Abstract
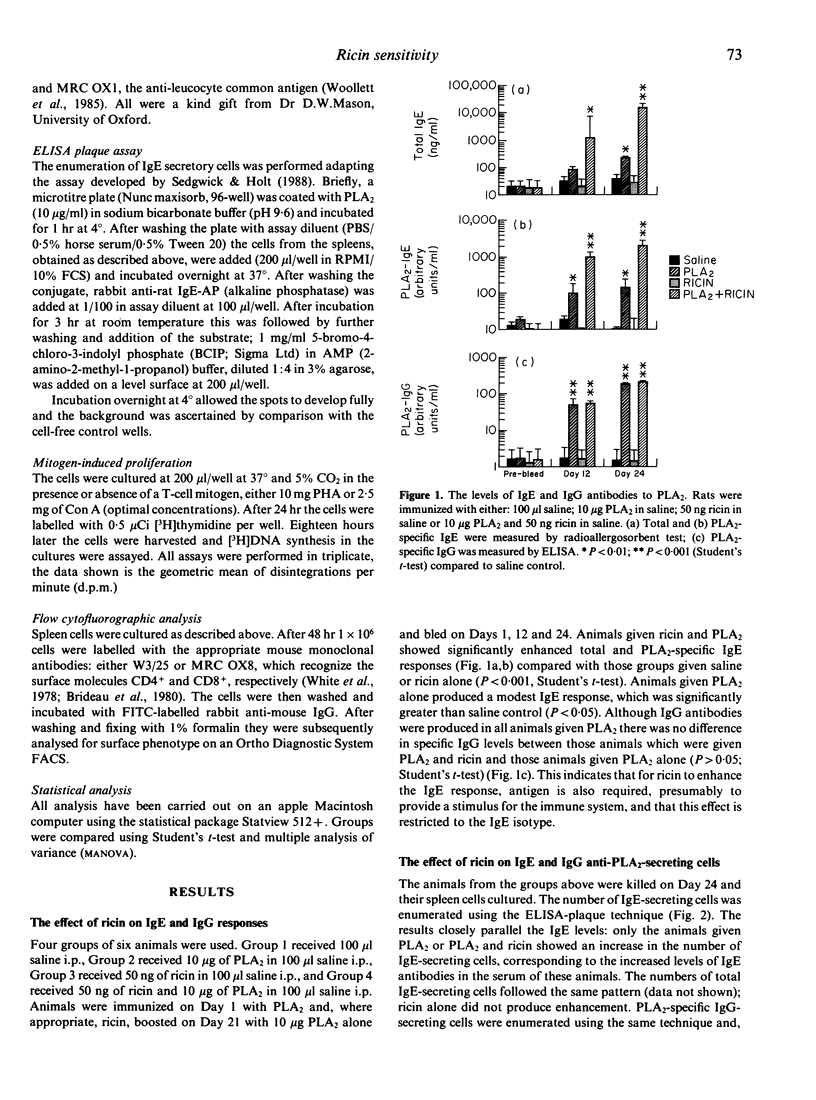
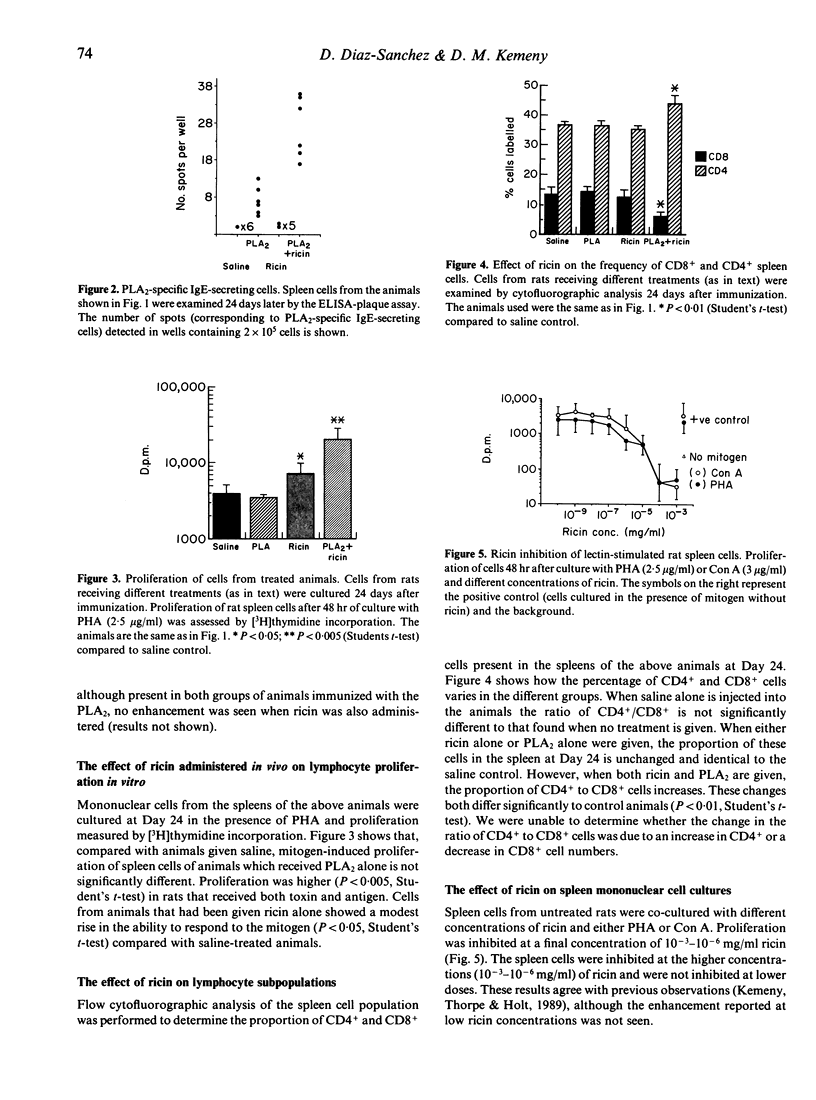
In a previous paper we described how the toxin ricin stimulates IgE but not IgG antibody responses in rats. In this study we have examined the cellular basis for this observation. The proportion of CD4- and CD8-positive cells present in the spleen at the peak of the IgE response was determined. Those animals injected with both ricin and antigen produced a substantial IgE response (50-fold increase). Their CD4+/CD8+ ratio was also markedly increased (P less than 0.001) compared with animals given toxin or antigen alone. In addition, mitogen-stimulated proliferation of mononuclear cells from spleens of the IgE-producing rats was enhanced nearly five-fold compared with cells from animals given toxin or allergen alone. The sensitivity of CD4- and CD8-positive rat spleen cells from unexposed animals to ricin in vitro was also studied. Spleen cells from untreated rats were co-cultured with optimal doses of mitogen and varying amounts of ricin. Mitogen-driven proliferation was inhibited at 10(-3) - 10(-6) mg/ml ricin. This effect was abrogated by the addition of as little as 0.01 M lactose but not by as much as 10 mg/ml mannan to the culture. Cultures depleted of CD4+ cells by rosetting were approximately 100 times more sensitive to ricin (P less than 0.01). Furthermore, the proportion of CD8+ to CD4+ cells present after culture of untreated cells with mitogen and ricin was significantly reduced. These results show (i) that the ability of ricin to increase the IgE response depends on the administration of antigen together with the toxin; (ii) that CD8+ spleen cells are more sensitive to ricin than CD4+ cells; (iii) that increased IgE responsiveness is associated with a reduction in the proportion of CD8- relative to CD4-positive cells in the spleen and increased responsiveness to mitogen. We believe that enhancement of the IgE responses by ricin may be due to inactivation of IgE-specific T-suppressor cells generated by immunization with antigen and speculate that these may be some or all of those bearing the CD8 marker.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Ceska M., Eriksson R., Varga J. M. Radioimmunosorbent assay of allergens. J Allergy Clin Immunol. 1972 Jan;49(1):1–9. doi: 10.1016/0091-6749(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Closs O., Saltvedt E., Olsnes S. Stimulation of human lymphocytes by galactose-specific abrus and ricinus lectins. J Immunol. 1975 Oct;115(4):1045–1048. [PubMed] [Google Scholar]
- Hamaoka T., Katz D. H., Benacerraf B. Hapten-specific IgE antibody responses in mice. II. Cooperative interactions between adoptively transferred T and B lymphocytes in the development of IgE response. J Exp Med. 1973 Sep 1;138(3):538–556. doi: 10.1084/jem.138.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattevig G., Kjellman B., Johansson S. G., Björkstén B. Clinical symptoms and IgE responses to common food proteins in atopic and healthy children. Clin Allergy. 1984 Nov;14(6):551–559. doi: 10.1111/j.1365-2222.1984.tb02243.x. [DOI] [PubMed] [Google Scholar]
- Holt P. G., Warner L. A., Mayrhofer G. Macrophages as effectors of T suppression: T-lymphocyte-dependent macrophage-mediated suppression of mitogen-induced blastogenesis in the rat. Cell Immunol. 1981 Sep 1;63(1):57–70. doi: 10.1016/0008-8749(81)90028-9. [DOI] [PubMed] [Google Scholar]
- Hsiung L., Barclay A. N., Brandon M. R., Sim E., Porter R. R. Purification of human C3b inactivator by monoclonal-antibody affinity chromatography. Biochem J. 1982 Apr 1;203(1):293–298. doi: 10.1042/bj2030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett E. E., Hall E., Karlsson T., Bennich H. Adjuvants in the induction and enhancement of rat IgE responses. Clin Exp Immunol. 1980 Jan;39(1):183–189. [PMC free article] [PubMed] [Google Scholar]
- Kemeny D. M., Thorpe S. C., Holt P. G. Effect of ricin on the proliferation of rat spleen and mesenteric lymph node cells. Int Arch Allergy Appl Immunol. 1989;88(1-2):231–233. doi: 10.1159/000234794. [DOI] [PubMed] [Google Scholar]
- Marsh D. G., Lichtenstein L. M., Norman P. S. Induction of IgE-mediated immediate hypersensitivity to group I rye grass pollen allergen and allergoids in non-allergic man. Immunology. 1972 Jun;22(6):1013–1028. [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Simmonds S. J. The autonomy of CD8+ T cells in vitro and in vivo. Immunology. 1988 Oct;65(2):249–257. [PMC free article] [PubMed] [Google Scholar]
- ORDMAN D. An outbreak of bronchial asthma in South Africa, affecting more than 200 persons, caused by castor bean dust from an oil-processing factory. Int Arch Allergy Appl Immunol. 1955;7(1):10–24. doi: 10.1159/000228201. [DOI] [PubMed] [Google Scholar]
- Oehler J. R., Herberman R. B., Campbell D. A., Jr, Djeu J. Y. Inhibition of rat mixed lymphocyte cultures by suppressor macrophages. Cell Immunol. 1977 Mar 15;29(2):238–250. doi: 10.1016/0008-8749(77)90319-7. [DOI] [PubMed] [Google Scholar]
- Okumura K., Tada T. Regulation of homocytotropic antibody formation in the rat. VI. Inhibitory effect of thymocytes on the homocytotropic antibody response. J Immunol. 1971 Dec;107(6):1682–1689. [PubMed] [Google Scholar]
- PANZANI R. Respiratory castor bean dust allergy in the South of France with special reference to Marseilles. Int Arch Allergy Appl Immunol. 1957;11(3-4):224–236. doi: 10.1159/000228420. [DOI] [PubMed] [Google Scholar]
- Sedgwick J. D., Holt P. G. Induction of IgE-secreting cells and IgE isotype-specific suppressor T cells in the respiratory lymph nodes of rats in response to antigen inhalation. Cell Immunol. 1985 Aug;94(1):182–194. doi: 10.1016/0008-8749(85)90095-4. [DOI] [PubMed] [Google Scholar]
- Simmons B. M., Stahl P. D., Russell J. H. Mannose receptor-mediated uptake of ricin toxin and ricin A chain by macrophages. Multiple intracellular pathways for a chain translocation. J Biol Chem. 1986 Jun 15;261(17):7912–7920. [PubMed] [Google Scholar]
- Tada T., Taniguchi M., Okumura K. Regulation of homocytotropic antibody formation in the rat. II. Effect of X-irradiation. J Immunol. 1971 Apr;106(4):1012–1018. [PubMed] [Google Scholar]
- Taniguchi M., Tada T. Regulation of homocytotropic antibody formation in the rat. IV. Effects of various immunosuppressive drugs. J Immunol. 1971 Aug;107(2):579–585. [PubMed] [Google Scholar]
- Thorpe S. C., Kemeny D. M., Panzani R., Lessof M. H. Allergy to castor bean. I. Its relationship to sensitization to common inhalant allergens (atopy). J Allergy Clin Immunol. 1988 Jul;82(1):62–66. doi: 10.1016/0091-6749(88)90052-8. [DOI] [PubMed] [Google Scholar]
- Thorpe S. C., Murdoch R. D., Kemeny D. M. The effect of the castor bean toxin, ricin, on rat IgE and IgG responses. Immunology. 1989 Nov;68(3):307–311. [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Kojima S., Ovary Z. Suppression of IgE antibody production in SJL mice. I. Nonspecific suppressor T cells. J Exp Med. 1976 Apr 1;143(4):833–845. doi: 10.1084/jem.143.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. A., Mason D. W., Williams A. F., Galfre G., Milstein C. T-lymphocyte heterogeneity in the rat: separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978 Sep 1;148(3):664–673. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]


