Abstract
Knockout of the murine retinoic acid (RA)-synthesizing enzyme retinaldehyde dehydrogenase 2 (RALDH2) gene leads to early morphogenetic defects and embryonic lethality. Using a RA-responsive reporter transgene, we have looked for RA-generating activities in Raldh2-null mouse embryos and investigated whether these activities could be ascribed to the other known RALDH enzymes (RALDH1 and RALDH3). To this end, the early defects of Raldh2−/− embryos were rescued through maternal dietary RA supplementation under conditions that do not interfere with the activity of the reporter transgene in WT embryos. We show that RALDH2 is responsible for most of the patterns of reporter transgene activity in the spinal cord and trunk mesodermal derivatives. However, reporter transgene activity was selectively detected in Raldh2−/− embryos within the mesonephric area that expresses RALDH3 and in medial-ventral cells of the spinal cord and posterior hindbrain, up to the level of the fifth rhombomere. The craniofacial patterns of RA-reporter activity were unaltered in Raldh2−/− mutants. Although these patterns correlated with the presence of Raldh1 and/or Raldh3 transcripts in eye, nasal, and inner ear epithelia, no such correlation was found within forebrain neuroepithelium. These data suggest the existence of additional RA-generating activities in the differentiating forebrain, hindbrain, and spinal cord, which, along with RALDH1 and RALDH3, may account for the development of Raldh2−/− mutants once these have been rescued for early lethality.
Retinoic acid (RA), the active derivative of vitamin A (retinol), acts as an embryonic hormonal signal that controls many aspects of morphogenesis and organogenesis (1). The RA signal is transduced through two families of ligand-dependent transcriptional regulators, RA receptors (RARs) and retinoid X receptors (RXRs), that act as heterodimers to control the expression of target genes containing RA response elements (RAREs) (2). Gene knockout studies in the mouse have shown that RARs and RXRs can function in a partly redundant manner during development (3).
RA is produced in embryonic tissues by the oxidation of retinol, which, in placental vertebrates, originates from maternal blood. The first reaction, which converts retinol to retinaldehyde, is carried out by members of the alcohol dehydrogenase (ADH) family. Several ADH genes exhibit tissue-specific embryonic expression patterns; however, knockout studies have indicated that these enzymes may act in a redundant manner during development (4). Three retinaldehyde dehydrogenases (RALDH1, RALDH2, and RALDH3, renamed as ALDH1A1, ALDH1A2, and ALDH1A3) efficiently oxidize retinaldehyde into RA. These enzymes were identified as being responsible for the dorsal (RALDH1) and ventral (RALDH2 and RALDH3) RA-generating activities present in embryonic retina (5–8).
In situ hybridization (9) and enzymatic studies (10) have shown that RALDH2 is the prominent RALDH activity during early mouse embryogenesis. Raldh2 expression is first detected in the embryonic mesoderm when RA becomes detectable by HPLC or reporter systems. Subsequently, Raldh2 is expressed mostly in mesodermal derivatives including the somites, flank mesoderm, posterior hindbrain mesenchyme, and posterior heart mesoderm, and in various organs at fetal stages (9). Knockout of the Raldh2 gene results in early embryonic lethality [embryonic day (E)9.5–10.5] caused by severe trunk, hindbrain, and heart defects (11–13) that are reminiscent of the effects of full vitamin A deficiency in quail (14–16) and rat (17, 18) embryos. Using a RARE-lacZ reporter transgene, Raldh2−/− null embryos were shown to lack any detectable RA activity, except in the eye field, which contains other RALDH enzymes (see below).
In the present study, we took advantage of the possibility to rescue lethality of Raldh2−/− embryos through short-term maternal RA supplementation during early embryogenesis. Although the resulting mutants exhibit specific organ defects, such as persistent truncus arteriosus (13), which do not allow postnatal survival, they appear mostly similar to WT embryos up to at least E14.5. Using this RA rescue system, we analyzed Raldh2−/− mutants carrying a RARE-hsp68-lacZ reporter transgene at various developmental stages from E10.5 to E14.5 to determine which of the previously described embryonic RA-response patterns (19) could be independent of RALDH2 function. Furthermore, we analyzed in mutant embryos the expression patterns of the two other known Raldh genes (Raldh1 and Raldh3). We show that specific expression of either one of these enzymes can account for some of the patterns of RA-reporter transgene activity in mutant embryos, especially within the craniofacial region. However, several regions of RA-reporter activity are clearly distinct from those expressing Raldh1 or Raldh3, suggesting the existence of additional, yet uncharacterized embryonic activities in the mouse embryo.
Methods
Raldh2-null mutant mice have been described (11). Raldh2+/− heterogygous mutants were crossed with the RARE-hsp68-lacZ reporter transgenic line (19), which harbors a tetrameric repeat of the RARβ2 RARE linked to the hsp68 minimal promoter and has been widely used as a RA-reporter transgene (e.g., ref. 20). To circumvent the early lethality of Raldh2−/− mutants, all-trans RA (Sigma) from a 5 mg/ml ethanol stock suspension was diluted in 50 ml of water and mixed with 30 g of powdered food (R03 breeding diet from UAR, Villemoisson, France) at a final concentration of 100 μg/g food (E7.5–E8.5 treatments) or 250 μg/g (E8.5–E9.5 treatments). The resulting food paste was provided to pregnant mice in a Petri dish wrapped in aluminum foil. Whole-mount 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) assays were as described (19). In situ hybridization with 35S-labeled Raldh1 and Raldh3 riboprobes (21, 22) and X-Gal reactions (23) were performed on frozen sections as described.
Results
Some Domains of RA Responsivity Are Selectively Maintained in Rescued Raldh2-Null Mutant Embryos.
Our initial rescue experiments of Raldh2−/− defects involved administration of multiple subteratogenic RA doses through maternal oral gavage (11). As this could lead to ectopic activation of the RARE-hsp68-lacZ transgene (19), we looked for a more steady mode of administration that may rescue the viability and morphological defects of Raldh2−/− embryos without interfering with the RARE-lacZ expression pattern in WT embryos. RA in suspension was mixed with powdered food, which was given daily as the only source of food. Several RA doses were tested; 100 μg/kg body weight from E7.5 to E8.5 and 250 μg/kg from E8.5 to E9.5 safely avoided teratogenic effects and did not lead to detectable ectopic expression of the RARE-lacZ transgene (Fig. 1, and data not shown), even when embryos were analyzed at E9.5 during the period of RA administration (24).
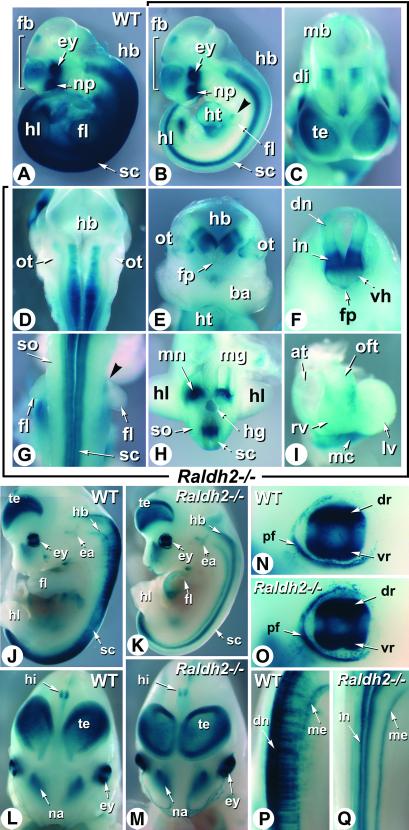
Fig 1.
RARE-hsp68-lacZ reporter transgene activity in E10.5 and E12.5 rescued Raldh2−/− embryos. (A and B) WT and mutant (Raldh2−/−) embryos obtained after dietary RA supplementation from E7.5 to E8.5 were concomitantly stained with X-Gal. Shown are profile views. The Raldh2−/− embryo exhibits a hypoplastic forelimb bud (see ref. 23), but otherwise no external defect. (C–I) Details of the RA reporter patterns in the Raldh2−/− embryo. Frontal view of the forebrain region (C), dorsal view of the hindbrain region after removal of the roof plate (D), transverse section of the hindbrain just rostrally to the otocysts (E), transverse section of the cervical spinal cord (F), dorsal view of the forelimb bud region (G), transverse section of the trunk region at the posterior edge of the hindlimb buds (H), frontal view of the dissected heart (I). Arrowheads in B and G point to a patch of transgene expression in the proximal-rostral aspect of the mutant forelimb bud. (J and K) WT and mutant E12.5 transgenic embryos obtained after dietary RA supplementation from E7.5 to E9.5. Shown are profile views. Mutant forelimb development is selectively altered under these conditions (see ref. 24). (L–Q) Comparison of the RA reporter patterns in the craniofacial and forebrain regions (L and M), eye (N and O), and cervical spinal cord (P and Q) of WT and Raldh2−/− embryos, respectively. at, atria; ba, first branchial arch; di, diencephalon; dn, dorsal neurons; dr, dorsal retina; ea, ear; ey, eye; fb, forebrain; fl, forelimb bud; fp, floor plate; hb, hindbrain; hg, hindgut; hi, hippocampal region; hl, hindlimb bud; ht, heart; in, interneurons; lv, left ventricle; mb, midbrain; mc, mesocardium; me, meninges; mg, midgut; mn, mesonephros; na, nasal region; np, nasal placode; oft, outflow tract; ot, otocyst; pf, palpebral fissure; rv, right ventricle; sc, spinal cord; so, somites; te, telencephalic vesicle; vh, ventral horn; vr, ventral retina. (Magnifications: ×4, J and K; ×6, A and B; ×8, L and M; ×10, D; ×12, C, E–H, P, and Q; ×15, I; ×20, N and O.)
To investigate the patterns of RA response in the absence of RALDH2, Raldh2−/− mutants were analyzed at various developmental stages, at least 48 h after cessation of RA supplementation. Analysis of E10.5 Raldh2−/− embryos revealed a marked down-regulation of transgene activity throughout the trunk region, when compared with control littermates (Fig. 1 A and B). Transgene down-regulation was almost complete in somitic and lateral mesoderm derivatives, except for a patch of weak expression in the upper proximal part of the forelimb buds (Fig. 1 B and G, arrowheads). Strong reporter activity was selectively seen in the posterior mesonephric area, which is adjacent to the hindlimb buds (Fig. 1 B and H) and expresses the RALDH3 enzyme (21). The RA-reporter transgene was also selectively activated in the posterior heart mesocardium (Fig. 1 B and I), and at lower levels in part of the heart outflow tract (Fig. 1I).
Contrasting with the altered trunk mesodermal expression pattern, there was no down-regulation of the RA-reporter transgene in the craniofacial region of Raldh2−/− mutants. As in WT embryos, the reporter was highly expressed in the developing eye and nasal region (Fig. 1 A and B; see below), and more weakly in specific forebrain areas (telencephalic vesicles, prospective hippocampal region, and near the forebrain/midbrain junction; Fig. 1 A–C) and in the medial portion of the developing otocyst (Fig. 1E). RA-reporter activity was selectively seen in mutants along the entire ventral spinal cord and part of the hindbrain neuroepithelium, extending rostrally up to the level of the fifth rhombomere (r5) that is adjacent to the otocyst (Fig. 1 B and D). On cross sections, lacZ activity appeared to be restricted to specific columns of the ventral hindbrain (Fig. 1E) and spinal cord (prospective interneuron region) neuroepithelium (Fig. 1 F and H). Weak activity was also seen in the floor plate at cervical levels (Fig. 1F).
Analysis of E12.5 embryos confirmed the down-regulation of the reporter in the Raldh2−/− trunk (Fig. 1 J and K). At this stage, its activity was restricted mostly to the spinal cord in both control (Fig. 1J) and mutant (Fig. 1K) embryos. RARE-lacZ activity in WT embryos was most prominent in spinal cord dorsal cells (Fig. 1P). Along the anteroposterior (AP) axis, transgene activity was enhanced in the cervical/upper thoracic and lumbar regions (Fig. 1J). No RARE-lacZ activity was seen within spinal cord dorsal cells in Raldh2−/− embryos (Fig. 1 K and Q). However, reporter transgene expression was maintained along the spinal cord midventral columns (Fig. 1 K and Q), where its activity was evenly distributed along the AP axis (Fig. 1K). Transgene activity was also seen in the ventral meningeal layer (Fig. 1 K and Q; see below).
Except for the spinal cord, all other areas of reporter transgene activity were similar in Raldh2−/− and WT embryos. Transgene expression in Raldh2−/− eyes was indistinguishable from that observed in control eyes. Two strong expression domains in the dorsal and ventral retina were separated by a central region of lower expression (Fig. 1 N and O), as reported in WT (7, 25). The RARE-lacZ reporter was also similarly expressed in the inner ear (Fig. 1 J and K), nasal region (Fig. 1 L and M), dorsal telencephalon (Fig. 1 J–M), and hippocampal area (Fig. 1 L and M) of WT and mutant embryos.
Not All of the Maintained RA-Responsive Domains Can Be Ascribed to Raldh1 and Raldh3 Activities.
We compared at E12.5 and E14.5 the pattern of reporter activity with those of Raldh1 and Raldh3 expression to determine which of the reporter activity domains that are maintained in mutant embryos could not be correlated with the expression of these enzymes, and therefore may correspond to additional RA-producing activities. Serial histological sections of WT and Raldh2−/− RARE-lacZ transgenic embryos were processed for X-Gal staining or in situ hybridization with Raldh1 and Raldh3 riboprobes to allow a direct spatial comparison of the expression patterns.
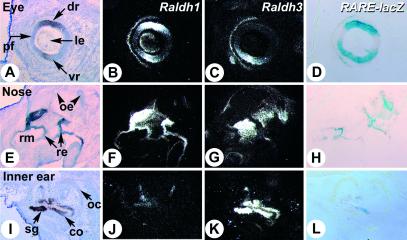
As expected, the RARE-lacZ activity in developing mutant eyes closely correlated with the combined expression of Raldh1 and Raldh3 genes, in both WT (data not shown) and Raldh2−/− embryos (Fig. 2 A–D). The reporter activity (Fig. 2D) matched that of Raldh1 in the dorsal retina (Fig. 2B), while it correlated in the ventral retina with the Raldh3 transcript domain (Fig. 2C), which outflanks the Raldh1 ventral domain. Both enzymes were expressed at the level of the palpebral fissure, which also exhibited lacZ activity. A looser correlation between reporter and Raldh gene expression was found in the nasal region. Raldh1 was strongly expressed in the respiratory epithelium (Fig. 2F), whereas Raldh3 was expressed in the underlying mesenchyme and part of the olfactory epithelium (Fig. 2G; refs. 8 and 21). RARE-lacZ activity was mostly epithelial (Fig. 2H) and partly overlapped with Raldh1-expressing cells in the respiratory region. LacZ-positive cells were also found in the olfactory epithelium, where they partly overlapped with Raldh3-expressing cells (Fig. 2 G and H; see also Fig. 3 G and H), as well as in a discrete region of the E14.5 inner ear epithelium (Fig. 2L), which was much more limited than the expression domain of Raldh3 (Fig. 2K) and was distinct, although adjacent, to that of Raldh1 (Fig. 2J).
Fig 2.
Correlation between RA reporter activity and Raldh1 and/or Raldh3 gene expression in developing sensory epithelia. Serial histological sections of the eye (A–D), nasal region (E and H), and inner ear (I–L) of an E14.5 transgenic Raldh2−/− embryo were hybridized with Raldh1 (B, F, and J) and Raldh3 (C, G, and K) riboprobes, or X-Gal-stained (D, H, and L). In situ hybridization signal grain appears white on dark-field illumination. Bright-field views of the Raldh1-hybridized (A and E) or Raldh3-hybridized (I) sections are shown for histology. co, cochlea; dr, dorsal retina; le, lens vesicle; oc, otic capsule; oe, olfactory epithelium; pf, palpebral fissure; re, respiratory epithelium; rm, respiratory mesenchyme; sg, spiral ganglion; vr, ventral retina. (Magnifications: ×8, A–H; ×12, I–L.)
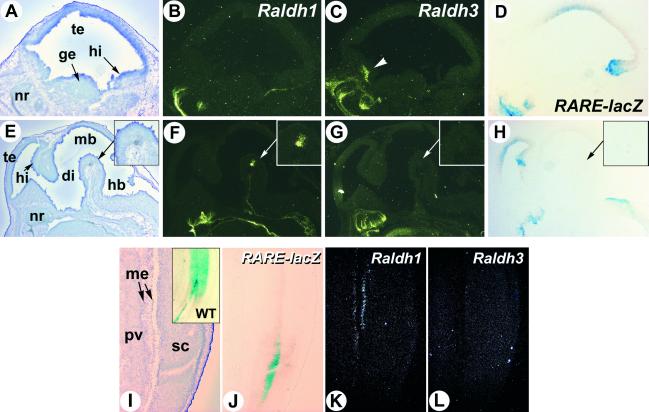
Fig 3.
RA reporter activity in the Raldh2−/− brain and spinal cord does not correlate with Raldh1 or Raldh3 gene expression. (A–H) Sagittal sections through the lateral region of the forebrain (A–D) and the near-midline brain structures (E–H) of an E14.5 transgenic Raldh2−/− embryo were hybridized with Raldh1 (B and F) and Raldh3 (C and G) riboprobes, or X-Gal-stained (D and H). A higher magnification of the ventral midbrain Raldh1-expressing area is shown (Insets) (arrows in E–H). Raldh3-specific expression in the rostro-ventral telencephalon is indicated by an arrowhead. (I–L) Serial sections through the lumbar spinal spinal cord of an E14.5 transgenic Raldh2−/− embryo were X-Gal-stained (J) or hybridized with Raldh1 (K) and Raldh3 (L) riboprobes. (I Inset) The X-Gal pattern obtained in a WT spinal cord. di, diencephalon; ge, ganglionic eminence; hb, hindbrain; hi, hippocampal region; mb, midbrain; me, meninges; nr, nasal region; pv, prevertebrae; sc, spinal cord; te, telencephalon. (Magnifications: ×5, E–H; ×8, A–D; ×10, I–L.)
In contrast, analysis of brain sections showed no spatial correlation between RARE-lacZ reporter activity and Raldh1 or Raldh3 expression. Raldh1 prenatal expression is limited to ventral midbrain cells that will become part of the nigro-striatal system (refs. 26 and 27; Fig. 3F). This area showed no lacZ reporter activity (Fig. 3H). None of the domains of RARE-lacZ activity (in dorsal telencephalon, hippocampal region and hindbrain floor) expressed Raldh1 or Raldh3 transcripts (Fig. 3 B–D and F–H). Note that Raldh3 is specifically expressed in a rostroventral telencephalic area encompassing part of the lateral ganglionic eminence and ventral septum (refs. 7 and 27; Fig. 3C).
We also investigated whether the lacZ reporter activity seen in the spinal cord of the Raldh2−/− mutants could reflect the presence of RALDH1 or RALDH3. While WT reporter transgene activity was found mostly in the dorsal spinal cord cells at cervical and lumbar levels (Figs. 3I and 4H), transgene activity was restricted to a medio-ventral column in Raldh2−/− mutants (Fig. 3J). This activity did not correlate with Raldh1 or Raldh3 expression (Fig. 3 K and L). Whereas the former was weakly expressed in the meningeal layer (Fig. 3K), the latter showed no expression in the spinal cord of mutant (Fig. 3L) or WT (data not shown) embryos.
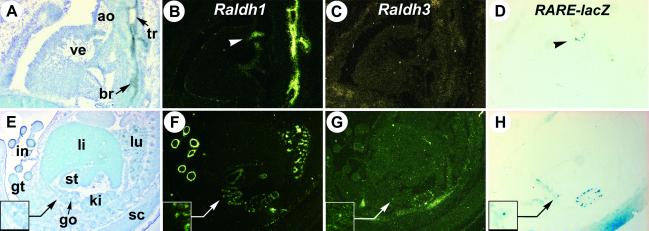
Fig 4.
RA reporter transgene activity and Raldh1/Raldh3 gene expression in developing trunk organs. (A–D) Sections through the heart of an E14.5 Raldh2−/− transgenic embryo. Raldh1 and RARE-lacZ transgene expression in the atrioventricular valve region is indicated (arrowheads). (E–H) Sections through the abdominal cavity of a littermate WT transgenic embryo. The mesonephric duct area is enlarged (Insets). ao, ascending aorta; br, bronchus; go, gonad; gt, genital tubercle; in, intestine; ki, kidney; li, liver; lu, lung; sc, spinal cord; st, stomach; tr, trachea; ve, ventricle. (Magnifications: ×5, E–H; ×10, A–D.)
Except for the CNS and sensory organs, few other areas expressed the RA-responsive reporter transgene, whether in mutant (Fig. 4 A–D) or WT embryos (Fig. 4 E–H; data not shown). The developing kidney showed conspicuous lacZ reporter activity (Fig. 4H), which correlated with the presence of both Raldh1 (Fig. 4F) and Raldh3 (Fig. 4G) transcripts. RARE-lacZ expression in the mesonephric duct correlated with that of Raldh3 (Fig. 4 G and H Insets). RARE-lacZ expression was restricted to the atrioventricular valves of the E14.5 heart (Fig. 4D), which express Raldh1 (Fig. 4B, arrowhead). Most of the developing trunk organs were devoid of Raldh3 expression (Fig. 4 C and G, and data not shown). In contrast, Raldh1 was expressed at high levels in the intestinal mesenchyme, the tracheal and bronchial epithelium, and at lower levels in the stomach mesenchyme or the developing gonad (Fig. 4 B and F; ref. 21). None of these tissues, though, showed concomitant RARE-lacZ activity (Fig. 4H).
Discussion
We have reported (13) that nonteratogenic RA dietary supplementation from E7.5 to E8.5/9.5 can rescue Raldh2−/− null mutants deficient in RA synthesis during early embryogenesis (11), with limited phenotypic defects up to E14.5. Here we used a RARE-lacZ reporter transgene to show that these rescued embryos exhibit several sites of RA production that are independent of RALDH2 function. We further show that only a subset of the RARE-lacZ reporter expression domains in Raldh2−/− embryos correlate with the expression of one (or both) of the other known RALDH enzymes (RALDH1 and RALDH3). These findings suggest the presence of other, yet uncharacterized, tissue-specific RA-producing activities in the mammalian embryo. Our data are consistent with those published after completion of this study by Mic et al. (28), who used another mode of RA rescue of Raldh2−/− embryos.
RALDH2 Expression Is Crucial for Most Embryonic RA Synthesis in Trunk Mesoderm and Spinal Cord.
Despite the extensive morphologic rescue of Raldh2−/− null embryos on early RA supplementation, it is striking that these embryos still exhibit a marked down-regulation of the RA reporter in their trunk region. Among mesodermal derivatives, strong reporter activity is seen only in the posterior mesonephric epithelium and surrounding mesenchyme. This activity can be ascribed to the presence of RALDH3 in mesonephric and ureteric bud epithelia (21, 28). Interestingly, it is spatially adjacent to the proximal margin of the hindlimb buds. We have reported that RA-rescued Raldh2−/− mutants exhibit defects in forelimb growth and patterning, which have no counterpart in the hindlimbs (24). One possible explanation for this difference could be that RA produced by the RALDH3 activity present in the mesonephric area diffuses in the hindlimb buds, thereby compensating for the lack of RALDH2-synthesizing activity. It will therefore be interesting to investigate whether the genetic ablation of Raldh3 alters hindlimb development in a Raldh2−/− null background.
Our data show that RALDH2 activity is crucial for proper RA production within the embryonic trunk. RALDH2-mediated RA synthesis is likely to begin in the embryonic mesoderm as early as E7.5 (9). From E8.5 to E10.5, Raldh2 is expressed mostly in the somitic mesoderm, in a cranial to caudal gradient, whereas no expression is detected within the spinal cord (9). However, reporter transgene expression is down-regulated in both the trunk mesoderm and spinal cord of E10.5 Raldh2−/− mutants. This finding suggests that RA normally produced by RALDH2 in the mesoderm of WT embryos diffuses to the spinal cord neuroepithelium, where it activates the RARE reporter transgene.
Raldh2 expression appears by ≈E11.5 within the lateral motor columns of the cervical/brachial and lumbar spinal cord (29). The contribution of these Raldh2-expressing cells to RARE-lacZ transgene activity is clearly evidenced when WT and Raldh2−/− embryos are compared at E12.5. In WT embryos, transgene expression is strongly enhanced, within the spinal cord, at cervical/upper thoracic and lumbar levels (including dorsally, which indicates that RA produced ventrally diffuses through the plane of the spinal cord: Fig. 1J). These region-specific lacZ patterns are absent in the Raldh2-null spinal cord (Fig. 1K). Some of the Hoxb genes fail to be up-regulated at similar levels of the spinal cord in E11.5 Raldh2−/− embryos (T. Oosterveen and J. Deschamps, personal communication), thus establishing a link between RALDH2-mediated RA signaling and Hox gene regulation.
RALDH1 and RALDH3 Are Involved in RA Production in the Developing Ear, Eye, and Nasal Epithelium.
In contrast with the situation in the trunk region, we found no alteration of the craniofacial expression domains of the RA-responsive reporter transgene in Raldh2−/− embryos. The pattern of transgene activity in the developing dorsal and ventral retina precisely correlates with the expression domains of Raldh1 and Raldh3, respectively, whereas the central RA-free region can been ascribed to the presence of the RA-metabolizing enzyme CYP26A1 (25). However, reporter transgene expression in the nasal epithelium correlated only partly, both in WT and mutants, with the presence of Raldh1 and Raldh3 transcripts in the respiratory and olfactory regions, respectively. Similarly, reporter activity was much more restricted than Raldh3 transcript distribution in the E14.5 inner ear epithelium. Thus, as suggested for the developing retina (25), additional mechanisms are likely to restrict RA availability (e.g., through the presence of RA-metabolizing enzymes; see ref. 30) and/or RA response (e.g., through restricted receptor expression; see ref. 31) in these epithelia.
An Additional, As Yet Unknown RA-Generating Activity in the Developing CNS?
Our study clearly reveals that, in Raldh2−/− as well as in WT embryos, some of the regions expressing the RA-responsive reporter do not correspond to sites of Raldh1 or Raldh3 expression. This is the case in the E12.5–E14.5 forebrain for the dorsal telencephalic vesicles and hippocampal region. Raldh1 expression in the developing brain is restricted to prospective dopaminergic neurons in the ventral midbrain, whereas Raldh3 expression appears by E11.5 in a streak of cells within the ventral telencephalon (26, 27). However, Raldh3 is expressed at earlier stages (E8.5–E10.5) in the ventral head ectoderm (mostly in olfactory placodes), which lies in close contact with the developing telencephalic vesicles (7, 27). RA produced by RALDH3 in the ectoderm could possibly act in a paracrine manner to activate the reporter transgene in the prospective telencephalic vesicles, and stability of the β-galactosidase enzyme could explain the persistent X-Gal labeling observed at later stages in these structures (which actually fades by E14.5, unlike that seen in the hippocampal region). Analysis of Raldh3−/− knockout mutants will reveal whether RALDH3 is in fact involved in the expression of the RA reporter in the prospective telencephalic vesicles.
Activity of the RA-responsive transgene along the ventral spinal cord and hindbrain of Raldh2-null mutants also does not correlate with Raldh1 or Raldh3 expression. Unlike the telencephalon, there is no early expression of Raldh1 or Raldh3 within (or near) the spinal cord/hindbrain that may activate the transgene before E10.5 (7, 8, 21). The RARE-lacZ pattern of activity does not correlate with the hindbrain/spinal cord distribution of cellular RA-binding proteins or RA-metabolizing enzymes, making it unlikely that it may result from an uneven sequestration of the maternally administered RA. Interestingly, this neuroepithelial activity extends along the hindbrain floor until the fifth rhombomere. Unrescued Raldh2−/− embryos undergo marked defects of the caudal hindbrain, characterized by a size reduction, lack of segmentation, and caudal expansion of rostral hindbrain markers (12). This phenotype is, however, less severe than that seen in vitamin A-deficient quail embryos (15), which led to the suggestion that RA signaling might still operate, albeit at a reduced level, in the Raldh2−/− hindbrain (32). We now provide evidence that a RA response occurs in the caudal (r5-r8) hindbrain region of E10.5–14.5 Raldh2−/− embryos. It is noteworthy that under the present maternal RA supplementation, caudal hindbrain patterning of Raldh2−/− embryos is almost completely rescued (unpublished results). One possible explanation would be that RA produced by a novel (RALDH1- and RALDH3-independent) hindbrain and spinal cord RA-generating activity, together with RA of maternal origin, may result in sufficient RA levels in the r5-r8 region to allow the retinoid-dependent patterning events (33) to proceed almost normally, even in the absence of RA synthesized by the mesodermal RALDH2.
Altogether, our data strongly suggest the existence of an additional RA-producing activity within specific hindbrain, spinal cord, and forebrain (hippocampal) regions. Whether this activity involves a novel member of the aldehyde dehydrogenase (ALDH) family (or other enzyme classes reported to have a retinaldehyde-oxidizing activity; refs. 34 and 35) deserves further investigation.
A RALDH1 Function Unrelated to RA Synthesis?
Most, if not all, of the few regions that express RALDH3 during development (8, 21) correspond to sites of RA production, as evidenced by the expression of the RA reporter transgene. RALDH1 is much more widely expressed than RALDH3 during mid-late gestation (21, 36). However, only a few of the Raldh1-expressing tissues (e.g., the dorsal retina, developing heart valves, and kidney, which also expresses Raldh3) stain positively for the RA-responsive transgene and several regions of high Raldh1 expression, such as the tracheo-bronchial epithelium, intestine epithelium, or midbrain dopaminergic cells, do not express the reporter transgene. Although it is not excluded that silencing of the RARE-lacZ reporter transgene could occur during differentiation of these cell types, it is tempting to speculate that RALDH1 could be only marginally involved (with respect to RALDH2 and RALDH3) in the RA synthetic pathway.
The liver is a major postnatal site of RALDH1 expression, where it is likely to be involved in the detoxification of endogenous aldehydes. A similar function may be performed in other differentiating organs, such as the intestine or kidney, which also express Raldh1 during adult life (21, 37). It is noteworthy that, among the three RALDH enzymes, RALDH1 has the lowest affinity for retinaldehyde and is ≈10-fold less efficient at producing RA in cultured cells (38–40). Thus, RALDH1 may have initially functioned as a detoxifying enzyme (e.g., in the eye lens, where it may act on peroxidic aldehydes produced by UV light absorption; ref. 41), and only subsequently could have been co-opted in the RA synthetic pathway within specific tissues (e.g., the developing retina). Similarly, some of the cytochrome P450 enzymes may have evolved to accommodate RA, thereby allowing the appearance of tissue-specific RA catabolic functions (20, 42). Thus, the evolution of RA synthetic and catabolic activities within two enzyme families with differential expression patterns may have created the possibility to finely tune the RA signal along various embryonic structures such as the differentiating trunk/tail bud region, eye, and hindbrain.
Acknowledgments
We thank Dr. J. Rossant for providing the RARE-hsp68-lacZ transgenic line, S. Bronner and B. Schuhbaur for technical assistance, and M. Le Meur and S. Falcone for animal care. This work was supported by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Collège de France, the Hôpitaux Universitaires de Strasbourg, the Fondation pour la Recherche Médicale, and Bristol-Myers Squibb. J.V. was supported by fellowships from the Ministère de la Recherche and the Association pour la Recherche sur le Cancer.
Abbreviations
E(n), embryonic day
RA, retinoic acid
RALDH, retinaldehyde dehydrogenase
RARE, retinoic acid response element
X-Gal, 5-bromo-4-chloro-3-indolyl β-d-galactoside
References
- 1.Morriss-Kay G. M. & Ward, S. J. (1999) Int. Rev. Cytol. 188, 73-131. [DOI] [PubMed] [Google Scholar]
- 2.Chambon P. (1996) FASEB J. 10, 940-954. [PubMed] [Google Scholar]
- 3.Mark M., Ghyselinck, N. B., Wendling, O., Dupé, V., Mascrez, B., Kastner, P. & Chambon, P. (1999) Proc. Nutr. Soc. 58, 609-613. [DOI] [PubMed] [Google Scholar]
- 4.Deltour L., Foglio, M. H. & Duester, G. (1999) J. Biol. Chem. 274, 16796-16801. [DOI] [PubMed] [Google Scholar]
- 5.McCaffery P., Posch, K. C., Napoli, J. L., Gudas, L. & Dräger, U. C. (1993) Dev. Biol. 158, 390-399. [DOI] [PubMed] [Google Scholar]
- 6.Zhao D., McCaffery, P., Ivins, K. J., Neve, R. L., Hogan, P., Chin, W. W. & Dräger, U. C. (1996) Eur. J. Biochem. 240, 15-22. [DOI] [PubMed] [Google Scholar]
- 7.Li H., Wagner, E., McCaffery, P., Smith, D., Andreadis, A. & Dräger, U. C. (2000) Mech. Dev. 95, 283-289. [DOI] [PubMed] [Google Scholar]
- 8.Mic F. A., Molotkov, A., Fan, X., Cuenca, A. E. & Duester, G. (2000) Mech. Dev. 97, 227-230. [DOI] [PubMed] [Google Scholar]
- 9.Niederreither K., McCaffery, P., Dräger, U. C., Chambon, P. & Dollé, P. (1997) Mech. Dev. 62, 67-78. [DOI] [PubMed] [Google Scholar]
- 10.McCaffery P. & Dräger, U. C. (1995) Adv. Exp. Med. Biol. 372, 173-183. [DOI] [PubMed] [Google Scholar]
- 11.Niederreither K., Subbarayan, V., Dollé, P. & Chambon, P. (1999) Nat. Genet. 21, 444-448. [DOI] [PubMed] [Google Scholar]
- 12.Niederreither K., Vermot, J., Schuhbaur, B., Chambon, P. & Dollé, P. (2000) Development (Cambridge, U.K.) 127, 75-85. [DOI] [PubMed] [Google Scholar]
- 13.Niederreither K., Vermot, J., Messaddeq, N., Schuhbaur, B., Chambon, P. & Dollé, P. (2001) Development (Cambridge, U.K.) 128, 1019-1031. [DOI] [PubMed] [Google Scholar]
- 14.Dersch H. & Zile, M. H. (1993) Dev. Biol. 160, 424-433. [DOI] [PubMed] [Google Scholar]
- 15.Gale E., Zile, M. & Maden, M. (1999) Mech. Dev. 89, 43-54. [DOI] [PubMed] [Google Scholar]
- 16.Zile M. H., Kostetskii, I., Yuan, S., Kostetskaia, E., St. Amand, T. R., Chen, Y. P. & Jiang, W. (2000) Dev. Biol. 223, 323-338. [DOI] [PubMed] [Google Scholar]
- 17.Dickman E. D., Thaller, C. & Smith, S. M. (1997) Development (Cambridge, U.K.) 124, 3111-3121. [DOI] [PubMed] [Google Scholar]
- 18.White J. C., Highland, M., Kaiser, M. & Clagett-Dame, M. (2000) Dev. Biol. 220, 263-284. [DOI] [PubMed] [Google Scholar]
- 19.Rossant J., Zirnbigl, R., Cado, D., Shago, M. & Giguère, V. (1991) Genes Dev. 5, 1333-1344. [DOI] [PubMed] [Google Scholar]
- 20.Sakai Y., Meno, C., Fujii, H., Nishino, J., Shiratori, H., Saijoh, Y., Rossant, J. & Hamada, H. (2001) Genes Dev. 15, 213-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niederreither K., Fraulob, V., Garnier, J. M., Chambon, P. & Dollé, P. (2002) Mech. Dev. 110, 165-171. [DOI] [PubMed] [Google Scholar]
- 22.Niederreither K. & Dollé, P. (1998) in Methods in Molecular Biology: Retinoids, ed. Redfern, C. P. F. (Humana, Totowa, NJ), pp. 247–267. [DOI] [PubMed]
- 23.Brocard J., Warot, X., Wendling, O., Messaddeq, N., Vonesch, J. L., Chambon, P. & Metzger, D. (1997) Proc. Natl. Acad. Sci. USA 94, 14559-14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niederreither K., Vermot, J., Schuhbaur, B., Chambon, P. & Dollé, P. (2002) Development (Cambridge, U.K.) 129, 3563-3574. [DOI] [PubMed] [Google Scholar]
- 25.Wagner E., McCaffery, P. & Dräger, U. C. (2000) Dev. Biol. 222, 460-470. [DOI] [PubMed] [Google Scholar]
- 26.Wallen A., Zetterstrom, R. H., Solomin, L., Arvidsson, M., Olson, L. & Perlmann, T. (1999) Exp. Cell. Res. 253, 737-746. [DOI] [PubMed] [Google Scholar]
- 27.Smith D., Wagner, E., Koul, O., McCaffery, P. & Dräger, U. C. (2001) Cereb. Cortex 11, 894-905. [DOI] [PubMed] [Google Scholar]
- 28.Mic F. A., Haselbeck, R. J., Cuenca, A. E. & Duester, G. (2002) Development (Cambridge, U.K.) 129, 2271-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sockanathan S. & Jessell, T. M. (1998) Cell 94, 503-514. [DOI] [PubMed] [Google Scholar]
- 30.Abu-Abed S., MacLean, G., Fraulob, V., Chambon, P., Petkovich, M. & Dollé, P. (2002) Mech. Dev. 110, 173-177. [DOI] [PubMed] [Google Scholar]
- 31.Romand R., Sapin, V. & Dollé, P. (1998) J. Comp. Neurol. 393, 298-308. [DOI] [PubMed] [Google Scholar]
- 32.Gavalas A. & Krumlauf, R. (2000) Curr. Opin. Genet. Dev. 10, 380-386. [DOI] [PubMed] [Google Scholar]
- 33.Dupé V., Ghyselinck, N. B., Wendling, O., Chambon, P. & Mark, M. (1999) Development (Cambridge, U.K.) 126, 5051-5059. [DOI] [PubMed] [Google Scholar]
- 34.Roberts E. S., Vaz, A. D. & Coon, M. J. (1992) Mol. Pharmacol. 41, 427-433. [PubMed] [Google Scholar]
- 35.Tomita S., Tsujita, M. & Ichikawa, Y. (1993) FEBS Lett. 336, 272-274. [DOI] [PubMed] [Google Scholar]
- 36.Haselbeck R. J., Hoffmann, I. & Duester, G. (1999) Dev. Genet. 25, 353-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhat P. V., Marcinkiewicz, M., Li, Y. & Mader, S. (1998) J. Histochem. Cytochem. 46, 1025-1032. [DOI] [PubMed] [Google Scholar]
- 38.Grün F., Hirose, Y., Kawauchi, S., Ogura, T. & Umesono, K. (2000) J. Biol. Chem. 275, 41210-41218. [DOI] [PubMed] [Google Scholar]
- 39.Duester G. (2000) Eur. J. Biochem. 267, 4315-4324. [DOI] [PubMed] [Google Scholar]
- 40.Lamb A. L. & Newcomer, M. E. (1999) Biochemistry 38, 6003-6011. [DOI] [PubMed] [Google Scholar]
- 41.King G. & Holmes, R. (1997) Adv. Exp. Med. Biol. 414, 19-27. [PubMed] [Google Scholar]
- 42.Abu-Abed S., Dollé, P., Metzger, D., Beckett, B., Chambon, P. & Petkovich, M. (2001) Genes Dev. 15, 226-240. [DOI] [PMC free article] [PubMed] [Google Scholar]