Abstract
Although signal transducer and activator of transcription 1 (STAT1) is an essential signaling molecule in many IFN-α-regulated processes, some biological responses to IFN-α can occur independently of STAT1. To establish the role of STAT1 in mediating the biological actions of IFN-α in the CNS, transgenic mice [termed glial fibrillary acidic protein (GFAP)-IFN-α] with astrocyte production of IFN-α were bred to be null for the STAT1 gene. Surprisingly, GFAP-IFN-α mice deficient for STAT1 developed earlier onset and more severe neurological disease with increased lethality compared with GFAP-IFN-α mice sufficient for STAT1. Whereas the brain of 2- to 3-month-old GFAP-IFN-α mice showed little, if any abnormality, the brain from GFAP-IFN-α mice deficient for STAT1 had severe neurodegeneration, inflammation, calcification with increased apoptosis, and glial activation. However, the cerebral expression of a number of IFN-regulated STAT1-dependent genes increased in GFAP-IFN-α mice but was reduced markedly in GFAP-IFN-α STAT1-null mice. Of many other signaling molecules examined, STAT3 alone was activated significantly in the brain of GFAP-IFN-α STAT1-null mice. Thus, in the absence of STAT1, alternative signaling pathways mediate pathophysiological actions of IFN-α in the living brain, giving rise to severe encephalopathy. Finally, STAT1 or a downstream component of the JAK/STAT pathway may protect against such IFN-α-mediated injury in the CNS.
IFN-α has a critical role in antiviral and antitumor protection and immune regulation (1, 2). This cytokine also is implicated in the pathogenesis of certain clinical disorders such as NeuroAIDS (3), the familial neurological disorder Aicardi–Goutières syndrome (4), and insulin-dependent diabetes mellitus (5). IFN-α therapy often is associated with significant adverse side effects including neuropsychiatric disorder (6), myasthenia gravis (7), insulin-dependent diabetes mellitus (8), and autoimmune hepatitis (9). That IFN-α may be a causal factor in disease is supported by studies in transgenic mice with tissue-specific targeting of IFN-α gene expression to brain (10, 11), pancreatic islet beta cells (12), and testes (13), which develop tissue degeneration, inflammation, and calcification leading to neurological disease, insulin-dependent diabetes mellitus, and sterility, respectively. The precise mechanisms that underlie IFN-α-mediated disease pathogenesis in these different tissues currently are unknown.
How IFN-α modulates cellular gene expression is well described and involves the activation of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) tyrosine protein kinase pathway (14, 15). Binding of IFN-α to its receptor triggers activation of receptor associated Tyk2 and JAK1 kinases, which then phosphorylate STAT2 and STAT1 sequentially, leading to the formation of a STAT1/STAT2 heterodimer. This STAT heterodimer translocates to the nucleus and subsequently associates further with IFN regulatory factor (IRF)-9 to form a complex termed IFN-stimulated gene factor 3 (ISGF3). Finally, ISGF3 interacts with a specific DNA motif (termed the IFN-stimulated response element or ISRE) present in the 5′ regulatory region of IFN-regulated target genes, thus modulating their transcriptional activity. Consistent with the importance of this pathway in mediating the actions of IFN-α, mice that lack either STAT1 (16, 17) or STAT2 (18) have impaired IFN-regulated, ISGF3-dependent gene expression and are highly sensitive to viral infection. However, despite the central role of the JAK/STAT pathway in mediating IFN-dependent gene regulation, there is accumulating evidence showing that IFN-α can control certain cellular functions, e.g., cell proliferation, in a STAT1-independent fashion (19–21). Moreover, IFN-α-regulated, STAT1-independent signaling pathways are physiologically important because STAT1-null mice are more resistant to infection with viruses than are mice lacking expression of both the IFN-α/β and IFN-γ receptors (21).
Notwithstanding the studies noted above, very little is known concerning the role of IFN-α-regulated, STAT1-independent signaling in mediating biological responses directly to IFN-α in vivo. Therefore, here we asked, what is the role of STAT1 in mediating the biological actions of IFN-α in the CNS in vivo? This question was addressed in transgenic mice (termed glial fibrillary acidic protein or GFAP-IFN-α; refs. 10 and 11) with astrocyte production of IFN-α that were bred to be null for the STAT1 gene. The results demonstrated, surprisingly, that GFAP-IFN-α mice deficient for STAT1, despite compromised expression of known ISGF3-regulated genes in the brain, have more severe and earlier onset of inflammatory encephalopathy. Thus, alternative STAT1-independent signaling pathways can mediate pathophysiological actions of IFN-α, which, in the living brain, produce severe neurological disease. Moreover, these findings suggest that STAT1 or a downstream component of the JAK/STAT pathway protects against such IFN-α-mediated injury in the CNS.
Materials and Methods
Animals.
The two lines (termed GIFN12 and GIFN39) of GFAP-IFN-α (strain CB6 F1) transgenic mice (10, 11) and the STAT1-null (strain 129/Sv) mice (16) used in the present study were described previously. GIFN STAT1-null mice were produced by interbreeding, and all genotypes were verified by PCR analysis of tail DNA. Handling of mice and experimental procedures were conducted in accordance with the National Institutes of Health guidelines for animal care and use.
RNase Protection Assay (RPA).
Poly(A)+ RNA was isolated from snap-frozen brain by an oligo(dT) cellulose (Ambion, Austin, TX) method (10), and RPAs were performed and analyzed as described (22). Multiprobe sets used for RPA analysis included cytokine (23), chemokine (22), STAT transcription factors (24), and IFN and IFN-regulated genes (25).
Immunocytochemistry and in Situ Cell Death Staining.
Brains were removed, one hemisphere was fixed overnight in ice-cold 4% (wt/vol) paraformaldehyde in PBS (pH 7.4) and embedded in paraffin, and 8-μm sagittal sections were prepared. For immunophenotyping, the other brain hemisphere was embedded in OCT compound (Sakura Finetek, Torrance, CA) and snap-frozen in liquid nitrogen. Sagittal sections (10 μm) were cut with a cryomicrotome. The following rat mAbs were used: mouse pan-leukocytes (to detect CD45; Becton Dickinson), lymphocytes (CD4, CD8, and B220; Becton Dickinson), neutrophils (7/4; Serotec), and macrophage/microglia (Mac-1; ATCC). For in situ cell death detection, a commercially available kit (Roche Diagnostics) was used according to the manufacturer's instructions. Combined cell death and cell-typing staining was performed with cell marker-specific antibodies to identify astrocytes (anti-GFAP; Dako), neurons (anti-neurofilament; Sternberger–Meyer, Jarrettsville, MD), and cells of the monocyte/macrophage lineage (lectin from Lycopersicon esculentum; Sigma) by using alkaline phosphatase (cell death) and peroxidase (cell type) substrates, respectively.
Preparation and Treatment of Splenic Leukocytes.
Spleens were removed from wild-type or STAT1-null mice, and a single cell suspension was prepared and red blood cells were lysed in hypotonic buffer (0.15 M NH4Cl/1 mM KHCO3/0.1 mM Na2EDTA, pH 7.4). After washing, the cells were resuspended to 4 × 107 viable cells per ml in RPMI medium 1640 (GIBCO/BRL) containing 1% BSA (maintenance medium). For treatment, 4 × 107 viable cells were pelleted by centrifugation and resuspended in maintenance medium plus or minus murine IFN-α (IFN-αA, 1,000 units/ml; PBL, New Brunswick, NJ) and placed at 37°C for 40 min. After incubation, the cells were pelleted by centrifugation and solubilized immediately for immunoblot analysis as described below.
Immunoblot Analysis.
Dissected brain regions or splenic leukocytes were homogenized with 50 mM Tris⋅HCl buffer, pH 7.4, containing 1% Nonidet P-40, 10% (vol/vol) glycerol, 1 mM EDTA, 1 mM Na3VO4, 1 mM NaF, 1 mM DTT, 4.5 mM sodium pyrophosphate, 10 mM β-glycerophosphate, and a protease inhibitor tablet (Roche Diagnostics). Protein lysates were fractionated by SDS/PAGE. Proteins then were transferred to nitrocellulose membranes, blocked with 5% (vol/vol) BSA or 5% nonfat dried milk in 20 mM TBS plus 0.05% Tween-20, and reacted with the following rabbit polyclonal antibodies: STAT2 (Chris Schindler, Columbia University), phospho-STAT3(Tyr-705), phospho-STAT5, phospho-ERK1/2, phospho-SAPK, SAPK, phospho-IκBα, IκBα, phospho-p38MAPK and p38MAPK (Cell Signaling Technology, Beverly, MA), STAT3 (BioSource International, Camarillo, CA), actin and STAT5 (Santa Cruz Biotechnology), ERK1/2 (Sigma), and phospho-CaMPKII and CaMPKII (Upstate Biotechnology, Lake Placid, NY). Bound antibodies were visualized by using peroxidase-conjugated secondary antibody followed by detection with an enhanced chemiluminescence kit (Pierce). For reblotting, the membrane was stripped in buffer [62.5 mM Tris⋅HCl, pH 6.8/2% (vol/vol) SDS/100 mM 2-mercaptoethanol] for 30 min at 50°C.
Results
Marked Exacerbation of Neurological Disorder in GFAP-IFN-α Mice with STAT1 Deficiency.
As noted (10, 11), the transgenic expression of IFN-α in the mouse brain resulted in transgene-dose and age-related neurologic abnormalities (Table 1). In contrast, STAT1-null mice seemed healthy and exhibited no abnormal neurologic signs. However, contrary to expectation, dramatically increased disease severity of earlier onset occurred in GFAP-IFN-α mice with STAT1 deficiency (Table 1). Thus, in the low IFN-α transgene-expressing line GIFN12, STAT1-null animals were, by 1 month of age, moribund, with many surviving for <3 months of age. In contrast, age-matched GIFN12 and GIFN12 mice heterozygous for STAT1 deficiency seemed healthy. The impact of the loss of STAT1 was more dramatic in the higher IFN-α transgene-expressing line, GIFN39. No viable GIFN39 mice were obtained that were STAT1 null, whereas the number of GIFN39 progeny heterozygous for STAT1 deficiency was considerably reduced and viable animals were severely debilitated and died within 4 weeks of their birth. This phenotype of the GIFN STAT1-null mice was not observed when GIFN mice of either line were interbred with wild-type 129/Sv mice (not shown). Thus, the exacerbation of neurological disease in the GIFN STAT1-null mice could not be explained simply by the influence of the genetic background.
Table 1.
Severe neurologic disease in GFAP-IFN-α mice deficient for STAT1
| Genotype | IFN-α | Phenotype | Onset, months |
|---|---|---|---|
| Wild type | — | — | — |
| STAT1−/− | — | — | — |
| GIFN12 | + | + | >8 |
| GIFN12 STAT1−/+ | + | + | >8 |
| GIFN12 STAT1−/− | + | +++ | <1 |
| GIFN39 | ++ | ++ | >1 |
| GIFN39 STAT1−/+ | ND | +++++ | <1 |
| GIFN39 STAT1−/− | ND | No viable mice | |
ND, not done.
Moribund, ataxic, seizure, premature death.
Severe Encephalopathy with Inflammation in GFAP-IFN-α Mice with STAT1 Deficiency.
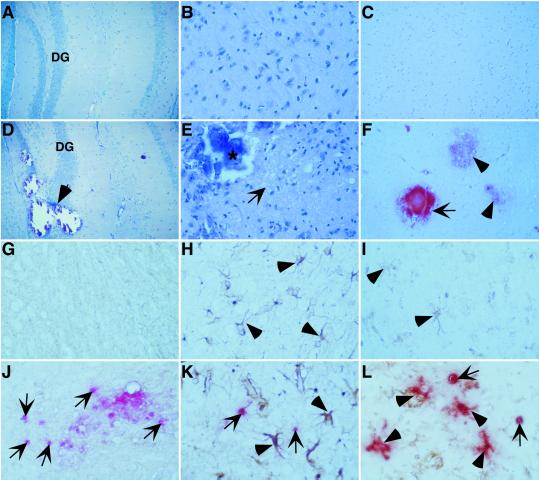
To determine the basis for the debilitating phenotypes exhibited by the GFAP-IFN-α mice deficient for STAT1, routine histological examination of the brain was performed on the different genotypes. In 2- to 3-month-old, wild-type (not shown), STAT1-null (not shown), and GIFN12 (Fig. 1 A and B) mice, the brain seemed normal. By contrast, the brain from similarly aged GIFN12 STAT1-null mice exhibited gross pathological alterations predominantly affecting the hippocampal and ventral and medial forebrain regions (Fig. 1 D and E). In these regions, large mineral deposits were common and produced marked structural damage (Fig. 1D, arrow) with surrounding brain tissue showing a significant loss of neurons (Fig. 1E). Two 1-month-old GIFN39 STAT1 heterozygous mice with severe clinical disease that were available for examination exhibited essentially the same pathologic changes with the exception that these were considerably more severe and widespread throughout the brain and included the cerebellum (not shown). Brain sections from GIFN12 mice were negative when stained with Alizarin red S (Fig. 1C) whereas in GIFN12 STAT1-null mice, extensive staining of the mineral deposits was seen, confirming that these were composed primarily of calcium (Fig. 1F). In GIFN12 mice deficient for STAT1, at higher magnification, numerous cells with the morphological appearance of being apoptotic were visible throughout the brain lesion areas. Apoptosis was examined further by terminal deoxynucleotidyltransferase-mediated dUTP end labeling (TUNEL) staining. Similar to wild-type (not shown) and STAT1-null (not shown) mice, few, if any, TUNEL-positive cells were present in brain from GIFN12 mice (Fig. 1 G–I). However, in GIFN12 STAT1-null mice, numerous TUNEL-positive cells were evident in the lesion areas (Fig. 1 J–L, arrows). Because of low staining, it was not possible to resolve whether any TUNEL-positive cells costained for the neurofilament neuronal marker (Fig. 1J). However, compared with the GIFN12 control (Fig. 1H), astrocytes that were grossly hypertrophied in GIFN12 STAT1-null mice (Fig. 1K, arrowheads) were TUNEL-negative. Similarly, compared with the GIFN12 control (Fig. 1I), microglia in lesion areas of the brain from the GIFN12 STAT1-null mice were hypertrophied and exhibited increased lectin staining; interestingly, many showed diffuse TUNEL staining (Fig. 1L, arrowheads), suggestive of active phagocytosis of TUNEL-positive apoptotic cells. These results indicated that in the absence of STAT1, GFAP-IFN-α mice developed an accelerated and more severe degenerative encephalopathy with calcification, neurodegeneration associated with increased cell death, and gliosis.
Fig 1.
Severe pathological changes in the brain of GIFN12 STAT1-null mice. Brain from 3-month-old GIFN12 (A–C) or GIFN12 STAT1-null (D–F) mice is shown. Luxol fast blue stains (A and D) showed mineral deposits (D, arrow) in the dentate gyrus (DG) region of the hippocampus, causing marked disruption of the dentate granule neurons in the GIFN12 STAT1-null specimen (both show original magnification, ×100). Hematoxylin/eosin stains (B and E) showed gross loss of neurons and spongiform changes (E, arrow) in proximity to the mineral deposits (*) in the basal ganglia region from this GIFN12 STAT1-null specimen (B and E, original magnification, ×400). Alizarin Red S-stained (C and F) advanced (F, arrow) as well as early (F, arrowheads) mineral deposits in brain from GIFN12 STAT1-null mice (C and F, original magnification, ×200) are shown. TUNEL staining revealed markedly increased cell death (arrows) in the brain from GIFN12 STAT1-null mice (J–L) compared with GIFN12 (G–I) animals. TUNEL staining combined with cell marker-specific staining for neurons (neurofilament; G and J), astrocytes (GFAP; H and K, arrowheads), and macrophage/microglia (tomato lectin; I and L, arrowheads) is shown. The region shown in G–L is the basal ganglia at an original magnification of ×600.
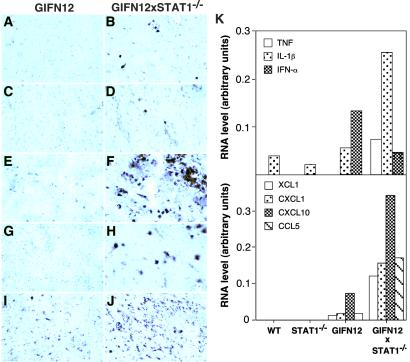
The routine examination above also revealed the presence of modest leukocyte infiltrates in the brain of GIFN12 mice deficient for STAT1 that were not present in control animals. Next, the identity of these leukocytes was examined by immunohistochemistry. Compared with GIFN12 (Fig. 2E), wild-type (not shown) and STAT1-null brain (not shown), increased numbers of cells positive for the pan-leukocyte marker CD45, were present in the brain from GIFN12 STAT1-null mice (Fig. 2F) and included CD4-positive (Fig. 2B) and CD8-positive (Fig. 2D) T cells, 7/4-positive neutrophils (Fig. 2H), and Mac-1-positive macrophage/microglia (Fig. 2J). Therefore, in addition to degenerative pathology, an increased presence of immune cells was apparent in the brain of GIFN12 mice that lacked STAT1. To determine whether the inflammatory process in the brain of the GIFN12 STAT1-null mice was associated with evidence of a functional immune pathology, RPA analysis was used to examine for the expression of a number of proinflammatory cytokine (Fig. 2K Upper) and chemokine (Fig. 2K Lower) genes previously shown by us to be elevated in the CNS of GFAP-IFN-α mice (10, 26). IFN-α RNA, which was detectable in the brain from GIFN12 but not wild-type or STAT1-null mice, was decreased to about half that level in GIFN12 STAT1-null mice (Fig. 2K Upper). Therefore, the exacerbation of the inflammatory encephalopathy in the brain of the GIFN12 STAT1-null mice was not caused simply by the increased expression of transgene-encoded IFN-α in the brain. In contrast to IFN-α and compared with wild type, STAT1 null, and GIFN12 controls, the level of expression of the IL-1β and TNF genes was increased 4- to 5-fold in the GIFN12 STAT1-null mice (Fig. 2K Upper). In addition, expression of the chemokine genes including XCL1 (lymphotactin), CCL5 (RANTES), CXCL1 (MIP-2), and CXCL10 (IP-10) also was increased markedly in the brain from GIFN12 STAT1-null mice (Fig. 2K Lower). In all, these findings suggested that an active immune pathology was induced in the brain of GIFN12 STAT1-null mice.
Fig 2.
Increased inflammatory response in brain of GIFN12 STAT1-null mice. Analysis of brain from 3-month-old GIFN12 (A, C, E, G, and I) or GIFN12 STAT1-null (B, D, F, H, and J) mice immunostained for CD4 T cells (A and B), CD8 T cells (C and D), CD45 pan-leukocyte (E and F), 7/4-neutrophil (G and H), and Mac-1 macrophage/microglia (I and J). Basal ganglia region is shown. (A–H, ×600; I and J, ×200.) (K) RPA analysis (two samples per group; each sample contained pooled RNA prepared from two mice) for cytokine and chemokine gene expression. Autoradiographs were quantified by densitometry, and the values were normalized to housekeeping gene L32 by using NIH IMAGE V.1.57 software. Only those cytokine (Upper) or chemokine (Lower) genes that had major changes are shown.
Compromised IFN-Regulated Gene Expression in GFAP-IFN-α Mice with STAT1 Deficiency.
To evaluate the impact of STAT1 molecule deficiency on the gene transcriptional program in GFAP-IFN-α mice, the cerebral expression of a number of prototypic IFN-regulated genes was examined. In GIFN12 mice, expression of the key IFN-α signaling molecule genes, STAT1, STAT2, and IRF9, was increased compared with wild-type and STAT1-null mice (Fig. 3 A and C). In GIFN12 STAT1-null mice, whereas STAT1 RNA was, as expected, not detectable, expression of the STAT2 and IRF9 genes was not significantly different from wild-type or STAT1-null mice (Fig. 3 A and C). Strong induction in the expression of the PKR, TGTP, and 2′5′OAS but not PKIp58, CIITA, IRF-1, and IRF2 genes was observed in GIFN12 mice compared with wild-type or STAT1-null animals (Fig. 3 B and D). By contrast, in GIFN12 STAT1-null mice, the expression of the PKR, TGTP, and 2′5′OAS genes was induced to only a minor level. Thus, these findings confirmed that the expression of a number of ISRE-modulated genes known to be induced by IFN-α via formation of the ISGF3 signaling complex was severely compromised in the brain of GIFN12 mice lacking STAT1.
Fig 3.
Cerebral expression of IFN-regulated genes is compromised in GIFN12 STAT1-null mice. (A) RPA analysis for various signal-transduction and other IFN-regulated (B) genes in poly(A)+ RNA (1 μg per sample) extracted from the brain of 3-month-old mice. Samples in each lane are derived from individual animals. (C and D) Autoradiographs were quantified by densitometry, and the values were normalized to the housekeeping gene L32 by using NIH IMAGE V.1.57 software.
STAT3 Alone Is Activated in the Brain of GFAP-IFN-α Mice with STAT1 Deficiency.
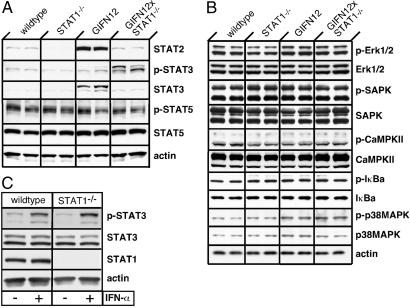
To determine the possible involvement of alternative signaling pathways in mediating the action of IFN-α in the CNS, we next examined the total and phosphoprotein levels for a number of different signaling molecules in the brain. In parallel with the RNA transcripts (Fig. 3), increased levels of STAT2 protein also were observed in the brain of GIFN12 mice but not GIFN12 STAT1-null mice (Fig. 4A). For technical reasons, it was not possible to determine the levels of phospho-STAT2 in the brain. No significant alterations were found in the steady-state levels of STAT5 or phopsho-STAT5 proteins in any of the brains examined. However, steady-state levels of the STAT3 protein were increased in GIFN12 but not in GIFN12 STAT1-null mice. On the other hand, phospho-STAT3 levels clearly were increased in the brain of GIFN12 STAT1-null mice compared with GIFN12 or wild-type and STAT1-null controls. A variety of molecules from the stress kinase, calcium-dependent kinase, and NF-κB signaling pathways also were evaluated (Fig. 4B). No significant changes were found in either the steady-state or phosphoprotein levels of SAPK/JNK, p38MAPK, ERK, IκB-α, and CaMPKII in any of the brains examined. Thus, of all these signaling molecules examined, only the activation state of STAT3 was altered significantly in the CNS of GIFN12 STAT1-null mice.
Fig 4.
Activation of STAT 3 but not other signal-transduction molecules in the brain of GIFN12 STAT1-null mice. (A) Immunoblot analysis of STAT and various other signal-transduction molecules (B) in total protein lysates (100 μg per lane) from the basal forebrain of 3-month-old mice. (C) STAT3 activation in splenic leukocytes after treatment (40 min) with murine IFN-α (1,000 units/ml). Immunoblot analysis of total protein lysates (200 μg per lane) also is shown.
We asked whether the activation of STAT3 found in the absence of STAT1 could be mediated directly by IFN-α. Whether from wild-type or STAT1-null mice, splenic leukocytes responded to IFN-α treatment with a robust increase in the levels of phospho-STAT3 (Fig. 4C). Therefore, the activation of STAT3 is not only mediated directly by IFN-α but is also independent of STAT1.
Discussion
Here, we showed that an absence or even a reduced level of STAT1 unexpectedly and markedly exacerbated the neuropathogenic actions of IFN-α within the living CNS. Thus, these findings indicated that dysfunctional type I IFN receptor signaling because of a deficiency in STAT1 actually can have pronounced pathophysiological consequences in vivo. Although of earlier onset and greatly enhanced in their severity, many of the pathologic changes including calcification, inflammation, and neurodegeneration, found in the brain of the GFAP-IFN-α STAT1-null mice, also occurred in older GFAP-IFN-α mice sufficient for STAT1 (10, 11). In particular, the presence of calcification seems to be a prototypic feature of the chronic production of IFN-α in the brain. Although STAT1-null mice are known to be very sensitive to viral infection (16, 17, 21), the overlapping brain pathology noted above and the concordance between severity of the neurological disorder with the level of transgene-encoded IFN-α make it unlikely that the phenotype of the GFAP-IFN-α STAT1 mice was caused by a viral infection. Additionally, no evidence of either systemic or CNS viral infection has been detected in the control STAT1-null mice. Therefore, we conclude not only that the IFN-regulated processes giving rise to cellular pathologies in the brain occur in the absence of STAT1, but that STAT1 itself, or a downstream component of the JAK/STAT pathway, plays a protective role in reducing such deleterious processes. Currently, the identity of these putative, deleterious processes and the basis for such protection remain unknown. Recently, Chen et al. (27) showed that STAT1 protects insulinoma cells against cytotoxicity mediated by the combination of IFN-γ and IL-1β. Therefore, these findings, together with those here, provide convincing evidence of a major protective function for STAT1 against IFN-mediated cellular injury.
Consistent with IFN-α activation of the JAK/STAT pathway and formation of ISGF3, in GIFN12 mice, expression of prototypic ISRE-containing genes such as 2′5′OAS and PKR as well as STAT1, STAT2, and IRF-9 is induced in the brain (ref. 28 and Fig. 3). In the case of STAT1 and 2′5′OAS, neurons in general express the highest levels of these genes, indicating that these cells are also major targets for the action of IFN-α. However, STAT1 deficiency seriously compromised ISRE-dependent, IFN-α-regulated gene expression in the brain of the GFAP-IFN-α mice. Thus, these studies not only confirmed in vivo the reported impairment in ISRE-dependent, IFN-α-regulated gene expression in the STAT1-deficient environment of these mice (16), they also demonstrated that genes regulated by this pathway are not necessary for the evolution of some of the neuropathologic changes such as calcification and inflammation induced by IFN-α in the CNS.
Although it is well established that the expression of a large number of genes can be regulated by IFN-γ in a STAT1-independent fashion (21, 29), whether this is also the case for the type I IFNs such as IFN-α remains to be determined. Biological responses to IFN-α, such as inhibition of B lymphopoiesis (19) and inhibition of granulocyte/macrophage colony-stimulating factor-stimulated bone marrow macrophage proliferation (21), can occur in the absence of STAT1. A limited number of genes were shown to be stimulated by IFN-α in the absence of STAT1. Thus, the IFN-α-mediated, STAT1-independent inhibition of IL-7-stimulated proliferation of B cell lineage progenitors involves induction of the Fas death domain-associated protein Daxx (30). In addition, IFN-α is an effective inducer of IFN-γ gene expression by splenic mononuclear cells in the absence of STAT1 (31). In the present study, IFN-α production in the CNS in the absence of STAT1 evoked a number of responses. Together, these findings suggest that, like IFN-γ, IFN-α can regulate the gene-transcription program of cells in the absence of STAT1. One such response observed by us was inflammation with leukocyte infiltration of the brain and increased expression of some proinflammatory cytokine genes and a number of chemokine genes. Although these observations indicate that STAT1 is not required for an IFN-α-driven inflammatory process to develop in the brain, because of the complexity of the in vivo milieu and severity of the neuropathologic alterations that occurred in these animals, it is not possible to say whether the inflammation was caused by the direct action of IFN-α or secondary to other perturbations such as increased cell death. Further studies will be necessary, for example, employing enriched neuronal or glial cell cultures derived from the STAT1-deficient mice, to determine the extent to which IFN-α can regulate directly the gene transcriptional program and other responses of these cells.
A variety of alternative signaling molecules and pathways can be activated in cells by type I IFNs. These include other STAT transcription factors, STAT3 (32–34) and STAT5 (34), as well as p38 MAP kinase (35), phosphatidylinositol 3′-kinase (36), p42/44 MAP kinase (36, 37), and NF-κB (38). Activation of p38 MAP kinase (35) and NF-κB (38) signaling by type I IFNs mediates effects on cell survival, indicating that such alternative signaling can regulate physiological responses. Whether the activation of such alternative signaling pathways in response to IFN requires STAT1 or not is unclear. Our investigation indicated that only STAT3 activation was enhanced significantly in the brain of GFAP-IFN-α mice deficient for STAT1. Furthermore, our studies showed that IFN-α can directly activate STAT3 in STAT1-null splenic leukocytes. Thus, the activation of STAT3 in the brain of the GFAP-IFN-α STAT1-null mice might be mediated directly by the action of IFN-α on target cells. Interestingly, embryonic STAT3-null fibroblasts, when stimulated with IL-6, show an IFN-γ-like response because of activation of STAT1 via the IL-6/gp130 receptor complex (39). Whether a switch to STAT3 usage and, thus, signaling occurred in the brain of the GFAP-IFN-α mice lacking STAT1 is unclear. In any event, the biological consequences of IFN-stimulated STAT3 activation in the CNS remain unknown, and it is unclear as to the extent to which or how activated STAT3 signaling mechanisms could contribute to the increased neuropathogenic actions of IFN-α seen in the absence of STAT1. In ongoing studies, we hope to define further the nature and impact of IFN-α-stimulated, STAT1-independent STAT3 activation in the brain.
IFN-α is a double-edged sword in the CNS, with both physiological and pathophysiological actions (6, 10). Based on the findings here, we propose that IFN receptor signaling is complex, involving the coexistence of the JAK/STAT and alternative pathways. The balance in the activity of these pathways ultimately determines the repertoire of CNS responses regulated by IFN-α. Primary signaling would occur via the JAK/STAT pathway, leading to the induction of genes such as 2′5′OAS that play a beneficial role, for example, in antiviral defense. A second, distinct pathway that mediates many neuropathologic responses to IFN-α is counteracted by STAT1 or factors downstream in the JAK/STAT signaling cascade. Under conditions of STAT1 deficiency, the counteractive influence involving this molecule is removed and the alternative pathway(s) becomes the primary signaling pathway such that accelerated and more severe neuropathological responses occur even in the presence of very low concentrations of IFN-α. The finding of alternative pathways that are involved in the pathophysiological actions of IFN-α raises some important issues. First, the signal-transduction pathways that mediate the IFN-α-regulated, STAT1-independent gene expression and cellular responses in the brain remain to be identified. Second, the question remains of whether deficiency of other components, i.e., STAT2 and IRF9, of the ISGF3 signaling complex also would give rise to increased and similar neuropathogenic responses to IFN-α. The recent development of STAT2 (18) and IRF-9-null (40) mice offers tools to address this question. Third, the pathophysiologic states in which IFN-α responses might be evoked in a setting of STAT1 deficiency need to be ascertained. Our findings suggest that STAT1 could be a susceptibility factor for certain neurological disorders; however, pathologic states in which STAT1 deficiency might occur in the CNS currently remain undefined. Nevertheless, situations are known to arise, where STAT1 is inhibited or depleted, that conceivably could produce dysfunctional IFN signaling. Thus, a number of different viruses have the capacity to block the activation or induction of STAT1 by IFN (41–43). Physiological inhibitors of STAT1 also exist: for example, the suppressor of cytokine signaling 1 (44, 45) and protein inhibitor of STAT 1 (46) molecules. Finally, a partially dominant germ-line mutation in human STAT1 producing haploinsufficiency was identified recently (47). The clarification of all these issues is an important goal that we are now undertaking.
Acknowledgments
We thank Heather J. Weinkauf, Nyan Pham-Mitchell, and Mary Kies-Wagner for their technical support and Dr. Christian Schindler (Columbia University) for providing the STAT2 antibody. This study was supported by National Institutes of Health Grants MH62231 and MH62261 (to I.L.C.) and CA43059 (to R.D.S.). This manuscript is no. 15087-NP from The Scripps Research Institute.
Abbreviations
GFAP, glial fibrillary acidic protein
JAK, Janus kinase
STAT, signal transducer and activator of transcription
RPA, RNase protection assay
IRF, IFN regulatory factor
ISRE, IFN-stimulated response element
TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP end labeling
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pestka S., Langer, J. A., Zoon, K. C. & Samuel, C. E. (1987) Annu. Rev. Biochem. 56, 727-777. [DOI] [PubMed] [Google Scholar]
- 2.Belardelli F. (1995) Acta Pathol. Microbiol. Immunol. Scand. 103, 161-179. [DOI] [PubMed] [Google Scholar]
- 3.Rho M. B., Wesselingh, S., Glass, J. D., McArthur, J. C., Choi, S., Griffin, J. & Tyor, W. R. (1995) Brain Behav. Immunol. 9, 366-377. [DOI] [PubMed] [Google Scholar]
- 4.Lebon P., Badoual, J., Ponsot, G., Goutieres, F., Hemeury-Cukier, F. & Aicardi, J. (1988) J. Neurol. Sci. 84, 201-208. [DOI] [PubMed] [Google Scholar]
- 5.Huang X., Yuang, J., Goddard, A., Foulis, A., James, R. F., Lernmark, A., Pujol-Borrell, R., Rabinovitch, A., Somoza, N. & Stewart, T. A. (1995) Diabetes 44, 658-664. [DOI] [PubMed] [Google Scholar]
- 6.Bocci V. (1988) J. Biol. Regul. Homeostat. Agents 2, 107-118. [PubMed] [Google Scholar]
- 7.Mase G., Zorzon, M., Biasutti, E., Vitrani, B., Cazzato, G., Urban, F. & Frezza, M. (1996) J. Neurol. Neurosurg. Psychiatry 60, 348-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabris P., Betterle, C., Floreani, A., Greggio, N. A., de Lazzari, F., Naccarato, R. & Chiaramonte, M. (1992) Lancet 340, 548. [DOI] [PubMed] [Google Scholar]
- 9.Cianciara J. & Laskus, T. (1995) Dig. Dis. Sci. 40, 1842-1844. [DOI] [PubMed] [Google Scholar]
- 10.Akwa Y., Hassett, D. E., Eloranta, M. L., Sandberg, K., Masliah, E., Powell, H., Whitton, J. L., Bloom, F. E. & Campbell, I. L. (1998) J. Immunol. 161, 5016-5026. [PubMed] [Google Scholar]
- 11.Campbell I. L., Krucker, T., Steffensen, S., Akwa, Y., Powell, H. C., Lane, T., Carr, D. J., Gold, L. H., Henriksen, S. J. & Siggins, G. R. (1999) Brain Res. 835, 46-61. [DOI] [PubMed] [Google Scholar]
- 12.Stewart T. A., Hultgren, B., Huang, X., Pitts-Meek, S., Hully, J. & MacLachlan, N. J. (1993) Science 260, 1942-1946. [DOI] [PubMed] [Google Scholar]
- 13.Hekman A. C., Trapman, J., Mulder, A. H., van Gaalen, J. L. & Zwarthoff, E. C. (1988) J. Biol. Chem. 263, 12151-12155. [PubMed] [Google Scholar]
- 14.Darnell J. E., Kerr, I. M. & Stark, G. R. (1994) Science 264, 1415-1421. [DOI] [PubMed] [Google Scholar]
- 15.Stark G. R., Kerr, I. M., Williams, B. R. G., Silverman, R. H. & Schreiber, R. D. (1998) Annu. Rev. Biochem. 67, 227-264. [DOI] [PubMed] [Google Scholar]
- 16.Meraz M. A., White, J. M., Sheehan, K. C., Bach, E. A., Rodig, S. J., Dighe, A. S., Kaplan, D. H., Riley, J. K., Greenlund, A. C., Campbell, D., et al. (1996) Cell 84, 431-442. [DOI] [PubMed] [Google Scholar]
- 17.Durbin J. E., Hackenmiller, R., Simon, M. C. & Levy, D. E. (1996) Cell 84, 443-450. [DOI] [PubMed] [Google Scholar]
- 18.Park C., Li, S., Cha, E. & Schindler, C. (2000) Immunity 13, 795-804. [DOI] [PubMed] [Google Scholar]
- 19.Gongora R., Stephan, R. P., Schreiber, R. D. & Cooper, M. D. (2000) J. Immunol. 165, 2362-2366. [DOI] [PubMed] [Google Scholar]
- 20.Lee C.-K., Rao, D. T., Gimeno, R. & Levy, D. E. (2000) J. Immunol. 165, 3571-3577. [DOI] [PubMed] [Google Scholar]
- 21.Gil M. P., Bohn, E., O'Guin, A. K., Ramana, C. V., Levine, B., Stark, G. R., Virgin, H. W. & Schreiber, R. D. (2001) Proc. Natl. Acad. Sci. USA 98, 6680-6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asensio V. C. & Campbell, I. L. (1997) J. Virol. 71, 7832-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobbs M. V., Weigle, W. O., Noonan, D. J., Torbett, B. E., McEvilly, R. J., Koch, R. J., Cardenas, G. J. & Ernst, D. N. (1992) J. Immunol. 150, 3602-3614. [PubMed] [Google Scholar]
- 24.Maier J., Kincaid, C., Pagenstecher, A. & Campbell, I. L. (2002) Am. J. Pathol. 160, 271-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asensio V. C., Maier, J., Milner, R., Boztug, K., Kincaid, C., Moulard, M., Phillipson, C., Lindsley, K., Krucker, T., Fox, H. S., et al. (2001) J. Virol. 75, 7067-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asensio V. C., Lassmann, S., Pagenstecher, A., Steffensen, S. C., Henriksen, S. J. & Campbell, I. L. (1999) Am. J. Pathol. 154, 1181-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen G., Hohmeier, H. E. & Newgard, C. B. (2001) J. Biol. Chem. 276, 766-772. [DOI] [PubMed] [Google Scholar]
- 28.Campbell I. L. (2001) Prog. Brain Res. 132, 491-508. [DOI] [PubMed] [Google Scholar]
- 29.Ramana C. V., Gil, M. P., Han, Y., Ransohoff, R. M., Schreiber, R. D. & Stark, G. R. (2001) Proc. Natl. Acad. Sci. USA 98, 6674-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gongora R., Stephan, R. P., Zhang, Z. & Cooper, M. D. (2001) Immunity 14, 727-737. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen K. B., Cousens, L. P., Doughty, L. A., Pien, G. C., Durbin, J. E. & Biron, C. A. (2000) Nat. Immunol. 1, 70-76. [DOI] [PubMed] [Google Scholar]
- 32.Rani M. R., Leaman, D. W., Han, Y., Leung, S., Croze, E., Fish, E. N., Wolfman, A. & Ransohoff, R. M. (1999) J. Biol. Chem. 274, 32507-32511. [DOI] [PubMed] [Google Scholar]
- 33.Yang C. H., Murti, A. & Pfeffer, L. M. (1998) Proc. Natl. Acad. Sci. USA 95, 5568-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fasler-Kan E., Pansky, A., Wiederkehr, M., Battegay, M. & Heim, M. H. (1998) Eur. J. Biochem. 254, 514-519. [DOI] [PubMed] [Google Scholar]
- 35.Uddin S., Majchrzak, B., Woodson, J., Arunkumar, P., Alsayed, Y., Pine, R., Young, P. R., Fish, E. N. & Platanias, L. C. (1999) J. Biol. Chem. 274, 30127-30131. [DOI] [PubMed] [Google Scholar]
- 36.Uddin S., Fish, E. N., Sher, D. A., Gardziola, C., White, M. F. & Platanias, L. C. (1997) J. Immunol. 158, 2390-2397. [PubMed] [Google Scholar]
- 37.Nguyen V. A., Chen, J., Hong, F., Ishac, E. J. & Gao, B. (2000) Biochem. J. 349, 427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang C. H., Murti, A., Pfeffer, S. R., Basu, L., Kim, J. G. & Pfeffer, L. M. (2000) Proc. Natl. Acad. Sci. USA 97, 13631-13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa-Pereira A. P., Tininini, S., Strobl, B., Alonzi, T., Schlaak, J. F., Is'harc, H., Gesualdo, I., Newman, S. J., Kerr, I. M. & Poli, V. (2002) Proc. Natl. Acad. Sci. USA 99, 8043-8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura T., Kadokawa, Y., Harada, H., Matsumoto, M., Sato, M., Kashiwazaki, Y., Tarutani, M., Tan, R. S., Takasugi, T., Matsuyama, T., et al. (1996) Genes Cells 1, 115-124. [DOI] [PubMed] [Google Scholar]
- 41.Cebulla C. M., Miller, D. M. & Sedmak, D. D. (1999) Intervirology 42, 325-330. [DOI] [PubMed] [Google Scholar]
- 42.Hariya Y., Yokosawa, N., Yonekura, N., Kohama, G.-I. & Fujii, N. (2000) Microbiol. Immunol. 44, 537-541. [DOI] [PubMed] [Google Scholar]
- 43.Parisien J.-P., Lau, J. F., Rodriguez, J. J., Ulane, C. M. & Horvath, C. M. (2002) J. Virol. 76, 4190-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Starr R. & Hilton, D. J. (1999) BioEssays 21, 47-52. [DOI] [PubMed] [Google Scholar]
- 45.Hilton D. J. & Nicholson, S. E. (1998) J. Leukocyte Biol. 63, 665-668. [DOI] [PubMed] [Google Scholar]
- 46.Liu B., Liao, J., Rao, X., Kushner, S. A., Chung, C. D., Chang, D. D. & Shuai, K. (1998) Proc. Natl. Acad. Sci. USA 95, 10626-10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dupuis S., Dargemont, C., Fieschi, C., Thomassin, N., Rosenzweig, S., Harris, J., Holland, S. M., Schreiber, R. D. & Casanova, J. L. (2001) Science 293, 300-303. [DOI] [PubMed] [Google Scholar]