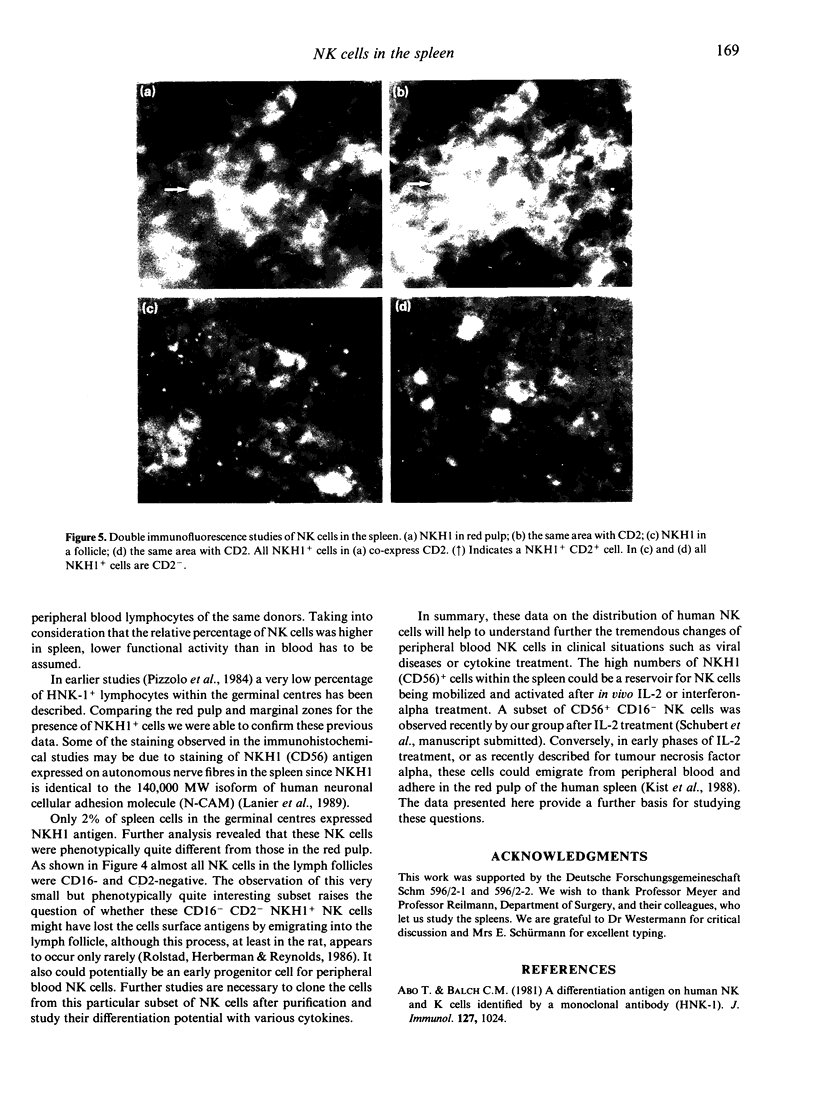
Abstract
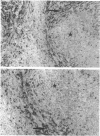
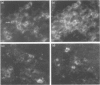
Natural killer cells have an important role in tumour and viral defence and immunoregulation. In the present study the pan-NK cell monoclonal antibody NKH1 was utilized to study the heterogeneity of NK cells in the human spleen. Using one and two colour analysis it could be demonstrated that the majority of NKH1+ NK cells are located in the red pulp and marginal zone whereas only a minor subset is found in the lymph follicles. In the red pulp, NK cells resemble those of peripheral blood in terms of function and phenotype. In contrast, the few NK cells in the lymph follicles are mostly NKH1+, CD2- and CD16- and thus express a unique phenotype. The analysis of tissue distribution provides a basis for further studies on NK cell kinetics and differentiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. I. Two distinct phenotypes of human NK cells with different cytotoxic capability. J Immunol. 1982 Oct;129(4):1752–1757. [PubMed] [Google Scholar]
- Bunn P. A., Jr, Linnoila I., Minna J. D., Carney D., Gazdar A. F. Small cell lung cancer, endocrine cells of the fetal bronchus, and other neuroendocrine cells express the Leu-7 antigenic determinant present on natural killer cells. Blood. 1985 Mar;65(3):764–768. [PubMed] [Google Scholar]
- Caligiuri M., Murray C., Buchwald D., Levine H., Cheney P., Peterson D., Komaroff A. L., Ritz J. Phenotypic and functional deficiency of natural killer cells in patients with chronic fatigue syndrome. J Immunol. 1987 Nov 15;139(10):3306–3313. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Hercend T., Griffin J. D., Bensussan A., Schmidt R. E., Edson M. A., Brennan A., Murray C., Daley J. F., Schlossman S. F., Ritz J. Generation of monoclonal antibodies to a human natural killer clone. Characterization of two natural killer-associated antigens, NKH1A and NKH2, expressed on subsets of large granular lymphocytes. J Clin Invest. 1985 Mar;75(3):932–943. doi: 10.1172/JCI111794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercend T., Schmidt R. E. Characteristics and uses of natural killer cells. Immunol Today. 1988 Oct;9(10):291–293. doi: 10.1016/0167-5699(88)91317-5. [DOI] [PubMed] [Google Scholar]
- Herrmann F., Schmidt R. E., Ritz J., Griffin J. D. In vitro regulation of human hematopoiesis by natural killer cells: analysis at a clonal level. Blood. 1987 Jan;69(1):246–254. [PubMed] [Google Scholar]
- Kist A., Ho A. D., Räth U., Wiedenmann B., Bauer A., Schlick E., Kirchner H., Männel D. N. Decrease of natural killer cell activity and monokine production in peripheral blood of patients treated with recombinant tumor necrosis factor. Blood. 1988 Jul;72(1):344–348. [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Lanier L. L., Testi R., Bindl J., Phillips J. H. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989 Jun 1;169(6):2233–2238. doi: 10.1084/jem.169.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzolo G., Semenzato G., Chilosi M., Morittu L., Ambrosetti A., Warner N., Bofill M., Janossy G. Distribution and heterogeneity of cells detected by HNK-1 monoclonal antibody in blood and tissues in normal, reactive and neoplastic conditions. Clin Exp Immunol. 1984 Jul;57(1):195–206. [PMC free article] [PubMed] [Google Scholar]
- Ritchie A. W., James K., Micklem H. S. The distribution and possible significance of cells identified in human lymphoid tissue by the monoclonal antibody HNK-1. Clin Exp Immunol. 1983 Mar;51(3):439–447. [PMC free article] [PubMed] [Google Scholar]
- Ritz J., Schmidt R. E., Michon J., Hercend T., Schlossman S. F. Characterization of functional surface structures on human natural killer cells. Adv Immunol. 1988;42:181–211. doi: 10.1016/s0065-2776(08)60845-7. [DOI] [PubMed] [Google Scholar]
- Rolstad B., Herberman R. B., Reynolds C. W. Natural killer cell activity in the rat. V. The circulation patterns and tissue localization of peripheral blood large granular lymphocytes (LGL). J Immunol. 1986 Apr 15;136(8):2800–2808. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Schmidt R. E., Bartley G., Levine H., Schlossman S. F., Ritz J. Functional characterization of LFA-1 antigens in the interaction of human NK clones and target cells. J Immunol. 1985 Aug;135(2):1020–1025. [PubMed] [Google Scholar]
- Schmidt R. E., MacDermott R. P., Bartley G., Bertovich M., Amato D. A., Austen K. F., Schlossman S. F., Stevens R. L., Ritz J. Specific release of proteoglycans from human natural killer cells during target lysis. Nature. 1985 Nov 21;318(6043):289–291. doi: 10.1038/318289a0. [DOI] [PubMed] [Google Scholar]
- Schmidt R. E., Michon J. M., Woronicz J., Schlossman S. F., Reinherz E. L., Ritz J. Enhancement of natural killer function through activation of the T11 E rosette receptor. J Clin Invest. 1987 Jan;79(1):305–308. doi: 10.1172/JCI112800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Emmons S. L., Pollack S. B. Analysis and enrichment of murine natural killer cells with the fluorescence-activated cell sorter. J Immunol. 1980 Feb;124(2):650–655. [PubMed] [Google Scholar]
- Vyakarnam A., Brenner M. K., Reittie J. E., Houlker C. H., Lachmann P. J. Human clones with natural killer function can activate B cells and secrete B cell differentiation factors. Eur J Immunol. 1985 Jun;15(6):606–610. doi: 10.1002/eji.1830150614. [DOI] [PubMed] [Google Scholar]
- Werfel T., Uciechowski P., Tetteroo P. A., Kurrle R., Deicher H., Schmidt R. E. Activation of cloned human natural killer cells via Fc gamma RIII. J Immunol. 1989 Feb 15;142(4):1102–1106. [PubMed] [Google Scholar]