Abstract
Adoptive T cell therapy, involving the ex vivo selection and expansion of antigen-specific T cell clones, provides a means of augmenting antigen-specific immunity without the in vivo constraints that can accompany vaccine-based strategies. A phase I study was performed to evaluate the safety, in vivo persistence, and efficacy of adoptively transferred CD8+ T cell clones targeting the tumor-associated antigens, MART1/MelanA and gp100 for the treatment of patients with metastatic melanoma. Four infusions of autologous T cell clones were administered, the first without IL-2 and subsequent infusions with low-dose IL-2 (at 0.25, 0.50, and 1.0 × 106 units/m2 twice daily for the second, third, and fourth infusions, respectively). Forty-three infusions of MART1/MelanA-specific or gp100-specific CD8+ T cell clones were administered to 10 patients. No serious toxicity was observed. We demonstrate that the adoptively transferred T cell clones persist in vivo in response to low-dose IL-2, preferentially localize to tumor sites and mediate an antigen-specific immune response characterized by the elimination of antigen-positive tumor cells, regression of individual metastases, and minor, mixed or stable responses in 8 of 10 patients with refractory, metastatic disease for up to 21 mo.
The identification of T cell-defined tumor antigens in melanoma has led to the development of clinical trials that target cancer cells by augmenting the antigen-specific cellular immune response (1). This approach has been pursued by vaccination strategies, in which the antigens are presented in a potentially immunogenic context to induce T cell responses or by adoptive cellular therapy, in which antigen-specific T cells are isolated and expanded ex vivo and then infused to increase the number of effector cells in vivo.
Although some clinical responses have been observed in vaccine trials, the magnitude of the induced T cell response has been generally low or undetectable and has correlated poorly with clinical responses. In contrast to vaccination strategies, adoptive therapy strategies can overcome the in vivo constraints that influence the magnitude and avidity of the targeted response. T cells of a given specificity, function, and avidity for tumor can be selected in vitro and then expanded to achieve in vivo frequencies in the peripheral blood that are higher than generally attained by current immunization regimens and are consistent with levels predicted by murine tumor therapy models to be required to mediate tumor elimination (2, 3).
In this study of adoptive therapy, we target the T cell-defined melanoma antigens MART1/Melan-A (4, 5) and gp100 (6), which belong to a group of tumor-associated differentiation antigens expressed by normal melanocytes. Targeting such self proteins overexpressed by malignant cells represents an increasingly important paradigm in tumor immunology (7) but has potential limitations, including the fact that normal tissues may be recognized and injured. We report results of a phase I study of adoptive T cell therapy using tumor-reactive CD8+ T cell clones generated from patients with metastatic melanoma.
Materials and Methods
Generation of MART-1 and gp100-Specific CD8+ T Cell Clones from Peripheral Blood of Patients with Metastatic Melanoma.
All studies using human subjects received prior approval by Institutional Review at the Fred Hutchinson Cancer Research Center. After informed consent, peripheral blood mononuclear cells (PBMCs) were obtained and antigen-specific cytotoxic T lymphocytes (CTLs) were generated as described by using autologous dendritic cells pulsed with the A2-restricted peptide epitope of MART-1 (M27: AAGIGILTV) or gp100 (G154: KTWGQYWQV) (8–10). After three cycles of stimulation at weekly intervals, T cells were cloned by and expanded for in vitro testing. CTL clones demonstrating specific lysis of antigen-positive tumor targets in a chromium release assay were selected. Clones were expanded in 14-day cycles by using anti-CD3 antibody (OKT3, Orthoclone; Ortho Biotech, Raritan, NJ) at 30 ng/ml, irradiated allogeneic PBMCs, at 106 cells/ml, irradiated allogeneic lymphoblastoid cell lines (2 × 105 cells/ml), and serial IL-2 (aldesleukin; Chiron) at 25–50 units/ml every 2–3 days (11). All clones were characterized as CD3+, CD4−, CD8+ and expressed the high-affinity IL-2 receptor (CD25) after antigen stimulation.
Adoptive T Cell Therapy.
Ten patients were enrolled in a phase I study of adoptive T cell therapy. All patients met the following entry criteria: histopathologic diagnosis of metastatic (stage IV) melanoma, age <75, no evidence of CNS metastasis and no clinically significant cardiac, hepatic, or pulmonary dysfunction. All patients expressed HLA-A2.
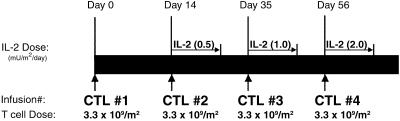
A total of four T cell infusions was planned, the first without low-dose IL-2 and subsequent infusions (second, third, and fourth) coadministered with increasing doses of s.c. IL-2 (0.25, 0.5, and 1.0 × 106 units/m2 twice daily for 14 days; Fig. 1). Patients were monitored closely by physical examination and serum chemistries for evidence of toxicity. Stopping rules included the appearance of serious (≥grade III toxicity by National Cancer Institute common toxicity criteria). The dose of IL-2 used is sufficient to saturate high-affinity IL-2 receptors in vivo and, when administered alone, has no anti-melanoma effect and minimal toxicity (12, 13).
Fig 1.
Treatment schedule. Four infusions of antigen-specific CD8+ CTL clones are scheduled at 2-wk intervals between the first and second infusion, and at 3-wk intervals for subsequent infusions given with a 14-day course of low-dose IL-2. The IL-2 is administered s.c., twice daily for 14 days at 0.50, 1.0, and 2.0 million units/m2/day for the second, third, and fourth infusions, respectively.
Construction of Peptide–MHC Tetramers.
Tetramers were made according to the protocol of Altman et al. (14). In brief, human β2-microglobulin and the soluble domain of the HLA-A2 heavy chain linked at its COOH terminus to a BirA substrate peptide were expressed in Escherichia coli and refolded together in vitro in the presence of peptide and biotinylated. After purification, tetrameric complexes were produced by mixing biotinylated heterodimer with NeutrAvidin–PE (Molecular Probes) at a molar ratio of 4:1. Tetramers presenting immunogenic epitopes of MART-1 (M27:AAGIGILTV), tyrosinase (T368:YMDGTMSQV), and gp100 (G154:KTWGQYWQV) were constructed.
Analysis of Antigen-Specific T Cell Frequency in the Peripheral Blood and Tissue Samples Using Peptide–MHC Tetramers.
To evaluate in vivo persistence of T cells in the peripheral blood, PBMCs were prepared from samples drawn on days 0 (preinfusion), 1, 7, 14, and 21 after each infusion and cryopreserved so all samples could be analyzed simultaneously. At the completion of the study, these samples were thawed, stained for 40 min at 22°C with peptide–MHC tetramer-PE (25–50 μg/ml), anti-CD8-FITC, and “dump” antibodies. Flow cytometric analysis was carried out on a minimum of 50,000 cells. The frequency of antigen–specific CTLs is presented as a fraction of tetramer-positive, CD8+ lymphocytes over the total number of CD8+ cells. This method detects with high specificity a frequency of antigen-specific T cells as low as 0.05% CD8+ cells (9, 15, 16). The median survival of transferred T cells is defined as the day on which one-half maximal frequency of tetramer+ T cells persists.
Immunohistochemical Analysis of Antigen Expression in Skin and Tumor Biopsies.
Staining for gp100, MART-1, and tyrosinase was performed by a peroxidase-labeled streptavidin–biotin method on an automated stainer (Ventana Medical Systems, Tucson, AZ). Paraffin tissue sections were mounted on aminoalkylsilane-treated glass slides and heat treated to optimize antigen retrieval. Immunohistochemical staining involved the sequential application of primary antibody, biotinylated goat secondary antibodies, and then peroxidase-labeled streptavidin. The antigens were visualized by incubation with aminoethylcarbazole substrate in the presence of hydrogen peroxide. Nonimmune mouse IgG in place of specific antibody was used for negative control sections.
Results
Study Patients.
A total of 43 T cell infusions were administered to 10 patients with metastatic melanoma (Table 1). Five patients received therapy with MART-1-specific CTLs (22 infusions total), and five received gp100-specific CTLs (21 infusions total). All 10 patients presented with stage IV disease. Patients were enrolled and underwent leukapheresis before beginning conventional therapy (chemotherapy, biochemotherapy, or high-dose IFN) for treatment of metastatic disease so that T cell clones could be generated in vitro and cryopreserved until needed. Only after failing conventional therapy (as documented by disease progression) did patients proceed to adoptive T cell therapy.
Table 1.
Patient demographics and clinical summary
| ID no.
|
Age
|
Sex
|
Pretreatment status | Target antigen
|
No. of infusions | Toxicity
|
Response | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Previous Tx | Disease sites | No IL-2 | +IL-2 | Type | Duration | |||||
| 1017-1 | 45 | F | IFN | Skin, LN | MART1 | 4 | 3 | F, M | Stable disease | 21.3 |
| 1017-2 | 46 | F | Chemo | Lu, LN, CW | MART1 | 1 | 3 | F, M, R | Minor | 2.0 |
| 1017-3 | 50 | F | Bio-chemo, IFN | Lu, liver | MART1 | 1 | 3 | None | Progressive disease | — |
| 1017-4 | 55 | M | IFN | Lu | gp100 | 1 | 3 | F, M | Stable disease | 3.6 |
| 1017-5 | 56 | F | Chemo | Skin, LN | MART1 | 1 | 2 | F, M | Stable disease | 15.2 |
| 1017-6 | 53 | F | Bio-chemo | Lu | gp100 | 1 | 3 | F, M | Mixed∥ | 6.8 |
| 1017-7 | 50 | M | Bio-chemo | Lu, LN | gp100 | 1 | 3 | F | Stable disease | 14.7 |
| 1017-8 | 47 | F | Chemo, IFN | Lu, BrPI | MART1 | 1 | 3 | F, M | Minor | 15.3 |
| 1017-9 | 59 | M | Bio-chemo | Lu, liver | gp100 | 1 | 5 | F, M | Stable disease | 7.0 |
| 1017-10 | 38 | F | Bio-chemo | Lu, liver | gp100 | 1 | 2 | F | Progressive disease | — |
| 49.9 | 13 | 30 | 10.9 | |||||||
The age, gender, pretreatment status, T cell regimen, toxicity, and clinical response following adoptive T cell therapy are summarized in Table 1. Overall, 43 infusions were administered to 10 patients with an average age of 50 (38–59). All patients presented with progressive disease following conventional therapy. T cell-related toxicity was limited to fever and myalgias and a skin rash in one patient. Eight of 10 patients experienced a minor, mixed, or stable response, with a median duration of 11 mo (2–21 mo).
Chemo, chemotherapy; IFN, high-dose interferon.
Lu, lung; LN, lymph nodes; CW, chest wall; BrPI, brachial plexus.
F, fever; M, myalgia; R, rash.
Duration in months.
Decreased LN disease.
∥ Mixed response in lung nodules.
Regain of brachial function, decreased/absent PET activity in disease site.
Safety of Adoptively Transferred Melanocyte Antigen-Specific CD8+ T Cells.
Patients were monitored for signs and symptoms of toxicity, including autoimmunity, because MART1/MelanA and gp100 may be expressed in normal tissues such as melanocytes. No grade III or IV toxicity was observed (National Cancer Institute common toxicity criteria; http://ctep.cancer.gov/reporting/ctc.html). Nine of 10 patients experienced myalgias, fever, and fatigue, which began 4–6 h after T cell infusion and resolved spontaneously within 72 h, symptoms consistent with a cytokine release syndrome.
One patient, 1017-2, developed a targetoid erythematous skin rash surrounding pigmented areas of skin on day 5 after an infusion of MART1-specific CTLs and low-dose IL-2. Detailed analysis of this rash has been previously reported documenting the accumulation of adoptively transferred CTLs accompanied by destruction of MART1-positive melanocytes (9).
Assessment of in Vivo Frequency of Transferred T Cells.
The in vivo median survival of the transferred cells was determined by measuring the frequency of transferred CTLs in serial samples of peripheral blood of treated patients by using peptide MHC tetramers. To affirm the sensitivity, and reproducibility of this approach, the CTL frequency was also determined in the initial 3 patients, by another structure-based assay, semiquantitative PCR with clone-specific primers designed within the hyervariable CDR3 region of the T cell receptor of the infused clone. When either method was used, the T cell frequencies and the duration of in vivo persistence represented by T cell median survival were nearly identical (e.g., 6.66 vs. 6.84 days, Fig. 2).
Fig 2.
Analysis of T cell frequency in PBMCs after infusion of MART1-specific CD8+ T cell clones without IL-2. Two methods were used: semiquantitative PCR analysis of a clone-specific CDR3 region (gel bands digitized and quantified based on dilution standards 10−2 to 10−5) and tetramer analysis by flow cytometry. The T cell frequency in both cases was characterized by a rapid rise on day +1 after the infusion followed by a steady decline to nearly undetectable levels by day +14 and a median T cell survival in each case of ≈7 days.
In Vivo Frequency and Persistence of Infused CTL Clones Either Administered Alone or Followed by Low-Dose IL-2.
In our initial study (protocol 1017.00) of 4 patients who received MART-1-specific CD8+ CTL clones alone, the median survival was ≈6.7 days (data not shown). To improve the in vivo persistence of transferred CD8+ T cells, increasing doses of IL-2 (0.0, 0.5, 1.0, and 2.0 million units IL-2/m2/day) were coadministered with the second, third, and fourth infusions (protocol 1017.01, Materials and Methods).
Among 10 patients treated in this study, analysis of a total of 13 infusions given without IL-2 demonstrated a pattern of T cell survival that was characterized by undetectable levels preinfusion and a peak T cell frequency on day 1 after infusion, comprising on average 1.47% of total peripheral blood CD8+ cells (range 0.64–2.0). This increase was followed by a steady decline to 0.48% (range 0.32–0.76) by day 7 and <0.01% by day 14. The median survival of CD8+ T cells given without IL-2 was 6.68 ± 0.93 days.
Analysis of infusion no. 2 among the 10 patients, which was followed by 14 days of IL-2 (0.5 million units/m2/day), demonstrated a similar peak on day 1 to an average of 1.52% of CD8+ cells, but, in contrast to T cells administered without IL-2, this frequency was sustained at an average of 0.97% until day 14, before falling to <0.01% by day 21.
With higher doses of IL-2 (infusions no. 3 and no. 4 administered with 1.0 and 2.0 million units/m2/day), no further increase in peak T cell frequency or median survival over that seen with the lowest dose of IL-2 was observed. Overall, analysis of the 30 infusions given with low-dose IL-2 (0.5, 1.0, and 2.0 million units/m2/day) demonstrated a median survival of CD8+ T cell clones of 16.92 ± 1.37 days (Table 2). Compared with the median survival of infusions given without IL-2, the coadministration of low-dose IL-2 significantly prolonged T cell survival in vivo (P < 0.001 by paired t test analysis). A representative frequency analysis is shown in Fig. 3 for a T cell clone infused alone initially and then followed by 0.5 million units/m2/day of IL-2.
Table 2.
Analysis of 43 T cell infusions in 10 patients (1017-1 to 1017-10)
| Patient
|
Infusion no.
|
Median T cell survival | |
|---|---|---|---|
| Without IL-2 | With IL-2 | ||
| 1017-1 | 1 | 5.3 | |
| 2 | 6 | ||
| 3 | 6.9 | ||
| 4 | 7.4 | ||
| 5 | 19 | ||
| 6 | 18.4 | ||
| 7 | 15.3 | ||
| 1017-2 | 1 | 6.6 | |
| 2 | 15.6 | ||
| 3 | 16 | ||
| 4 | 17.7 | ||
| 1017-3 | 1 | 5.9 | |
| 2 | 17.2 | ||
| 3 | 16.8 | ||
| 4 | 16.4 | ||
| 1017-4 | 1 | 7.2 | |
| 2 | 15.9 | ||
| 3 | 18.3 | ||
| 4 | 17.8 | ||
| 1017-5 | 1 | 7.3 | |
| 2 | 18.3 | ||
| 3 | 16.7 | ||
| 1017-6 | 1 | 6.3 | |
| 2 | 14.5 | ||
| 3 | 17.6 | ||
| 4 | 18.4 | ||
| 1017-7 | 1 | 8.2 | |
| 2 | 16.5 | ||
| 3 | 17.7 | ||
| 4 | 19.2 | ||
| 1017-8 | 1 | 6 | |
| 2 | 14.3 | ||
| 3 | 16.9 | ||
| 4 | 15.1 | ||
| 1017-9 | 1 | 5.6 | |
| 2 | 18.5 | ||
| 3 | 15.2 | ||
| 4 | 16.5 | ||
| 5 | 15.7 | ||
| 6 | 17.4 | ||
| 1017-10 | 1 | 8.1 | |
| 2 | 16.1 | ||
| 3 | 18.7 | ||
| No. of infusions | 13 | 30 | |
| Avg. median survival | 6.68 | 16.92 | |
| SD | 0.93 | 1.37 | |
Median T cell survival in vivo as determined by tetramer analysis for infusions without IL-2 and with low-dose IL-2. IL-2 doses of 250,000, 500,000, and 1,000,000 units/m2 were administered for 14 days, with infusions 2, 3, and 4, respectively (note exception for patient 1017-1).
Patient 1017-1 was enrolled on the modified protocol using low-dose IL-2 after four infusions without IL-2.
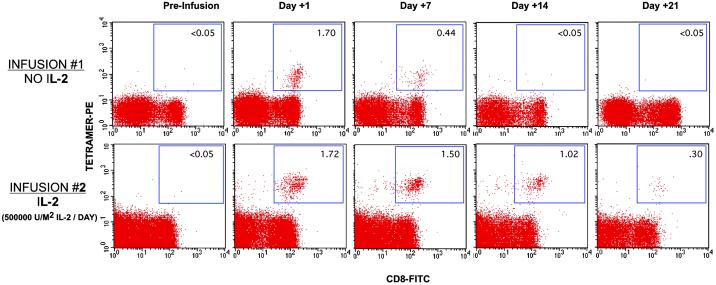
Fig 3.
Analysis of T cell persistence in vivo after infusion of MART1-specific CD8+ T cell clones without IL-2 (INFUSION #1) and with IL-2 (INFUSION #2). Peripheral blood samples from patient 1017-6 were analyzed pre-T cell infusion (day 0), days 1, 7, 14, and 21 after T cell infusion without IL-2 (INFUSION #1) and with low-dose IL-2 (INFUSION #2). Percentage of tetramer+, CD8+ T cells over total CD8+ T cells is displayed in the right upper quadrant. Sustained elevation in the frequency of MART1-tetramer+ T cells in the peripheral blood to at least day 14 (1.02 vs. <0.05%) is observed when low-dose IL-2 is given (INFUSION #2) compared with infusion of T cells when no IL-2 is given (INFUSION #1).
Migration of Adoptively Transferred T Cells to Sites of Tumor.
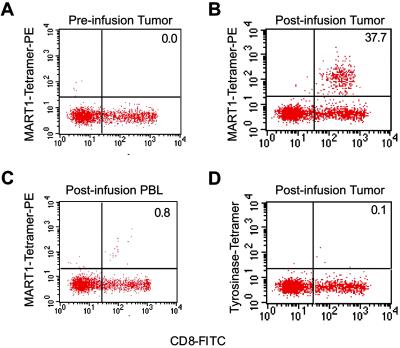
To determine whether transferred T cells accumulated at sites of disease, tumor samples (biopsies or aspirates) were obtained from 3 patients (1017-1, 1017-2, and 1017-9) who had accessible tumor sites both before and shortly after T cell infusions. Representative results are shown for a core biopsy of a chest wall/axillary mass from patient 1017-2 preinfusion and 3 days postinfusion of MART1-specific CTL (Fig. 4). In the preinfusion biopsy, minimal lymphocytic infiltration was present. Of this infiltration, <5% were CD8+ cells, and none were MART1-peptide tetramer+ (Fig. 4A). By contrast, in the postinfusion sample, MART-1-specific CD8+ T cell clones were found by tetramer staining to comprise 37.7% of infiltrating CD8+ cells (Fig. 4B). Given a simultaneous peripheral MART-1specific CTL frequency of 0.8% (Fig. 4C), these results are consistent with preferential localization of CTLs to tumor. The identity of these tetramer+ CD8+ T cells with the infused clone was confirmed by comparing the sequence of the CDR3 region of the sorted tetramer+ T cells in the tumor sample with that of the infused clone.
Fig 4.
Analysis of T cell infiltrate from tumor nodule harvested from patient 1017-2 after infusion of MART1-specific CD8+ T cell clones. Percentage of infused clone (tetramer+, CD8+ T cells) among all CD8+ T cells is displayed in the upper right quadrant. Significant infiltrate comprised of MART1-specific CD8+ T cells is seen in postinfusion tumor nodule (37.7%; A) compared with preinfusion nodule (0.0%; B) and postinfusion peripheral blood (0.8%; C) demonstrating preferential localization of infused clones to tumor site. Less than 0.2% of CD8 T cells in tumor nodule stain with an irrelevant tetramer (tyrosinase-peptide tetramer; D).
Clinical and Radiographic Response.
All patients in this study had, at the time of the first T cell infusion, progressive metastatic disease refractory to conventional therapy. Adoptive T cell therapy resulted in disease stabilization in 5 of 10 patients and minor or mixed responses in an additional 3 patients for periods up to 21 months (Table 1). Patient 1017-8, who experienced a minor response, presented with a progressive right brachial plexopathy secondary to metastasis, which manifested as right arm pain and inability to abduct the arm above shoulder level. After the second T cell infusion, these symptoms completely resolved and full range of motion without pain was restored. Patient 1017-1, whose pulmonary lesions stabilized after T cell therapy, returned to 100% functional status from 70% at time of study entry and full-time work as a nurse for a period of 1 year after treatment. Patient 1017-6 demonstrated a mixed response characterized by resolution of multiple pulmonary nodules and the appearance of a single new nodule on computed tomography (CT) scan (Fig. 5A). Patient 1017-2 experienced a minor response with a postinfusion decrease in paratracheal lymphadenopathy (Fig. 5B). Historically, patients presenting with advanced melanoma failing conventional therapy experience continued progressive disease and a median survival duration of 4 mo or less. Although the number of patients in this study is small, the outcomes suggest a favorable progression-free duration (Table 1).
Fig 5.
Radiographic response in metastatic lesions after adoptive T cell therapy. Two peripheral pulmonary nodules in the left lower lobe present pre-T cell therapy in patient 1017-6 are absent after the fourth infusion of MART1-specific T cell clones (A). However, a new 1+ cm nodule appears more in the lobe during this interval, demonstrating a mixed response to therapy. In patient 1017-2 (B), partial resolution of paratracheal adenopathy is observed after the fourth infusion of MART1-specific T cells.
Appearance of Antigen-Loss Variants After Adoptive T Cell Therapy.
Recurrent or residual s.c. tumor nodules were analyzed for expression of melanocytic antigens and compared with preinfusion tumor biopsies. If possible, the identical nodule was assayed pre- and postinfusion by fine needle aspiration or core needle biopsy to eliminate heterogeneity of antigen expression among different nodules as an explanation for observed differences. Tumors from a total of 5 patients were analyzed.
In three of the five patients studied, preinfusion tumor immunostaining demonstrated moderately intense expression of all three antigens (gp100, tyrosinase, and MART1), but the postinfusion relapsing or residual nodules revealed selective loss of the targeted antigen. A representative case is presented in Fig. 6. In patient 1017-2, the preinfusion core biopsy demonstrated expression of gp100, tyrosinase, and MART1. A repeat core biopsy of recurrent chest wall tumor obtained 3 wk after the final T cell infusion demonstrated selective loss of MART1 expression with retained expression of gp100 and tyrosinase. HLA-A2 expression, as determined by flow cytometric staining of single cell suspensions, in all postinfusion tumor specimens (including those without evidence of antigen-loss tumor variants), was also preserved, demonstrating that immune escape was not due to loss of the restricting HLA allele.
Fig 6.
Immunohistochemical analysis of antigen expression in preinfusion tumor and recurrent postinfusion tumor nodule appearing after MART1-specific T cell therapy. Gp100, tyrosinase, and MART1 expression are seen in the tumor sample preinfusion. Selective loss of MART1 expression is seen in postinfusion tumor cells, demonstrating the outgrowth of antigen-loss tumor variant.
Discussion
Adoptive therapy using ex vivo expanded T cell clones provides a means of treating patients with cancer by augmenting the antigen-specific immune response. This phase I study represents a demonstration that, after adoptive transfer, autologous antigen-specific CD8+ T cell clones targeting tumor-associated self proteins retain IL-2 responsiveness in vivo, traffic to tumor sites, and effect a clinical response in patients with advanced disease. In this study, T cells recognizing the tumor-associated melanoma antigens MART-1 and gp100 consistently attained frequencies of between 0.5% and 2.2% (of all CD8 T cells) after adoptive therapy. By comparison, peptide-based vaccine strategies generally achieved maximal frequencies of between 0 and 0.3% (18–20). In contrast to vaccine studies, where the magnitude and tumor avidity of the induced T cell response can be unpredictable, in adoptive T cell therapy, T cell clones can be selected on the basis of tumor-reactivity and a uniform population of T cells expanded ex vivo to desired magnitude (up to several billion in number) as shown in this study and others (21). Despite high frequencies of high avidity MART1 and gp100-specific CTLs in the peripheral blood, no serious toxicity was elicited in any patient after adoptive therapy.
In this study, the in vivo persistence of transferred CD8+ CTLs was prolonged by low-dose IL-2. In contrast to tumor-infiltrating lymphocyte (TIL) or lymphokine-activated killer cell (LAK) therapy, in which pharmacologic doses of IL-2 (up to 120 times higher) have been required to support the growth of infused cells, transferred antigen-specific CD8+ T cell clones used in this study respond to the lowest doses of IL-2 administered (0.5 million units/m2/day), thus avoiding serious toxicities associated with high-dose IL–2, such as vascular leak syndrome (22). Additionally, the use of peptide MHC tetramers in this study to track antigen-specific T cells allowed us to document preferential accumulation of antigen-specific T cells at tumor sites and to confirm the identity of these infiltrating cells as the infused clones based on their clone-specific CDR3 regions, in contrast to previous studies of vaccine-based therapies, where lymphocytic infiltration has been observed but the antigen-specificity of infiltrating cells was not well-defined (20, 23).
In part, the effectiveness of adoptive therapy as a treatment strategy may depend on generating and administering antigen-specific T cells in ways that mimic physiologic conditions to preserve normal function. In this study, antigen-specific T cell clones generated in vitro by cyclical stimulation with autologous dendritic cells and very low dose IL-2 retain responsiveness to low-dose IL-2 in vivo and the capacity to traffic to tumor sites. By contrast, two recent studies, using T cells generated by using xenogeneic stimulators (24) or exposed to pharmacologic doses of IL-2 (25), failed to demonstrate in vivo persistence or preferential localization of T cells to tumor sites (24, 25).
Historically, patients with progressive refractory metastatic disease experience a median survival of 4 mo or less (26). In this study, adoptive T cell therapy resulting in minor, mixed, or stable responses, for an average response duration of 11 mo, and as long as 21 mo, provides potential evidence of significant antitumor effects and meaningful clinical responses (27). Although infused T cells were not detectable in the peripheral blood by day 21, effector memory T cells have been shown to reside in extravascular sites (28, 29) where they may provide continued immunoprotection.
The appearance of antigen-loss variants has been sporadically reported (17, 20, 23, 30). In our study, a greater prevalence of this finding (in three of five patients studied) may have been due to the consistently high frequencies of tumor-reactive CD8+ CTLs achieved in all patients. These results demonstrating selective loss of the targeted antigen with continued expression of other melanosomal antigens suggest that expression of these proteins is not necessarily linked and support the rationale for directing therapy against multiple target antigens simultaneously.
In summary, detailed immunologic and clinical monitoring in a trial of adoptive T cell therapy has demonstrated that infused T cell clones targeting melanoma-associated antigens is safe, and that such clones persist in vivo, respond to very low-dose IL-2, traffic to tumor sites, mediate antigen-specific immunity, and can result in favorable clinical responses. Whereas the results of this study support the use of adoptively transferred T cells as a strategy for the treatment of melanoma, they also elucidate potential obstacles to more effective therapy and may be used to establish requirements for antigen-specific immunotherapy in general by defining the desired response quantitatively and validating the safety and efficacy of targeting potential tumor rejection antigens.
Acknowledgments
We acknowledge the clinical assistance of Drs. Philip Gold and Daniel Markowitz and the invaluable technical assistance of Karla Kenyon, Justyna Tarnawska, J. Zachary Reilly, and Jianhong Cao. These studies were performed with the assistance of the Clinical Research Center, University of Washington Medical Center, Seattle, WA. This work was supported by the Cancer Research Institute, the National Cancer Institute, and the Damon Runyon–Walter Winchell Cancer Research Foundation.
Abbreviations
CTL, cytotoxic T lymphocyte
PBMC, peripheral blood mononuclear cell
See commentary on page 15840.
References
- 1.Van den Eynde B. J. & van der Bruggen, P. (1997) Curr. Opin. Immunol. 9, 684-693. [DOI] [PubMed] [Google Scholar]
- 2.Yee C., Riddell, S. R. & Greenberg, P. D. (1997) Curr. Opin. Immunol. 9, 702-708. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg P. D. (1991) Adv. Immunol. 49, 281-355. [DOI] [PubMed] [Google Scholar]
- 4.Coulie P. G., Brichard, V., Van Pel, A., Wolfel, T., Schneider, J., Traversari, C., Mattei, S., De Plaen, E., Lurquin, C. & Szikora, J. P. (1994) J. Exp. Med. 180, 35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakami Y., Eliyahu, S., Delgado, C. H., Robbins, P. F., Rivoltini, L., Topalian, S. L., Miki, T. & Rosenberg, S. A. (1994) Proc. Natl. Acad. Sci. USA 91, 3515-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawakami Y., Eliyahu, S., Delgado, C. H., Robbins, P. F., Sakaguchi, K., Appella, E., Yannelli, J. R., Adema, G. J., Miki, T. & Rosenberg, S. A. (1994) Proc. Natl. Acad. Sci. USA 91, 6458-6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houghton A. N., Gold, J. S. & Blachere, N. E. (2001) Curr. Opin. Immunol. 13, 134-140. [DOI] [PubMed] [Google Scholar]
- 8.Yee C., Savage, P. A., Lee, P. P., Davis, M. M. & Greenberg, P. D. (1999) J. Immunol. 162, 2227-2234. [PubMed] [Google Scholar]
- 9.Yee C., Thompson, J. A., Roche, P., Byrd, D. R., Lee, P. P., Piepkorn, M., Kenyon, K., Davis, M. M., Riddell, S. R. & Greenberg, P. D. (2000) J. Exp. Med. 192, 1637-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai V., Southwood, S., Sidney, J., Sakaguchi, K., Kawakami, Y., Appella, E., Sette, A. & Celis, E. (1997) J. Immunol. 158, 1796-1802. [PubMed] [Google Scholar]
- 11.Riddell S. R. & Greenberg, P. D. (1990) J. Immunol. Methods 128, 189-201. [DOI] [PubMed] [Google Scholar]
- 12.Vlasveld L. T., Horenblas, S., Hekman, A., Hilton, A. M., Dubbelman, A. C., Melief, C. J. & Rankin, E. M. (1994) Ann. Oncol. 5, 179-181. [DOI] [PubMed] [Google Scholar]
- 13.Atzpodien J., Korfer, A., Evers, P., Franks, C. R., Knuver-Hopf, J., Lopez-Hanninen, E., Fischer, M., Mohr, H., Dallmann, I., Hadam, M., et al. (1990) Mol. Biother. 2, 18-26. [PubMed] [Google Scholar]
- 14.Altman J. D., Moss, P. A. H., Goulder, P. J. R., Barouch, D. H., McHeyzer-Williams, M. G., Bell, J. I., McMichael, A. J. & Davis, M. M. (1996) Science 274, 94-96. [DOI] [PubMed] [Google Scholar]
- 15.Lee P. P., Yee, C., Savage, P. A., Fong, L., Brockstedt, D., Weber, J. S., Johnson, D., Swetter, S., Thompson, J., Greenberg, P. D., Roederer, M. & Davis, M. M. (1999) Nat. Med. 5, 677-685. [DOI] [PubMed] [Google Scholar]
- 16.Ogg G. S. & McMichael, A. J. (1998) Curr. Opin. Immunol. 10, 393-396. [DOI] [PubMed] [Google Scholar]
- 17.Maeurer M. J., Gollin, S. M., Martin, D., Swaney, W., Bryant, J., Castelli, C., Robbins, P., Parmiani, G., Storkus, W. J. & Lotze, M. T. (1996) J. Clin. Invest. 98, 1633-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds S. R., Celis, E., Sette, A., Oratz, R., Shapiro, R. L., Johnston, D., Fotino, M. & Bystryn, J. C. (1998) J. Immunol. 161, 6970-6976. [PubMed] [Google Scholar]
- 19.Rosenberg S. A., Yang, J. C., Schwartzentruber, D. J., Hwu, P., Marincola, F. M., Topalian, S. L., Restifo, N. P., Dudley, M. E., Schwarz, S. L., Spiess, P. J., et al. (1998) Nat. Med. 4, 321-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurner B., Haendle, I., Roder, C., Dieckmann, D., Keikavoussi, P., Jonuleit, H., Bender, A., Maczek, C., Schreiner, D., von den Driesch, P., et al. (1999) J. Exp. Med. 190, 1669-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maus M. V., Thomas, A. K., Leonard, D. G., Allman, D., Addya, K., Schlienger, K., Riley, J. L. & June, C. H. (2002) Nat. Biotechnol. 20, 143-148. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg S. A., Lotze, M. T., Muul, L. M., Leitman, S., Chang, A. E., Ettinghausen, S. E., Matory, Y. L., Skibber, J. M., Shiloni, E., Vetto, J. T., et al. (1985) N. Engl. J. Med. 313, 1485-1492. [DOI] [PubMed] [Google Scholar]
- 23.Jager E., Gnjatic, S., Nagata, Y., Stockert, E., Jager, D., Karbach, J., Neumann, A., Rieckenberg, J., Chen, Y. T., Ritter, G., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 12198-12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell M. S., Darrah, D., Yeung, D., Halpern, S., Wallace, A., Voland, J., Jones, V. & Kan-Mitchell, J. (2002) J. Clin. Oncol. 20, 1075-1086. [DOI] [PubMed] [Google Scholar]
- 25.Dudley M. E., Wunderlich, J., Nishimura, M. I., Yu, D., Yang, J. C., Topalian, S. L., Schwartzentruber, D. J., Hwu, P., Marincola, F. M., Sherry, R., et al. (2001) J. Immunother. 24, 363-373. [DOI] [PubMed] [Google Scholar]
- 26.Balch C. M., Houghton, A. N., Sober, A. J. & Soong, S., (1998) Cutaneous Melanoma (Quality Medical Publishing, St. Louis).
- 27.Simon R. M., Steinberg, S. M., Hamilton, M., Hildesheim, A., Khleif, S., Kwak, L. W., Mackall, C. L., Schlom, J., Topalian, S. L. & Berzofsky, J. A. (2001) J. Clin. Oncol. 19, 1848-1854. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. (1999) Nature 401, 708-712. [DOI] [PubMed] [Google Scholar]
- 29.Masopust D., Vezys, V., Marzo, A. L. & Lefrancois, L. (2001) Science 291, 2413-2417. [DOI] [PubMed] [Google Scholar]
- 30.Jager E., Ringhoffer, M., Altmannsberger, M., Arand, M., Karbach, J., Jager, D., Oesch, F. & Knuth, A. (1997) Int. J. Cancer 71, 142-147. [DOI] [PubMed] [Google Scholar]