A, a pancreatic exocrine gene product not detected in IPCCs described in a previous study (2).
Fig 2.
Stage 5NL IPCCs express several characteristic pancreatic β cell markers. Images in A–J were obtained by confocal microscopy and are representative of at least 12 samples for each immunohistochemical probe. Samples were processed identically and in parallel for each immunohistochemical probe. Immunofluorescent staining of adult pancreatic islets and stage 5NL IPCCs for insulin and C-peptide (A and B), insulin and proinsulin (C and D), Pdx1 (E and F), GLUT2 (G and H), and glucokinase (I and J). (K) RT-PCR analysis of IPCC gene expression during stages 1–4, and in handpicked stage 5NB and stage 5NL IPCCs, and wild-type pancreatic islets. Islet-1 and neurogenin-3 are transcription factors required for islet formation in mice (29, 30) and nestin is an intermediary filament protein (2). In stage 5 IPCCs, we detected carboxypeptidase A but not pancreatic amylase, both products of pancreatic exocrine (acinar) cells. Amylase transcripts are routinely detected by RT-PCR in pancreatic islet preparations because of adherent acinar cells. Other gene products are described in the text. (L) insulin release by IPCCs on exposure to 25 mM glucose. Results show insulin release by 10 IPCCs per time point.
In contrast to these results with stage 5NL IPCCs, a significant proportion of insulin+ cells in stage 5NB IPCCs coexpressed glucagon or α-fetoprotein (Fig. 1 F and I), and stage 5NB IPCCs were comprised mainly of MAP2- or β tubIII-expressing cells (Fig. 1 L and O). Ki67 staining revealed that ≈25% of cells in stage 5NB IPCCs were proliferating (Fig. 1R). Thus, insulin+ cells produced by our methods displayed multiple molecular features of pancreatic β cells and comprised IPCCs with some architectural similarities to pancreatic islets.
Insulin Content and in Vitro Glucose-Stimulated Insulin Secretion.
Direct comparison revealed that intracellular insulin content (5.0 ± 1 ng per IPCC) in stage 5NL IPCCs was 10% of levels in isolated pancreatic islets (52 ± 6 ng per islet). Normalized to protein content, the insulin level in stage 5NL IPCCs was 11,300 ± 1,000 ng/mg protein, ≈30-fold greater than the insulin level in IPCCs produced by treatment of ES cells with nicotinamide and B27 (390 ± 50 ng/mg protein). To our knowledge, higher levels of insulin expression during IPCC differentiation from mouse or human ES cells have not been reported. Consistent with these results, all stage 5NL IPCCs were uniformly stained by dithizone, a chromogen (14) used to detect stored intracellular insulin in pancreatic β cells, whereas stage 5NB cells were not stained by dithizone (Fig. 1 A–C). We obtained similar results with a combination of wortmannin, a different PI3K inhibitor, and nicotinamide, but not with nicotinamide alone (data not shown). In contrast, simultaneous exposure to a combination of nicotinamide, LY294002, and B27 supplement (stage 5NBL) produced IPCCs containing only 620 ± 80 ng insulin/mg protein. Thus, the greatest increase of insulin content was achieved by addition of nicotinamide and LY294002, and simultaneous removal of B27 supplement.
We measured glucose-dependent insulin release by IPCCs in vitro (Fig. 2L). Stage 5NL IPCCs released insulin in response to a step-increase of glucose to 25 mM with the rapid kinetics characteristic of primary pancreatic islets, consistent with results from a previous study (2). In comparison, stage 5NB IPCCs released significantly lower levels of insulin in response to glucose stimulation (Fig. 2L). In this assay, stage 5NL IPCCs released insulin (0.65 ± 0.1 ng/IPCC) at 13% of levels secreted by isolated pancreatic islets (5.0 ± 0.1 ng per islet). Furthermore, the fraction of total intracellular insulin released by stage 5NL IPCCs (13 ± 0.5%) and primary pancreatic islets (11 ± 0.6%) was similar. These results show that insulin+ cells comprising stage 5NL IPCCs release insulin in response to physiologically relevant glucose concentrations in vitro.
IPCCs Remain Differentiated and Grow After Engraftment.
To determine the feasibility of transplanting ES cell-derived IPCCs to treat diabetes mellitus, we performed a series of IPCC grafting experiments in immunocompromised recipient mice. Undifferentiated or partially differentiated ES cell grafts often form tumors, particularly teratomas (18). In contrast, transplantation of pancreatic islets does not produce these lethal tumors (1). Stage 5NB and stage 5NL IPCC fates were monitored after engraftment in the left subcapsular renal space of NOD scid mice. Within 3 weeks after transplantation of stage 5NB IPCCs, tumors formed (Fig. 3A) with features characteristic of teratomas (data not shown). In contrast, stage 5NL grafts maintained high levels of insulin and C-peptide expression in clustered cells that did not coexpress glucagon, and did not form tumors for 8 weeks after IPCC transplantation (Fig. 3 B–D). Engrafted insulin+ cells were larger than stage 5NL insulin+ cells before engraftment, and were similar in size to pancreatic islet cells (Fig. 3 E–G). Exposure to PI3K inhibitors can reduce cell size (Fig. 3E, ref. 17), suggesting that engraftment of stage 5NL IPCCs in an environment lacking LY294002 permitted cell enlargement. Thus, engrafted insulin+ cells from stage 5NL survived, grew larger, produced insulin, and remained differentiated.
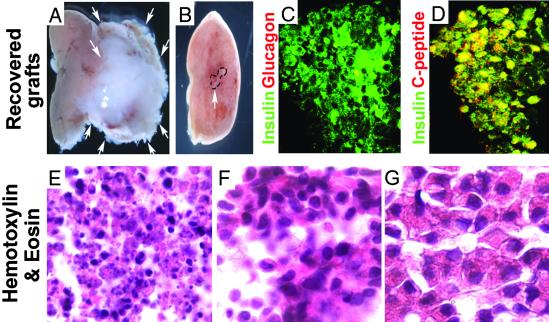
Fig 3.
IPCC graft analysis. (A) Teratoma (white arrows) formation 3 weeks after transplantation of 300 stage 5NB IPCCs under the left kidney capsule (pink tissue to left of tumor). (B) Lack of tumor growth after renal transplantation of 300 stage 5NL IPCCs (graft indicated by dashed lines and white arrow). (C) Immunohistochemical detection of insulin (green) and glucagon (red) expression in stage 5NL IPCCs 3 weeks after engraftment. Cell nuclei appear as black central spots surrounded by insulin, indicating cytoplasmic immunostaining. (D) Immunohistochemical detection of insulin (green) and C-peptide (red) expression in stage 5NL IPCCs 3 weeks after engraftment. (E–G) Hematoxylin and eosin staining of sectioned stage 5NL IPCC before transplantation (E) and sectioned stage 5NL IPCC grafts recovered from subcapsular renal transplantation site (F), compared with a sectioned adult pancreatic islet (G). Magnification in E–G is equal.
Complete Rescue of Survival After IPCC Transplantation in a Lethal Model of Diabetes Mellitus.
To investigate stage 5NL IPCC function in vivo, IPCCs were transplanted into NOD scid mice with STZ-induced diabetes mellitus (19). Seven days after STZ injection, NOD scid mice were engrafted with 300 IPCCs, the maximum dosage achieved (see Materials and Methods). Our in vitro measurements showed that glucose-stimulated insulin release by one IPCC was ≈13% of levels released by a pancreatic islet; from this data we estimated that transplantation of 300 IPCCs was similar, with respect to insulin replacement, to transplantation of ≈40 pancreatic islets. Thus, to control our IPCC transplantation studies, we engrafted STZ-treated NOD scid mice with 40 pancreatic islets, or performed a sham transplantation. In mice with STZ-induced diabetes, the most striking benefit of engraftment with stage 5NL IPCCs was complete rescue of survival (Fig. 4A). Survival of STZ-treated diabetic mice was similarly rescued by transplantation of 40 pancreatic islets (Fig. 4A). Endogenous pancreatic insulin content in IPCC-transplanted mice remained severely reduced at the completion of these transplantation experiments (Table 1), indicating that recovery of host pancreatic insulin secretion (20) did not account for the observed phenotypic rescue. In support of this conclusion, we observed that removal of the kidney containing the IPCC graft 21 days after transplantation resulted in rapid onset of severe hyperglycemia and death, whereas removal of the sham-transplanted kidney in surviving control mice had no detectable effects (Fig. 4 A and B). Together, these results show that improved outcomes after IPCC transplantation in mice with fatally severe diabetes mellitus resulted from the IPCC graft.
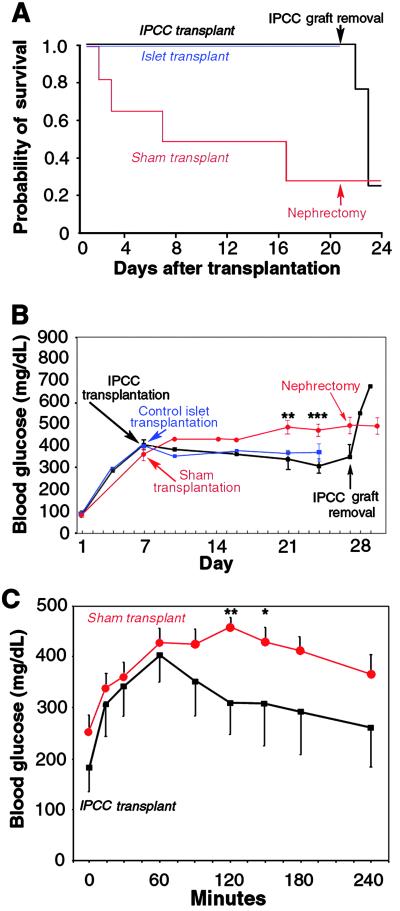
Fig 4.
Transplanted stage 5NL IPCCs function in vivo to rescue and ameliorate diabetic phenotypes. (A) Kaplan–Meier survival distribution after stage 5NL IPCC transplantation (n = 7, black lines), pancreatic islet transplantation (n = 3, blue lines), or sham transplant (n = 17, red lines). IPCC graft removal 3 weeks after engraftment resulted in increased mortality, in contrast to unilateral nephrectomy in surviving control sham-transplanted mice. (B) Random-fed blood glucose levels in STZ-treated mice with transplantation of stage 5NL IPCCs (n = 7, black lines), of 40 pancreatic islets (n = 3, blue lines), or in surviving sham-transplanted mice (n = 8, red lines). Comparing sham-transplanted mice to IPCC-transplanted mice: **, P < 0.01; ***, P < 0.005. In sham-transplanted mice that did not survive, average blood glucose on the day preceding death was 532 ± 33 mg/dl (n = 4). IPCC graft removal 3 weeks after transplantation resulted in comparable hyperglycemia (674 ± 117 mg/dl, n = 4). (C) i.p. glucose challenge in fasted mice 21 days after transplant with IPCCs (n = 7; black squares) or sham-transplant (n = 14; red circles). Blood glucose level was 135 ± 12 mg/dl in untreated control mice (n = 6) during random-feeding, and 85 ± 10 mg/dl (n = 6) after 15 h overnight fasting. **, P < 0.01; *, P < 0.05.
Table 1.
IPCC grafts increase circulating insulin levels in mice with STZ-induced diabetes mellitus
|
|
Blood | Pancreas | ||
|---|---|---|---|---|
| Insulin, ng/ml | Glucagon, pg/ml | Insulin, ng/g tissue | Glucagon, ng/g tissue | |
| Untreated | 1.65 ± 0.3 | 75 ± 9 | 97,800 ± 10,400 | 595 ± 71 |
| Sham transplant | 0.05 ± 0.01 | 107 ± 14 | 600 ± 145 | 700 ± 80 |
| IPCC transplant | 0.28 ± 0.06 | 151 ± 26 | 466 ± 66 | 520 ± 37 |
Insulin and glucagon in blood and pancreas from untreated control mice (n = 6), and in STZ-treated diabetic mice 3 weeks after receiving a sham transplant (n = 8) or IPCC transplant (n = 7).
Improved Glucose Regulation and Circulating Insulin Levels After IPCC Transplantation.
Twenty-one days after sham transplantation, surviving STZ-treated control mice had hyperglycemia (468 ± 37 mg/dl), a 17% average reduction in weight, and reduced serum insulin levels (0.05 ± 0.01 ng/ml; Table 1). The level of hyperglycemia (Fig. 4B) in surviving control mice underestimated the effects of STZ, because most STZ-treated mice had expired by this time. In STZ-treated NOD scid mice engrafted with stage 5NL IPCCs, hyperglycemia (305 ± 35 mg/dl) and weight loss (6%; P < 0.05) were attenuated, and serum insulin levels were increased ≈6-fold (0.28 ± 0.06 ng/ml; Table 1) compared with sham-transplanted controls. Thus, IPCC engraftment resulted in circulating in vivo insulin levels during random-feeding that were 17% of those in untreated control mice. Therefore, the persistence of mild hyperglycemia in random-fed mice after IPCC engraftment was consistent with the degree of partially restored circulating insulin levels. This improvement of random-fed glucose and circulating insulin levels observed after IPCC transplantation was similar to that achieved after transplantation of 40 pancreatic islets in STZ-treated mice (Fig. 4B). In these islet-transplanted mice, circulating insulin levels were 0.24 ± 0.16 ng/ml, comparable to levels achieved in IPCC-transplanted mice (Table 1). IPCC-engrafted mice also achieved moderately improved glucose regulation compared with sham-transplanted control mice in glucose tolerance tests after overnight fasting (Fig. 4C). Our data support the conclusion that transplantation of stage 5NL IPCCs in this diabetes model increased levels of circulating insulin, leading to better glucose regulation and maintenance of body mass. Further improvements in glycemic control by IPCC transplantation may be feasible by increasing the mass, insulin expression, or insulin secretion of transplanted IPCC grafts.
Discussion
It has been reported that ES cells can differentiate into heterogeneous multicellular structures containing insulin-producing cells (2, 3) but in vivo benefits in experimental models of diabetes mellitus were not demonstrated. A gene trap strategy (4) was used to isolate an insulin-producing cell line from ES cells, but in transplantation experiments with this cell line, graft removal to establish function was not performed. We report here that simple manipulations of ES cell culture conditions produce homogeneous clusters of insulin-secreting cells with important similarities to islets. Protein products essential for insulin production, glucose sensing, or insulin secretion in pancreatic β-cells like PDX1, GLUT2, and glucokinase, were expressed by insulin+ cells comprising IPCCs. To our knowledge, coexpression of these proteins by insulin+ cells derived from ES cells has not been previously demonstrated. Our studies have not yet determined whether production of insulin+ cells from ES cells recapitulates all of the steps thought to regulate pancreatic β-cell development (5, 21–23). However, the extent of shared IPCC and islet phenotypes raises the likelihood that the system for deriving IPCCs described here may prove useful for identifying conditions that promote development and function of pancreatic islet progenitor cells.
Our data show that, in addition to their similarities, stage 5NL IPCCs and islets are distinct in numerous ways. For example, stage 5NL IPCCs appeared to lack immunostainable pancreatic polypeptide- or somatostatin-producing cells, and the proportion of glucagon-producing cells in IPCCs was lower than the average proportion of glucagon-producing α-cells found in islets. Total intracellular insulin content in IPCCs was lower than average levels measured in pancreatic islets. We also detected more proinsulin accumulation in IPCCs than in pancreatic β-cells, suggesting that processing of precursor forms of mature insulin was less efficient in IPCCs than in pancreatic islets. Further studies to identify the molecular basis of phenotypic differences between IPCCs and pancreatic islets may elucidate the means to produce IPCCs that are functionally indistinguishable from islets.
Advances described here allowed ES cell-derived IPCCs to rescue and ameliorate disease phenotypes for the first time, to our knowledge, in an experimental model of diabetes. The modest levels of insulin replacement achieved by IPCC transplantation fully rescued survival in the mouse model of STZ-induced diabetes mellitus used here, consistent with observations by us and others that insulin replacement at moderate levels can completely rescue survival of otherwise lethal experimental diabetes without producing normoglycemia (24). Normoglycemia was achieved within 2–3 weeks after renal engraftment with 300 pancreatic islets in mice with STZ-induced diabetes (24). Our data showed that stage 5NL IPCCs produce insulin at 13% of levels found in pancreatic islets; thus, we estimate that engraftment of ≈2,500 stage 5NL IPCCs might restore normoglycemia to mice with STZ-induced diabetes. However, it is not yet possible to transplant this number of stage 5NL IPCCs in the mouse subcapsular renal space. We speculate that increased insulin production and secretion by IPCCs should be achievable, and may promote normoglycemia in this STZ diabetes model, or in other transplantation models. Further studies are also needed to assess the long-term fates of IPCC grafts.
β-cells in pancreatic islets are characteristically postmitotic, but much remains to be learned about the molecular basis of cell-cycle arrest during pancreatic β-cell maturation (6). PI3K inhibition is known to inhibit cell proliferation, and we hypothesized that reagents like LY294002 might enhance acquisition of β-cell phenotypes in our protocol by promoting a postmitotic state. Although additional studies are needed to show whether LY294002 and wortmannin promote IPCC maturation through a specific PI3K-dependent signaling pathway, our findings suggest that these or other purified reagents may be used to produce insulin-secreting cells from other multipotent cell lines, including human stem cell lines.
After engraftment, insulin+ cell fate appeared to be maintained, whereas cell size clearly increased. Thus, our methods may specify ES cells to a stable endocrine-like fate. Increased cell size is a well-established consequence of PI3K activation, and we speculate that the absence of LY294002 in IPCC grafts may lead to increased PI3K activity and subsequent cell growth. To the extent that cell growth indicated responsiveness to PI3K activation, these observations suggest that stage 5NL IPCCs may respond to PI3K-activating signals like insulin and IGF-1, protein ligands that are produced by islets and known to promote pancreatic islet functions (reviewed in ref. 25). Thus, IPCCs and pancreatic islets may be competent to respond to similar types of growth and differentiation cues.
Endocrine and exocrine cells are likely derived from a common precursor in the embryonic pancreas (26–28), and detection of both cell types associated with IPCCs is consistent with the possibility that a similar precursor cell may be produced and isolated during IPCC development in vitro. Expression of liver and pancreatic acinar cell markers during IPCC differentiation from ES cells suggests that our methods may also be adapted to model the differentiation of pancreatic exocrine cells, hepatocytes, and other endodermal derivatives, in addition to insulin-secreting cells. Growth in our understanding of ES cell development and the mechanisms governing differentiation of pancreatic progenitor cells may provide the basis for producing transplantable tissues that can completely replace pancreatic islet functions.
Acknowledgments
We thank Dr. R. Pedersen (University of Cambridge, Cambridge, U.K.) for the gift of JM1 and ROSA ES cells, J. Meneses and Dr. M. Firpo (University of California, San Francisco) for advice on growth and maintenance of these lines, Dr. S. Okabe (Tokyo Medical and Dental University, Tokyo) for assistance with IPCC differentiation, and I. Weissman, J. Shizuru, J. Baker, Å. Apelqvist, N. Smart, E. Harmon, and E. Rulifson for critical reading of earlier versions of the manuscript. Y.H. was supported by a Walter and Idun Berry fellowship and the Juvenile Diabetes Research Foundation, I.C.R. was supported by a Berry fellowship and a Katherine McCormick fellowship, J.J.H. and J.D.C. were supported as students in the Stanford Medical Scientist Training Program, and S.K.K. was supported by grants from the Pew Charitable Trusts and the Juvenile Diabetes Research Foundation.
Abbreviations
ES, embryonic stem
IPCC, insulin-producing cell clusters
PI3K, phosphoinositide 3-kinase
STZ, streptozotocin
MAP2, microtubule-associated protein 2
β tubIII, β-tubulin III
GLUT2, type 2 glucose transporter
References
- 1.Shapiro A. M., Lakey, J. R., Ryan, E. A., Korbutt, G. S., Toth, E., Warnock, G. L., Kneteman, N. M. & Rajotte, R. V. (2000) N. Engl. J. Med. 343, 230-238. [DOI] [PubMed] [Google Scholar]
- 2.Lumelsky N., Blondel, O., Laeng, P., Velasco, I., Ravin, R. & McKay, R. (2001) Science 292, 1389-1394. [DOI] [PubMed] [Google Scholar]
- 3.Shiroi A., Yoshikawa, M., Yokota, H., Fukui, H., Ishizaka, S., Tatsumi, K. & Takahashi, Y. (2002) Stem Cells 20, 284-292. [DOI] [PubMed] [Google Scholar]
- 4.Soria B., Roche, E., Berna, G., Leon-Quinto, T., Reig, J. A. & Martin, F. (2000) Diabetes 49, 157-162. [DOI] [PubMed] [Google Scholar]
- 5.Kim S. K. & Hebrok, M. (2001) Genes Dev. 15, 111-127. [DOI] [PubMed] [Google Scholar]
- 6.Rane S. G., Dubus, P., Mettus, R. V., Galbreath, E. J., Boden, G., Reddy, E. P. & Barbacid, M. (1999) Nat. Genet. 22, 44-52. [DOI] [PubMed] [Google Scholar]
- 7.Okabe S., Forsberg-Nilsson, K., Spiro, A. C., Segal, M. & McKay, R. D. (1996) Mech. Dev. 59, 89-102. [DOI] [PubMed] [Google Scholar]
- 8.Hazel T. & Müller, T. (1997) in Current Protocols in Neuroscience, eds. Crawley, J., Gerfen, C., Rogawski, M., Sibley, D., Skolnick, P., Wray, S. & McKay, R. (Wiley, New York), pp. 3.1.1–3.1.6.
- 9.Ptasznik A., Beattie, G. M., Mally, M. I., Cirulli, V., Lopez, A. & Hayek, A. (1997) J. Cell Biol. 137, 1127-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura K., Hattori, S., Kabuyama, Y., Shizawa, Y., Takayanagi, J., Nakamura, S., Toki, S., Matsuda, Y., Onodera, K. & Fukui, Y. (1994) J. Biol. Chem. 269, 18961-18967. [PubMed] [Google Scholar]
- 11.Qiu M., Bulfone, A., Martinez, S., Meneses, J. J., Shimamura, K., Pedersen, R. A. & Rubenstein, J. L. (1995) Genes Dev. 9, 2523-2538. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich G. & Soriano, P. (1991) Genes Dev. 5, 1513-1523. [DOI] [PubMed] [Google Scholar]
- 13.Zawalich W. S., Zawalich, K. C., Tesz, G. J., Sterpka, J. A. & Philbrick, W. M. (2001) Am. J. Physiol. Endocrinol. Metab. 280, E720-E728. [DOI] [PubMed] [Google Scholar]
- 14.Jindal R. M. (1995) Pancreas 11, 316-318. [DOI] [PubMed] [Google Scholar]
- 15.Kim S. K., Selleri, L., Lee, J. S., Zhang, A. Y., Gu, X., Jacobs, Y. & Cleary, M. L. (2002) Nat. Genet. 25, 430-435. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan E. L. & Meier, P. (1958) J. Am. Stat. Assoc. 53, 457-481. [Google Scholar]
- 17.Vanhaesebroeck B., Leevers, S. J., Ahmadi, K., Timms, J., Katso, R., Driscoll, P. C., Woscholski, R., Parker, P. J. & Waterfield, M. D. (2001) Annu. Rev. Biochem. 70, 535-602. [DOI] [PubMed] [Google Scholar]
- 18.Thomson J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S. & Jones, J. M. (1998) Science 282, 1145-1147. [DOI] [PubMed] [Google Scholar]
- 19.Eizirik D. L., Pipeleers, D. G., Ling, Z., Welsh, N., Hellerstrom, C. & Andersson, A. (1994) Proc. Natl. Acad. Sci. USA 91, 9253-9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guz Y., Nasir, I. & Teitelman, G. (2001) Endocrinology 142, 4956-4968. [DOI] [PubMed] [Google Scholar]
- 21.Sander M. & German, M. S. (1997) J. Mol. Med. 75, 327-340. [DOI] [PubMed] [Google Scholar]
- 22.Edlund H. (1998) Diabetes 47, 1817-1823. [DOI] [PubMed] [Google Scholar]
- 23.Kim S. & MacDonald, R. (2002) Curr. Opin. Genet. Dev. 12, 540-547. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman D. B., Platt, J. L., Rabe, F. L., Dunn, D. L., Bach, F. H. & Sutherland, D. E. (1990) J. Exp. Med. 172, 291-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saltiel A. R. & Kahn, C. R. (2001) Nature 414, 799-806. [DOI] [PubMed] [Google Scholar]
- 26.Herrera P. L. (2000) Development (Cambridge, U.K.) 127, 2317-2322. [DOI] [PubMed] [Google Scholar]
- 27.Gu G., Dubauskaite, J. & Melton, D. A. (2002) Development (Cambridge, U.K.) 129, 2447-2457. [DOI] [PubMed] [Google Scholar]
- 28.Kawaguchi Y., Cooper, B., Gannon, M., Ray, M., MacDonald, R. J. & Wright, C. V. (2002) Nat. Genet. 32, 128-134. [DOI] [PubMed] [Google Scholar]
- 29.Ahlgren U., Pfaff, S. L., Jessell, T. M., Edlund, T. & Edlund, H. (1997) Nature 385, 257-260. [DOI] [PubMed] [Google Scholar]
- 30.Gradwohl G., Dierich, A., LeMeur, M. & Guillemot, F. (2000) Proc. Natl. Acad. Sci. USA 97, 1607-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]