Abstract
Cholesterol and other sterols exit the body primarily by secretion into bile. In patients with sitosterolemia, mutations in either of two ATP-binding cassette (ABC) half-transporters, ABCG5 or ABCG8, lead to reduced secretion of sterols into bile, implicating these transporters in this process. To elucidate the roles of ABCG5 and ABCG8 in the trafficking of sterols, we disrupted Abcg5 and Abcg8 in mice (G5G8−/−). The G5G8−/− mice had a 2- to 3-fold increase in the fractional absorption of dietary plant sterols, which was associated with an ≈30-fold increase in plasma sitosterol. Biliary cholesterol concentrations were extremely low in the G5G8−/− mice when compared with wild-type animals (mean = 0.4 vs. 5.5 μmol/ml) and increased only modestly with cholesterol feeding. Plasma and liver cholesterol levels were reduced by 50% in the chow-fed G5G8−/− mice and increased 2.4- and 18-fold, respectively, after cholesterol feeding. These data indicate that ABCG5 and ABCG8 are required for efficient secretion of cholesterol into bile and that disruption of these genes increases dramatically the responsiveness of plasma and hepatic cholesterol levels to changes in dietary cholesterol content.
Keywords: ATP-binding cassette transporters, sitosterolemia, bile, knockout mice
Sitosterolemia is a rare autosomal recessive disorder characterized by the accumulation of plant and animal sterols in blood and tissues (1, 2). Affected subjects with this disorder develop large deposits of cholesterol in their skin, tendons, and coronary arteries. The accumulation of plant and animal sterols in the blood is caused by an increase in the fractional absorption of sterols from the diet and a decrease in the secretion of sterols into the bile, which is the major route of exit of sterols from the body (3, 4).
A striking feature of sitosterolemia is the precipitous fall in plasma cholesterol that follows reductions in dietary cholesterol intake, especially in young patients (5, 6). When normal individuals are switched from a high cholesterol, high fat diet to a low cholesterol, low fat diet, plasma levels of cholesterol fall ≈10–20%; in patients with sitosterolemia, plasma cholesterol can fall by >45% (5, 6). The hypercholesterolemia of sitosterolemia is also sensitive to treatment with bile-acid resins, which stimulate the conversion of cholesterol to bile acids, another pathway for removal of cholesterol from the body.
The pathognomonic feature of sitosterolemia is the elevation in plasma sitosterol, the most abundant plant sterol (1). Sitosterolemic patients also accumulate other sterols in plasma including a variety of plant sterols (campesterol, stigmasterol, and avenasterol) and shellfish sterols (brassicasterol, 24-methylene cholesterol, and 22-dehydrocholesterol) (1, 7). In normal individuals these sterols are poorly absorbed and preferentially secreted into the bile (8–10). These sterols comprise only ≈1% of plasma and tissue sterols in normal individuals but ≈15% of circulating and tissue sterols in sitosterolemia (11).
Studies of sitosterolemia have provided insights into the molecular basis of two important pathways in the trafficking of sterols: the absorption of dietary sterols in the intestine and the secretion of biliary sterols from the liver. The disease is caused by mutations in either of two oppositely oriented, closely apposed genes that encode the ATP-binding cassette (ABC) transporters ABCG5 and ABCG8 (12, 13). These genes, which are expressed almost exclusively in liver and small intestine, are coordinately up-regulated by the nuclear hormone receptor, LXRα, in response to dietary cholesterol (12, 14). High level expression of a human ABCG5 and ABCG8 transgene in mice results in an ≈50% reduction in the fractional absorption of dietary cholesterol and a dramatic increase in the biliary secretion of sterols (15). If coexpressed in the same cells, ABCG5 and ABCG8 are targeted to the apical (biliary) surface of polarized hepatocytes (16).
These data are consistent with the hypothesis that ABCG5 and ABCG8 form a functional complex that limits the accumulation of dietary sterols by secreting sterols from gut epithelial cells into the lumen and promoting secretion of hepatic sterols into bile. To test this hypothesis directly, we inactivated Abcg5 and Abcg8 in mice and examined the absorption and secretion of the major dietary plant and animal sterols.
Materials and Methods
Sterols were purchased from either Steraloids (Newport, RI) or Sigma–Aldrich (St. Louis), and the bile-acid standards were from Sigma. The [14C]taurocholic acid was obtained from American Radiolabeled Chemicals (St. Louis), and the [3H]H2O was from Perkin–Elmer Life Sciences. [26,26,26,27,27,27-2H6]cholesterol and [2,2,4,4,6-2H5]campesterol/sitosterol (40:60) were purchased from Medical Isotope (Pelham, NH). Deuterated cholestanol, campestanol, and sitostanol were synthesized by hydrogenation of the deuterated sterols using microwaves (17).
Generation of Mice Homozygous for a Disrupted Abcg5 and Abcg8 Allele.
Two DNA fragments were generated by PCR using genomic DNA from mouse liver (129S6/SvEv strain) as a template; a 1.3-kb fragment of intron 2 of Abcg5 was amplified by using primers containing XhoI sites at the 5′ ends (5′-ACAGTCTCGAGCTCCTGTACTTCTAAGGCAGGCTCTGG-3′ and 5′-ACAGTCTCGAGGGACTCACTCT CAGCTACACCCAACAG-3′), and a 9.6-kb fragment extending from intron 3 to exon 13 of Abcg8 was generated by using primers with NotI sites at the 5′ ends (5′-GCATATAGGCGGCCGCTGGCTTAGAGCACAGGTTCTCAGTCAGATAG-3′ and 5′-AGCATATAGGCGGCCGCCTACAGGAACAGGAAGCCGTAGCTGATGCC-3′). The fragments were cloned into a targeting vector (pJB1, kindly provided by Joachim Herz, University of Texas Southwestern Medical Center), which contained the neo gene driven by the murine phosphoglycerate kinase (PGK) promoter, flanked by loxP and frt sites, and followed by two copies of the herpes simplex virus thymidine kinase (HSV TK) genes.
129S6/SvEv mouse-derived embryonic stem cells (SM-1, Passage 9) were cultured on irradiated (10,000 rad) STO feeder cells (18, 19). Approximately 2 × 107 embryonic stem cells were electroporated with 100 μg of PmeI-linearized targeting construct (330 μF, 275 V, low resistance) by using Invitrogen Cell-Porator (Invitrogen). The cells were seeded onto STO feeder layers for 24 h before the addition of G418 (250 μg/ml). Forty-eight hours after electroporation the cells were selected with gancyclovir (2.5 μM) for 8–10 days. The embryonic stem-cell colonies were expanded and screened for homologous recombination by Southern blotting. Three positive clones were injected into C57BL/6J blastocysts. Eleven chimeric male and three chimeric female mice with ≈80% agouti coat color were fertile. One chimeric female had two female offspring with the disrupted Abcg5 and Abcg8 allele. A line was established by crossing these offspring to C57BL/6J male mice. Mice used in this study were offspring of matings between mice heterozygous for a disrupted Abcg5 and Abcg8 allele.
Animals and Diets.
Mice were housed in a temperature-controlled room (22°C) with a daylight cycle from 6 a.m. to 6 p.m. and fed ad libitum a cereal-based rodent-chow diet (Diet 7001, Harlan–Teklad, Madison, WI) containing 0.02% cholesterol and 4% fat. The sterol content of the chow diet was analyzed by GC and the relative amounts of cholesterol to sitosterol and to campesterol were 1:2.3 and 1:0.7, respectively. The mice used for breeding were fed a 6% fat diet (Diet 7002, Harlan–Teklad). All animal procedures were approved by the Institutional Animal Care and Research Advisory Committee at the University of Texas Southwestern Medical Center.
Production of Polyclonal Antibodies to Mouse ABCG5 and ABCG8.
Two DNA fragments encoding the N-terminal regions of mouse ABCG5 (residues 2–375) and ABCG8 (residues 2–400) were PCR-amplified from the ABCG5 and ABCG8 cDNAs and cloned into the pET30a(+) vector (Novagen). The constructs were used to transform BL21 (DE3) competent cells (Novagen), and the peptides expressed were purified by using the QIAexpressionist procedure with nickel-nitrilotriacetic acid columns in 8 M urea (Qiagen, Valencia, CA). Rabbits were injected every 2 weeks with 100 μg of purified protein to generate antibodies.
Metabolic Phenotyping.
Hepatic triglyceride levels were measured by using Infinity triglycerides reagent (catalog no. 343-500P; Sigma–Aldrich). Lipoproteins were size-fractionated by using fast protein liquid chromatography, and the sterol concentration in each fraction was measured by using Cholesterol/HP kits (catalog no. 1127771, Roche Diagnostics). The biliary lipid composition, fecal neutral sterol and bile-acid content, in vivo cholesterol-synthesis rates, and bile-acid pool sizes were measured as described (15, 20). Total cholesterol and noncholesterol sterol concentrations in liver and plasma samples were measured by GC as described (20, 21) with modifications. Briefly, tissues and plasma were saponified in 3% potassium hydroxide/ethanol at 65°C for 3 h, 5α-cholestane was added as a quantitative recovery standard, and the lipids were extracted by using petroleum ether. Samples were dried under nitrogen, and residual lipids were redissolved in Tri-Sil reagent (product no. 48999, Pierce) for analysis by GC.
Measurement of Intestinal Absorption of Dietary Sterols.
A mixture containing 4 mg of [26,26,26,27,27,27-2H6]cholesterol, 4 mg of [26,26,26,27,27,27-2H6]cholestanol, 10 mg of [2,2,4,4,6- 2H5]campesterol/sitosterol (40:60), and 4 mg of [2.2.4.4.6.-2H5]sitostanol was prepared (17) and dissolved in 2 ml of plant oil (Livio, Union Deutsche Lebensmittelwerke, Hamburg, Germany). A single dose of the deuterated sterol/stanol-oil mixture (50 μl) was gavaged to each mouse. Feces were collected for 3 days after the gavage, pooled, and processed as described (22–24). Fecal sterols and stanols were separated by GC, and the fractional absorption of sterols was calculated as described (22–24).
Statistical Analysis.
All data are reported as the mean ± SEM. The differences between the mean values were tested for statistical significance by the two-tailed Student's t test.
Results
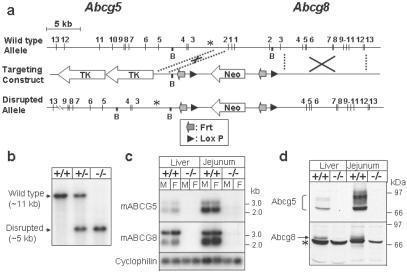
Similar to the human genes, the murine Abcg5 and Abcg8 genes are located within 400 bp of each other in a head-to-head orientation (Fig. 1a). To disrupt both genes, the region extending from intron 2 of Abcg5 to intron 3 of Abcg8 was replaced with a neomycin-containing cassette by homologous recombination. Gene disruption was confirmed by Southern blotting (Fig. 1b), RNA blotting (Fig. 1c), and immunoblotting (Fig. 1d). The deleted exons included the sequences encoding part of the Walker A consensus sequences of both genes. The Walker A sequence is part of the ATP-binding site, and its deletion should render the protein nonfunctional. ABCG5 and ABCG8 are both glycosylated, resulting in the generation of a series of higher molecular mass forms of the proteins (Fig. 1d). After N-glycosidase treatment, the higher molecular mass forms collapsed into one band (data not shown). The sizes and patterns of the precursor and mature glycosylated forms of the proteins were similar to those seen when the recombinant proteins are expressed in cultured cells (16). The amount of immunodetectable ABCG5 and ABCG8 (Fig. 1d) was proportional to the amounts of their respective transcripts in both tissues (Fig. 1c). No immunoreactive ABCG5 or ABCG8 protein was detected in the G5G8−/− mice (Fig. 1d).
Fig 1.
Targeted disruption of mouse Abcg5 and Abcg8 genes. (a) Schematic of the wild-type allele, the targeting construct, and the disrupted allele. The targeting construct was generated as described in Materials and Methods. B, BamHI restriction site. (b) Southern blot analysis of BamHI-digested genomic DNA from offspring of a heterozygote (G5G8+/−) mating. The probe used in the hybridization was a [α-32P]dCTP-labeled 275-bp DNA fragment (denoted by an asterisk in a), which was amplified from intron 2 of the Abcg5 gene by PCR using the primers 5′-CTCTGTGACAGGAGCTTGTCCTTC-3′ and 5′-CTAGACGCTCAAGGCAGAGTAATC-3′. (c) Northern blot analysis of hepatic and jejunal RNA from wild-type and G5G8−/− mice. RNA blotting was performed with pooled samples of total tissue RNA from mice of the indicated genotypes (n = 3 in each group) by using [α-32P]dCTP-labeled ABCG5 and ABCG8 cDNA probes. (d) Immunoblot analysis of ABCG5 and ABCG8 from liver and jejunum of wild-type and G5G8−/− mice. A total of 50 μg of pooled membrane protein (n = 3 in each group) was fractionated on SDS/PAGE and immunoblotted with rabbit anti-mouse ABCG5 and ABCG8 antisera developed as described in Materials and Methods. The filters were exposed to Kodak X-Omat Blue films for 5–30 s at room temperature. +/+, wild-type mice; −/−, G5G8−/− mice; *, nonspecific protein that reacts with the anti-ABCG8 antiserum.
The G5G8−/− mice were fertile, and their litter sizes were normal. No obvious physical differences were apparent between the G5G8−/− mice and wild-type mice. Routine histology of the liver, intestine, spleen, kidneys, ovaries, and testes and liver-function tests were normal in the G5G8−/− mice (data not shown).
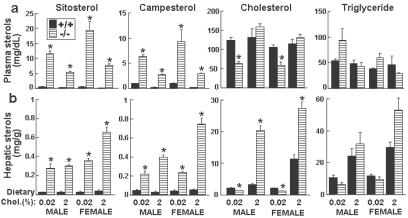
Because the hallmark of sitosterolemia is the accumulation of plant sterols, we measured the levels of plasma sterols in the G5G8−/− mice. The plasma levels of sitosterol, which were barely detectable in the wild-type animals, were elevated ≈30-fold in the G5G8−/− mice on a chow diet (Fig. 2a). The mean plasma level of campesterol, the second most plentiful plant sterol in the diet, was increased 8-fold in the G5G8−/− mice. When dietary cholesterol content was increased from 0.02% to 2%, the plasma levels of both sitosterol and campesterol fell by ≈50% in both wild-type and G5G8−/− mice. The levels of plant sterols in the liver were elevated dramatically in the G5G8−/− mice (Fig. 2b).
Fig 2.
Plasma and hepatic lipid levels in chow- and cholesterol-fed G5G8−/− and wild-type mice. Individually housed 20-week-old mice were fed a powdered chow diet (0.02% cholesterol) or the same diet containing 2% cholesterol for 21 days. The mice were killed in the daylight portion of the cycle. Blood and liver were collected. Plasma (a) and hepatic (b) levels of lipids were measured by GC as described in Materials and Methods. +/+, wild-type mice; −/−, G5G8−/− mice. *, P < 0.01 between wild-type and G5G8−/− mice (n = 5 in each group).
On a chow diet, the plasma cholesterol levels in the G5G8−/− mice were significantly lower than the wild-type controls (mean levels: 62 vs. 114 mg/dl). Fast protein liquid chromatography indicated that the distribution of sterols among the lipoproteins did not differ between knockout and wild-type mice (data not shown), and almost all the sterols were in high density lipoproteins (HDL). Plasma cholesterol levels did not increase significantly when the wild-type mice were challenged with a 2% cholesterol diet (Fig. 2a), whereas in the G5G8−/− mice cholesterol feeding resulted in a 2.4-fold increase in plasma cholesterol levels.
Hepatic cholesterol levels were significantly lower in the chow-fed G5G8−/− mice (mean levels: 1.33 vs. 2.21 mg/g) but increased 18-fold on the high cholesterol diet compared with a 3-fold increase in the wild-type animals. Both free cholesterol and esterified cholesterol levels were reduced in the knockout mice, and the ratio of free cholesterol to total cholesterol was increased significantly in both the female (0.92 vs. 0.78, P < 0.01) and male (0.91 vs. 0.84, P < 0.01) mice. Plasma and hepatic triglyceride levels varied over a wide range among the mice, and no significant differences were apparent between the chow-fed G5G8−/− and wild-type animals. Cholesterol feeding was associated with a significant increase in hepatic triglyceride levels in the G5G8−/− mice (Fig. 2b).
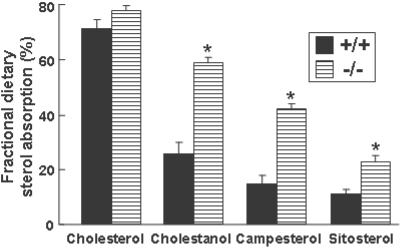
To measure the effect of ABCG5 and ABCG8 inactivation on the absorption of dietary sterols, mice were gavaged with an oil containing deuterated cholesterol, cholestanol, campesterol, sitosterol, and sitostanol (Fig. 3). Sitostanol was used as the nonabsorbable marker, because this sterol is only minimally absorbed in patients with sitosterolemia (23). The fractional absorption of dietary plant sterols and cholestanol was increased 2- to 3-fold in the G5G8−/− mice, but the fractional absorption of dietary cholesterol was not significantly different between the G5G8−/− and wild-type animals.
Fig 3.
Fractional absorption of dietary sterols in wild-type and G5G8−/− mice. Four-month-old female mice of the indicated genotypes (n = 8 in each group) were housed individually. Each mouse was gavaged with 50 μl of an oil mixture containing deuterated cholesterol, cholestanol, campesterol, sitosterol, and sitostanol. Feces were collected for 3 days, and the sterols were extracted. The fecal sterols were subjected to GC-MS as described in Materials and Methods. Deuterated sitostanol was used as a nonabsorbable fecal marker by which the fractional absorption of the other labeled sterols was calculated. +/+, wild-type mice; −/−, G5G8−/− mice. *, P < 0.05 between wild-type and G5G8−/− mice.
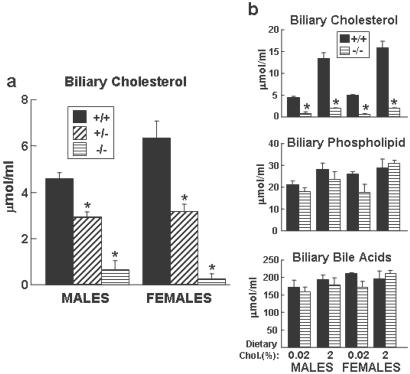
To determine whether the dramatic increase in hepatic cholesterol levels in response to the high cholesterol diet was due to impaired biliary cholesterol secretion, the cholesterol concentration of gallbladder bile was measured. Bile was collected from wild-type, G5G8+/−, and G5G8−/− animals after a 4-h fast. The concentration of biliary cholesterol in wild-type mice on a normal chow diet ranged between 4.6 and 6.3 μmol/ml, whereas the corresponding levels in the G5G8−/− mice ranged from <0.1 to 0.64 μmol/ml; the mean value in the G5G8−/− mice was only ≈9% of those in the control animals (Fig. 4a). Mice heterozygous for the disrupted genes had biliary cholesterol levels that were intermediate between those of the wild-type and G5G8−/− animals (Fig. 4a). The absolute differences in biliary cholesterol levels between wild-type and G5G8−/− mice increased when the animals were placed on a high cholesterol diet (≈1.9 vs. ≈14.6 μmol/ml) (Fig. 4b), suggesting that ABCG5 and ABCG8 play a crucial role in the transport of dietary cholesterol into the bile. These data indicate that much of the increase in hepatic cholesterol in the cholesterol fed G5G8−/− mice is attributable to a block in the biliary cholesterol secretion.
Fig 4.
Levels of biliary lipids in G5G8−/− mice and their littermate controls. (a) Male and female 16-week-old G5G8−/−, G5G8+/−, and G5G8+/+ mice (n = 4–5 in each group) maintained on a chow diet were killed after a 4-h fast. The gallbladder bile was collected, and lipid levels were measured as described in Materials and Methods. (b) Gallbladder bile was collected from the same mice as described for Fig. 2, and the levels of cholesterol, phospholipids, and bile acids were assayed as described in Materials and Methods. +/+, wild-type mice; +/−; heterozygotes; −/−, G5G8−/− mice. *, P < 0.01 between wild-type and G5G8−/− mice.
The other major lipid components of bile are phospholipids and bile acids. Phospholipids are required for biliary cholesterol secretion; mice lacking MDR2 (ABCB4), the biliary phosphatidylcholine transporter, have little or no detectable cholesterol in the bile (25). Inactivation of Abcg5 and Abcg8 resulted in modest, nonsignificant reductions in biliary phospholipid levels (Fig. 4b). The molar ratio of phospholipids to total biliary lipids (cholesterol, phospholipids, and bile acids) did not change significantly with disruption of Abcg5 and Abcg8 (10.7% in the wild-type mice and 9.8% in the G5G8−/− mice). In contrast, the cholesterol molar ratio was significantly lower in the G5G8−/− mice (0.37%) than in the littermate controls (2.2%). Thus the secretion of cholesterol into bile is not coupled quantitatively to the secretion of phospholipids.
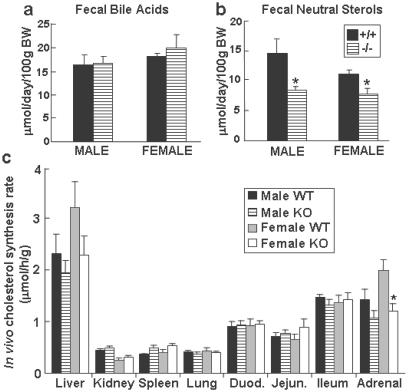
The G5G8−/− and wild-type mice also had similar concentrations of bile acids in the bile (Fig. 4b). Comparison of the amount of bile acid excreted into the stool (Fig. 5a), the bile-acid pool size, and the bile-acid composition (data not shown) revealed no significant differences between the G5G8−/− and wild-type mice. These data indicate that ABCG5 and ABCG8 are not required for the efficient secretion of bile acids into bile.
Fig 5.
The bile-acid (a) and neutral sterol (b) content of feces from G5G8−/− mice and their littermate controls and in vivo cholesterol synthesis (c). Feces were collected from individually housed 17-week-old mice of the indicated genotypes for 3 days while they consumed a chow diet. The daily excretion of total bile acids (a) and neutral sterols (b) was determined as described in Materials and Methods. (c) Four-month-old male and female wild-type (WT) and knockout (KO) mice (n = 6 in each group) maintained on a chow diet were injected i.p. with 40 mCi of [3H]H2O (1 Ci = 37 GBq). The mice were killed 1 h later (mid-daylight cycle), and the tissues were collected and processed as described in Materials and Methods. +/+, wild-type mice; −/−, G5G8−/− mice. *, P < 0.05 between wild-type and G5G8−/− mice.
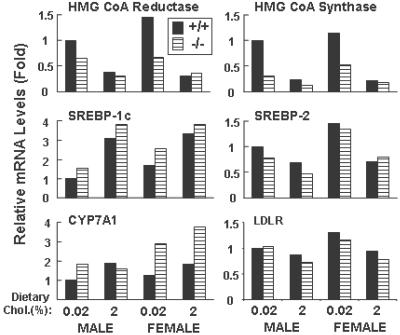
While eating a chow diet, the G5G8−/− animals had reduced amounts of fecal neutral sterols as compared with wild-type mice (Fig. 5b). The cholesterol synthesis rate, as measured by the incorporation of tritiated water into cholesterol, was significantly lower in the adrenal glands of the female G5G8−/− mice than the wild-type counterparts. Hepatic cholesterol synthesis was reduced by 17% and 29% in the male and female G5G8−/− mice, respectively, although these differences were not statistically significant (Fig. 5c). In support of hepatic cholesterol synthesis being reduced in the livers of the knockout animals was the finding that the hepatic mRNA levels for two highly regulated genes of cholesterol synthesis, HMG-CoA reductase and HMG-CoA synthase, were reduced by ≈50% in the G5G8−/− animals (Fig. 6). Expression microarray studies of pooled mRNA samples from the livers of G5G8−/− (n = 5) and wild-type (n = 5) mice revealed significant reductions in the mRNA levels of multiple genes encoding enzymes in the cholesterol biosynthetic pathway (data not shown).
Fig 6.
Quantitative real-time PCR of liver RNA from wild-type and knockout mice. Total RNA from the livers of mice in each group (n = 5) was isolated and pooled for real-time PCR as described (31, 32). Cyclophilin was used as an internal control for these studies. Each value represents the mRNA level relative to the amount of transcript in the chow-fed wild-type males, which was set arbitrarily to 1. +/+, wild-type mice; −/−, G5G8−/− mice.
The mRNA levels of cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme in the bile acid biosynthetic pathway, were 2-fold higher in the G5G8−/− animals (Fig. 6), although the fecal bile-acid excretion was not different between wild-type and G5G8−/− mice. The mRNA levels of the major transcriptional regulators of sterol synthesis, sterol regulatory element-binding protein 1c (SREBP-1c) and SREBP-2, and the low density lipoprotein receptor were similar in G5G8−/− mice and their wild-type littermates (Fig. 6), as were the levels of ABCA1 (data not shown).
Discussion
The major finding of this paper is that disruption of Abcg5 and Abcg8 in mice resulted in a selective and profound reduction in biliary cholesterol levels and an accumulation of cholesterol in the liver after cholesterol feeding. The G5G8−/− mice phenotypically resembled humans with sitosterolemia in a number of respects. First, the G5G8−/− mice had increased fractional absorption of plant sterols and a dramatic increase in plasma and tissue levels of plant sterols. The plasma levels of sitosterol in the G5G8−/− mice were ≈30-fold higher than those of their wild-type littermates, which is comparable with that observed in sitosterolemia. Second, plasma cholesterol levels were much more responsive to changes in dietary cholesterol content in the G5G8−/− mice. Third, the absence of ABCG5 and ABCG8 had no detectable effect on the amount or composition of bile acids.
The phenotype of the G5G8−/− mice differed from that of humans with sitosterolemia in four major respects. First, the reduction in fecal neutral sterols and cholesterol synthesis was less pronounced in the G5G8−/− mice than in humans with sitosterolemia. Second, the plasma and liver cholesterol levels were reduced by 50% in the chow-fed G5G8−/− mice despite the virtual absence of biliary cholesterol. Third, the plasma levels of cholesterol were not significantly higher in the knockout mice than in the wild-type mice even after dietary cholesterol challenge. And finally, no appreciable increase in the fractional absorption of dietary cholesterol was seen in the G5G8−/− animals.
Some of the differences in cholesterol metabolism between the knockout mice and patients with sitosterolemia may reflect differences in the composition of the mouse and human diets. Although the daily cholesterol intake of mice consuming the chow diet is higher than that of the typical Western diet (30 vs. 5 mg/kg/day) (26), the chow diet contains compounds that interfere with absorption of dietary cholesterol (27). Alternatively, we cannot rule out the possibility that the phenotypic differences between the knockout mice and sitosterolemic patients may be related to the fact that patients with sitosterolemia invariably have defects in only one gene (either ABCG5 or ABCG8), whereas both genes were disrupted in the mouse model. This possibility can be addressed only by selective inactivation of Abcg5 and Abcg8. Finally, the observed differences may reflect species-specific differences in the metabolism of dietary sterols.
The G5G8−/− mice share with their human counterpart an increased fractional absorption of dietary plant sterols. Humans absorb ≈45% of dietary cholesterol, 20% of dietary campesterol, and 5% of dietary sitosterol (23). The relative fractional absorption of these different neutral sterols was similar in the wild-type mice to that of humans, with the fractional absorption of cholesterol > campesterol > sitosterol. The rank order of these three dietary sterols is maintained in the G5G8−/− mice as it is in patients with sitosterolemia (23), indicating that the selectivity of the intestine for the absorption of different sterols is largely independent of ABCG5 and ABCG8.
A distinctive clinical feature of sitosterolemia is the responsiveness of the plasma cholesterol to changes in dietary cholesterol intake (5, 6). The plasma and liver cholesterol levels in the G5G8−/− mice were also much more responsive to changes in dietary cholesterol content than their littermate controls. The plasma and hepatic levels of cholesterol increased 2.4- and 18-fold, respectively, with cholesterol feeding in G5G8−/− mice, compared with no change in the plasma and a 3.0-fold increase in the liver of the wild-type animals. The exaggerated responsiveness to dietary cholesterol presumably results from the inability of the G5G8−/− mice to eliminate hepatic cholesterol by secretion into bile.
The plasma levels of sitosterol and campesterol fell with cholesterol feeding in both G5G8−/− and wild-type mice (Fig. 2). The reduction in plasma levels of plant sterols in mice is most likely due to competition for intestinal absorption of the sterols. The dietary cholesterol may compete with plant sterols for solubilization in micelles, uptake into the enterocyte, or incorporation into the nascent chylomicron particles.
Humans with sitosterolemia have a greater reduction in cholesterol synthesis (>70%) than was seen in the G5G8−/− mice (mean = 23%) (23, 28). This difference was reflected in the more modest reduction in fecal neutral sterols in the G5G8−/− mice (36% reduction) than has been reported for humans with sitosterolemia (>70% reduction). A larger proportion of fecal neutral sterols is derived from bile in humans than in mice. The daily intake of cholesterol in humans on a Western diet is ≈400 mg, and the daily output of cholesterol into the bile is estimated to be 2,000 mg (29). In contrast, in chow-fed mice, the daily dietary intake and biliary secretion of cholesterol are similar (≈1 mg each) (26). If dietary and biliary cholesterol are absorbed with equal efficiency, the bile would be expected to comprise a much larger fraction of the fecal neutral sterols in humans. Therefore, the disruption of biliary cholesterol secretion leads to a greater reduction in fecal neutral sterol excretion in humans than in mice.
A surprising finding in this study was that chow-fed G5G8−/− mice had lower hepatic cholesterol levels despite a marked reduction in biliary cholesterol secretion. Low hepatic levels of cholesterol should elicit a compensatory increase in cholesterol synthesis, which was not apparent in the mutant mice. We speculate that one or more plant sterols, or possibly other molecules normally secreted into the bile by ABCG5 and ABCG8, accumulate in the livers of the knockout mice and suppress cholesterol synthesis. Cholesterol synthesis was also significantly lower in the adrenal glands of the female knockout animals, and a similar trend was seen in the male mice. No mRNA expression of either ABCG5 and ABCG8 was detectable in the adrenal glands. The elevated levels of noncholesterol sterols may directly suppress cholesterol synthesis or may alter the intracellular distribution of cholesterol. Additional studies will be required to elucidate the mechanism responsible for the reduction in cholesterol synthesis in both the liver and adrenal glands in the G5G8 knockout mice.
The chow-fed G5G8−/− animals also had significantly lower plasma cholesterol levels than wild-type mice, most likely as a consequence of the reduced hepatic cholesterol levels. The increase in amount of noncholesterol sterols in the liver may interfere with the packaging of cholesterol into lipoproteins. Although the plasma levels of cholesterol in the G5G8−/− mice were more responsive to dietary cholesterol, the cholesterol-fed knockout mice were not hypercholesterolemic. Low density lipoprotein receptor expression was repressed only modestly in the cholesterol-fed G5G8−/− mice (Fig. 6) even in the presence of an 18-fold increase in hepatic cholesterol levels. For reasons that are still poorly understood, the murine low density lipoprotein receptor is not very responsive to changes in hepatic cholesterol content (30). Persistent hepatic low density lipoprotein receptor function in the G5G8−/− mice may maintain efficient clearance of plasma lipoproteins.
No difference was apparent between the fractional absorption of dietary cholesterol in G5G8−/− mice vs. wild-type mice. In humans with sitosterolemia, the relative increase in the fractional absorption of dietary cholesterol is much more modest than that seen for plant sterols (1, 23). Humans with sitosterolemia have fractional absorption of dietary cholesterol in the high normal range when compared with unaffected controls (1, 4). The lack of a detectable difference between the fractional absorption of cholesterol in the G5G8−/− mice and littermate controls suggests that ABCG5 and ABCG8 may not limit cholesterol absorption from the intestine in chow-fed mice; however, this conclusion is contingent on the assumption that sitostanol absorption is similar in the wild-type and G5G8−/− mice. Previous studies found no significant difference in the measurements of the fractional absorption of dietary sterols when using sitostanol or chromic oxide as the nonabsorbable fecal marker in individuals with sitosterolemia (23), but we cannot exclude the possibility that G5G8−/− mice absorb an increased fraction of dietary sitostanol, leading to an underestimation of the true fractional absorption of dietary sterols in these animals. Alternatively, a difference in fractional cholesterol absorption in the G5G8−/− mice may only be elicited by increasing the dietary content of cholesterol.
The data presented here indicate that lipid transit into the bile via ABCG5 and ABCG8 is selective (Fig. 4). Whereas disruption of Abcb4 (Mdr2) prevents biliary secretion of both phospholipids and cholesterol (25), the marked decrease in biliary cholesterol levels in G5G8−/− animals was not coupled with a significant reduction in biliary phospholipid levels. No difference was measured in the molar ratio of phospholipids to biliary lipids between G5G8−/− mice and control animals. These data indicate that ABCG5 and ABCG8 are specific for neutral sterols and that biliary phospholipid secretion can proceed in the absence of cholesterol secretion.
In conclusion, the results of these studies are consistent with ABCG5 and ABCG8 being the major hepatobiliary transporter of both dietary and endogenously synthesized neutral sterols. In humans and mice, the biliary secretion of cholesterol is crucial for the maintenance of cholesterol homeostasis and constitutes a major defense against the accumulation of cholesterol and plant sterols in blood and tissues. The G5G8−/− mice generated here provide a useful animal model to further probe the trafficking of sterols into and out of the body and to screen for agents that may affect this important pathway.
Acknowledgments
We especially thank Guosheng Liang for guidance in embryonic stem cell culture. We also thank Scott Grundy, Gloria Vega, and Anh Nguyen for measurement of tissue and plasma sterols; Robert Guzman, Yinyan Ma, Liz Lummus, Scott Clark, Richard Gibson, Jeffrey Cormier, Melody Kerr, Norma Anderson, Anja Kerksiek, and Silvia Winnen, Amanda Fletcher for excellent technical assistance; and Scott Grundy, Jay Horton, David Russell, Joseph Goldstein, and Michael Brown for helpful discussions. This work was supported by The Howard Hughes Medical Institute, National Institutes of Health Grant HL20948, The W. M. Keck Foundation, the Perot Family Foundation, The Donald W. Reynolds Cardiovascular Clinical Research Center at Dallas, and the Bundesministerium für Bildong, Forschung, Wissenschaft und Technologie Grant 01EC9402.
Abbreviations
ABC, ATP-binding cassette
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bhattacharyya A. K. & Connor, W. E. (1974) J. Clin. Invest. 53, 1033-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorkhem I., Boberg, K. & Leitersdorf, E. (2001) in The Metabolic and Molecular Bases of Inherited Disease, eds. Scriver, C., Beaudet, A., Sly, W. & Valle, D. (McGraw–Hill, New York), Vol. II, pp. 2961–2988. [Google Scholar]
- 3.Miettinen T. A. (1980) Eur. J. Clin. Invest. 10, 27-35. [DOI] [PubMed] [Google Scholar]
- 4.Salen G., Shore, V., Tint, G. S., Forte, T., Shefer, S., Horak, I., Horak, E., Dayal, B., Nguyen, L., Batta, A. K., et al. (1989) J. Lipid Res. 30, 1319-1330. [PubMed] [Google Scholar]
- 5.Morganroth J., Levy, R. I., McMahon, A. E. & Gotto, A. M., Jr. (1974) J. Pediatr. (Berlin) 85, 639-643. [DOI] [PubMed] [Google Scholar]
- 6.Belamarich P. F., Deckelbaum, R. J., Starc, T. J., Dobrin, B. E., Tint, G. S. & Salen, G. (1990) Pediatrics 86, 977-981. [PubMed] [Google Scholar]
- 7.Gregg R. E., Connor, W. E., Lin, D. S. & Brewer, H. B., Jr. (1986) J. Clin. Invest. 77, 1864-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoenheimer R. (1931) Science 74, 579-584. [DOI] [PubMed] [Google Scholar]
- 9.Gould R. G., Jones, R. J., LeRoy, G. V., Wissler, R. W. & Taylor, C. B. (1969) Metabolism 18, 652-662. [DOI] [PubMed] [Google Scholar]
- 10.Salen G., Ahrens, E. H., Jr. & Grundy, S. M. (1970) J. Clin. Invest. 49, 952-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salen G., Horak, I., Rothkopf, M., Cohen, J. L., Speck, J., Tint, G. S., Shore, V., Dayal, B., Chen, T. & Shefer, S. (1985) J. Lipid Res. 26, 1126-1133. [PubMed] [Google Scholar]
- 12.Berge K. E., Tian, H., Graf, G. A., Yu, L., Grishin, N. V., Schultz, J., Kwiterovich, P., Shan, B., Barnes, R. & Hobbs, H. H. (2000) Science 290, 1771-1775. [DOI] [PubMed] [Google Scholar]
- 13.Lee M. H., Lu, K., Hazard, S., Yu, H., Shulenin, S., Hidaka, H., Kojima, H., Allikmets, R., Sakuma, N., Pegoraro, R., et al. (2001) Nat. Genet. 27, 79-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Repa J. J., Berge, K. E., Pomajzl, C., Richardson, J. A., Hobbs, H. & Mangelsdorf, D. J. (2002) J. Biol. Chem. 277, 18793-18800. [DOI] [PubMed] [Google Scholar]
- 15.Yu L., Li-Hawkins, J., Hammer, R. E., Berge, K. E., Horton, J. D., Cohen, J. C. & Hobbs, H. H. (2002) J. Clin. Invest. 110, 671-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graf G. A., Li, W.-P., Gerard, R. D., Gelissen, I., White, A., Cohen, J. C. & Hobbs, H. H. (2002) J. Clin. Invest. 110, 659-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dayal B., Ertel, N. H., Rapole, K. R., Asgaonkar, A. & Salen, G. (1997) Steroids 62, 451-454. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi S., Brown, M. S., Goldstein, J. L., Gerard, R. D., Hammer, R. E. & Herz, J. (1993) J. Clin. Invest. 92, 883-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimano H., Shimonur, L., Hammer, R., Herz, J., Goldstein, J. L., Brown, M. S. & Horton, J. D. (1997) J. Clin. Invest. 100, 2115-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarz M., Russell, D. W., Dietschy, J. M. & Turley, S. D. (1998) J. Lipid Res. 39, 1833-1843. [PubMed] [Google Scholar]
- 21.Turley S. D., Herndon, M. W. & Dietschy, J. M. (1994) J. Lipid Res. 35, 328-339. [PubMed] [Google Scholar]
- 22.Lutjohann D., Meese, C. O., Crouse, J. R., III & von Bergmann, K. (1993) J. Lipid Res. 34, 1039-1046. [PubMed] [Google Scholar]
- 23.Lutjohann D., Bjorkhem, I., Beil, U. F. & von Bergmann, K. (1995) J. Lipid Res. 36, 1763-1773. [PubMed] [Google Scholar]
- 24.Lutjohann D., Bjorkhem, I. & Ose, L. (1996) Scand. J. Clin. Lab. Invest. 56, 229-240. [DOI] [PubMed] [Google Scholar]
- 25.Smit J. J., Schinkel, A. H., Elferink, R. P. O., Groen, A. K., Wagenaar, E., van Deemter, L., Mol, C. A., Ottenhoff, R., van der Lugt, N. M. & van Room, M. A. (1993) Cell 75, 451-462. [DOI] [PubMed] [Google Scholar]
- 26.Dietschy J. M. & Turley, S. D. (2002) J. Biol. Chem. 277, 3801-3804. [DOI] [PubMed] [Google Scholar]
- 27.Rudel L. L., Kelley, K., Sawyer, J. K., Shah, R. & Wilson, M. D. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 1818-1827. [DOI] [PubMed] [Google Scholar]
- 28.Bhattacharyya A. K., Connor, W. E., Lin, D. S., McMurry, M. M. & Shulman, R. S. (1991) Arterioscler. Thromb. 11, 1287-1294. [DOI] [PubMed] [Google Scholar]
- 29.Carey M. C. & Duane, W. C. (1994) in The Liver: Biology and Pathobiology, eds. Arias, I. M., Boyer, J. L., Fausto, N., Jakoby, W. B., Schachter, D. A. & Shafritz, D. A. (Raven, New York), pp. 719–767.
- 30.Horton J. D., Shimano, H., Hamilton, R. L., Brown, M. S. & Goldstein, J. L. (1999) J. Clin. Invest. 103, 1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J., Goldstein, J. L., Hammer, R. E., Moon, Y. A., Brown, M. S. & Horton, J. D. (2001) Proc. Natl. Acad. Sci. USA 98, 13607-13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang G., Yang, J., Horton, J. D., Hammer, R. E., Goldstein, J. L. & Brown, M. S. (2002) J. Biol. Chem. 277, 9520-9528. [DOI] [PubMed] [Google Scholar]