Abstract
In previous studies, we have shown that the myelopoiesis dependent upon myelosupportive stroma required production of growth factors and heparan-sulphate proteoglycans, as well as generation of a negatively charged sialidase-sensitive intercellular environment between the stroma and the myeloid progenitors. In the present study, we have investigated the production, distribution and role of gangliosides in an experimental model of in vitro myelopoiesis dependent upon AFT-024 murine liver-derived stroma. We used the FDC-P1 cell line, which is dependent upon GM-CSF (granulocyte/macrophage colony-stimulating factor) for both survival and proliferation, as a reporter system to monitor bioavailability and local activity of GM-CSF. GM3 was the major ganglioside produced by stroma, but not by myeloid cells, and it was required for optimal stroma myelosupportive function. It was released into the supernatant and selectively incorporated into the myeloid progenitor cells, where it segregated into rafts in which it co-localized with the GM-CSF-receptor α chain. This ganglioside was also metabolized further by myeloid cells into gangliosides of the a and b series, similar to endogenous GM3. In these cells, GM1 was the major ganglioside and it was segregated at the interface by stroma and myeloid cells, partially co-localizing with the GM-CSF-receptor α chain. We conclude that myelosupportive stroma cells produce and secrete the required growth factors, the cofactors such as heparan sulphate proteoglycans, and also supply gangliosides that are transferred from stroma to target cells, generating on the latter ones specific membrane domains with molecular complexes that include growth factor receptors.
Keywords: ganglioside, granulocyte/macrophage colony-stimulating factor (GM-CSF), intercellular environment, membrane raft, myelopoiesis
Abbreviations: CMF-BSS, calcium- and magnesium-free balanced salt solution; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; GM-CSF, granulocyte/macrophage colony-stimulating factor; GM-CSF-Rα, GM-CSF-receptor α subunit; HPTLC, high-performance TLC; IL, interleukin; PDMP, D,L-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
INTRODUCTION
Cells of the myeloid lineage can either dwell inside solid tissues in close contact with adjacent cells and the intercellular matrix or circulate free in liquid tissues, such as blood and lymph. The former situation is required for proliferation and commitment of myeloid cell progenitors, which occur mainly in the bone marrow. Myeloid cell populations can be amplified further in peripheral tissues. Circulation of leucocytes and myeloid cells in blood is only transitory, as the final site of their migration and activity occurs inside normal or injured tissues [1–3]. In both bone marrow and peripheral tissues, myeloid cell proliferation and activation are dependent upon the local stroma cells, the extracellular matrix and the cytokines or inflammatory mediators that are locally produced or brought into the tissues by blood [4–6].
Intercellular mediators and cytokines usually attain only relatively low concentrations in circulating biological fluids and their activity is dependent upon binding to the available membrane receptors on the target cells. Inside solid tissues, the intercellular space is commonly narrow and the mediators can attain high concentrations and steep gradients, interacting with components of the extracellular matrix or the cell glycocalyx. Their bioavailability and activity are thus largely dependent upon macromolecular complexes that surround the membrane receptors [3,7,8]. Moreover, while settling or moving inside the tissues, myeloid cells and their progenitors become polarized and redistribute the specialized membrane regions, which can fully segregate the cytokine receptors and the associated membrane components by capping. The cell can thus be highly responsive to a given cytokine on one of its sides and insensitive on the other. Under such conditions, the interaction of myeloid cells with the adjacent stroma can be highly specific and spatially ordered [9].
GM-CSF (granulocyte/macrophage colony-stimulating factor), IL (interleukin)-3 and IL-5 are central haemopoietins that act through a common β-receptor chain. They control proliferation, differentiation and activation of myeloid cells [10]. Besides their function in the bone marrow myelopoiesis, GM-CSF and IL-5 also participate in tissue repair and regeneration [11,12]. Chronic activation of GM-CSF production in injured tissues leads to exacerbated reactions, such as proliferation and activation of myofibroblasts and formation of granulation tissue and/or fibrosis [13,14]. GM-CSF expression also occurs in many cancer cells, correlating with the tumour aggressiveness and the extension of pericancerous stroma reaction [15]. Local controls of GM-CSF activities are thus relevant for pathobiology and evolution of tissue reactions in pathological states.
Experimental in vitro models of myelopoiesis have been well established [16]. One of the major findings is the fact that relatively high concentrations of haemopoietins are required to drive proliferation of myeloid progenitors in liquid cell cultures, whereas very low or barely detectable levels are sufficient in cultures provided with the myelosupportive stroma. Several studies have indicated that the mitogenic activity of GM-CSF can be modulated by the glycosaminoglycan moiety of heparan sulphate proteoglycans produced by stromal cells, which could either enhance or abrogate the biological availability or activity of the cytokine [17–19]. We have also shown [20] that the polarity of the intercellular micro-environment was determinant for optimal interaction between GM-CSF and glycosaminoglycans. This was sensitive to digestion with sialidase. In view of these findings, we proposed that clusters of negatively charged molecules, such as sialylated glycoproteins and glycosphingolipids (gangliosides), associated with membranes of stromal cells, haematopoietic progenitors or both cell types, could be determinants for the local physical and chemical properties leading to the decreased pH, which induces the conformational change in GM-CSF required for its optimal interaction with glycosaminoglycans [6,20,21]. In the present study, we have addressed the question of the production, distribution and role of gangliosides in the experimental model of in vitro myelopoiesis in the presence of a myelosupportive stroma. We have used the FDC-P1 cell line, which is dependent upon GM-CSF for both survival and proliferation, as a reporter system to monitor the bioavailability and the local activity of GM-CSF.
EXPERIMENTAL
Materials
DMEM (Dulbecco's modified Eagle's medium), RPMI 1640 medium, C6-NBD-ceramide {N-[7-(4-nitrobenzo-2-oxa-1,3-diazole)]-6-aminohexanoyl-D-erythro-sphingosine}, FITC-conjugated cholera toxin, rabbit anti-(cholera toxin) antibody and standard lipids were from Sigma–Aldrich; FBS (fetal bovine serum) was from Cultilab; D-[U-14C]galactose (300 mCi/mmol) was from Amersham BioSciences; plastic tissue-culture dishes were Nunc; Fluorsave® and PDMP (D,L-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol) were from Calbiochem; silica-gel HPTLC (high-performance TLC) plates were from Merck; a rabbit polyclonal antibody to GM-CSF-Rα (GM-CSF-receptor α subunit) was from Santa Cruz Biotechnology; FITC-conjugated goat anti-mouse serum, CY3-conjugated goat anti-mouse serum and TRITC-conjugated donkey anti-rabbit serum were from Jackson ImmunoResearch. DH2, a monoclonal anti-GM3 antibody, was generously supplied by Dr Sen-itiroh Hakomori (University of Washington, Seattle, WA, U.S.A).
Cells and cell cultures
The murine AFT-024, FDC-P1 and WeHi-3B cell lines were obtained from the Rio de Janeiro Cell Bank (PABCAM, Federal University, Rio de Janeiro, Brazil). AFT-024 (established originally from fetal liver) and WeHi-3B cells were maintained in RPMI 1640 and DMEM respectively, supplemented with 10% (v/v) FBS. The FDC-P1 cell line was maintained in RPMI 1640 medium containing 10% (v/v) FBS and 10% (v/v) supernatant of WeHi-3B cells, which secrete IL-3 and GM-CSF constitutively. Cultures were maintained under a humidified 5% CO2 atmosphere at 37 °C. For a typical co-culture experiment, FDC-P1 cells were washed with CMF-BSS (calcium- and magnesium-free balanced salt solution) in order to remove IL-3 and GM-CSF, inoculated on to the semi-confluent monolayers of AFT-024 cells in 24-well plates (1×105 cells/well) and maintained in culture for 48 h. Cell proliferation was monitored under a phase-contrast-equipped inverted microscope. For co-cultures under PDMP treatment, the AFT-024 culture was maintained with 10 μM PDMP for 48 h, FDC-P1 cells were then inoculated on to the semi-confluent monolayers, as described above, and maintained in culture for 24, 48 and 72 h in the presence of 10 μM PDMP. The PDMP-containing medium was renewed every 24 h. Controls were done using FDC-P1 cultures maintained in standard AFT-024 supernatant supplemented at the beginning of the culture with 10 μM PDMP.
Determination of the FDC-P1 proliferation rate
FDC-P1 cells were inoculated on to 24-well cultures plates at 1×105 cells/well and maintained in three different medium preparations: (i) RPMI supplemented with 10% (v/v) FBS and 50% (v/v) medium conditioned by AFT-024 cells; (ii) RPMI supplemented with 10% (v/v) FBS and 50% (v/v) medium conditioned by AFT-024 cells treated with 10 μM PDMP; (iii) RPMI supplemented with 10% (v/v) FBS and 50% (v/v) medium conditioned by AFT-024 cells and addition of 10 μM PDMP; and (iv) RPMI supplemented with 10% (v/v) FBS and 50% (v/v) medium conditioned by AFT-024 cells and 20% (v/v) DH2 hybridoma supernatant (producing monoclonal anti-GM3 antibody). After 24 h, the viable cells were counted on a haemocytometer. RPMI supplemented with only 10% (v/v) FBS was used as the negative control and RPMI supplemented with 10% (v/v) FBS and 10% (v/v) WeHi-3B-cell-conditioned medium was used as the positive control. In order to monitor the potential non-specific effect of DH2 over FDC-P1 proliferation, cells were maintained in positive-control medium with 20% (v/v) DH2.
Metabolic labelling and lipid extraction
Cultures of stromal cells that reached confluence and cultures of FDC-P1 cells were incubated with 0.5 μCi/ml [14C]galactose for 12 h. Subsequently, cells were washed three times with ice-cold PBS, harvested from the plate and pelleted by a brief centrifugation [22]. Lipids were extracted from radiolabelled cell pellets with chloroform/methanol (2:1, v/v), and glycosphingolipids were purified using a Sep-Pack C18 column [23]. Inhibition of PDMP was determined after incubation of stromal cells for 24 and 72 h with 10 μM PDMP and incubation with 0.5 μCi/ml [14C]galactose for the last 12 h of incubation.
Chromatography and analysis of gangliosides
The purified lipid extracts were evaporated under N2 and run on HPTLC silica gel 60 plates with two successive solvent systems: chloroform/methanol (4:1, v/v) and chloroform/methanol/0.25% aqueous CaCl2 (15:9:2, by vol.). Lipids were developed in a tank as described by Nores et al. [24]. Radiolabelled sphingolipids were visualized by exposing to a radiographic film at −70 °C, and their relative content was determined by densitometric scanning of the X-ray film in a CS 930 Shimadzu UV/vis densitometer. The standards were visualized by exposure to resorcinol/HCl [25]. Ganglioside designations are based on TLC co-migration with standards. The identity of labelled phosphatidylcholine was confirmed by alkaline hydrolysis and co-migration with purified phospholipid standard as described by Rosales-Fritz et al. [26].
Immunocytochemistry
Semi-confluent monolayers of AFT-024 and co-cultures (maintained for 12 h) grown on coverslips were fixed in 4% (v/v) paraformaldehyde for 30 min, washed in PBS and incubated in 3% (w/v) BSA-containing PBS for 1 h at 37 °C to block non-specific binding sites. FDC-P1 cells were washed with PBS, and 5×104 cells were suspended in 100 μl of PBS. They were cytocentrifuged at 40 g on to coverslips, fixed in 4% (v/v) paraformaldehyde for 30 min and the non-specific binding sites were blocked as described above. The immunocytochemistry procedures were done as described by Crespo et al. [25]. The primary antibodies were: mouse monoclonal anti-GM3 (clone DH2) and rabbit polyclonal anti-(GM-CSF-Rα) (1:200) antibodies. The secondary antibodies were: CY3-conjugated goat anti-mouse Ig (1:1000), FITC-conjugated goat anti-(mouse Ig) (1:40) or TRITC-conjugated goat anti-(rabbit Ig) (1:500) antibodies. After a final washing with PBS, specimens were mounted with Fluorsave. Confocal images were obtained using a Carl Zeiss LSM5 Pascal laser scanning confocal microscope (Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Argentina) equipped with an argon/helium/neon laser and a 63× (1.4 numerical aperture) oil-immersion objective (Zeiss Plan-Apochromat). For GM1 immunostaining, two methods were used: (i) fixed cells were incubated with 4 μg/ml non-toxic cholera toxin B subunit, followed by mouse monoclonal anti-(cholera toxin) antibody; and (ii) fixed cells were incubated with FITC-conjugated non-toxic cholera toxin B subunit. Other immunocytochemistry procedures were the same as described above.
Shedding of gangliosides
The AFT-024 and FDC-P1 cells were metabolically labelled with 0.5 μCi/ml [14C]galactose for 12 h, washed and cultured in fresh medium for 48 h. Subsequently, the culture supernatant was collected, dialysed against distilled water for 24 h, lyophilized and the total lipids were extracted from the lyophilized powder with chloroform/methanol (1:1, v/v). The cells were harvested and lipids extracted with chloroform/methanol (2:1, v/v) as indicated above. The gangliosides present in the cells and in the supernatant were purified and analysed [27].
Uptake of radioactive lipids from AFT-024-conditioned medium by FDC-P1 cells
AFT-024 cells were metabolically labelled with 0.5 μCi/ml [14C]galactose for 12 h, washed and cultured in fresh medium for 48 h. The culture supernatant was collected and centrifuged at 1200 g for 20 min to eliminate cells and debris. Subsequently, FDC-P1 cells were cultivated overnight in RPMI supplemented with 10% (v/v) FBS and 50% (v/v) AFT-024 cell-conditioned medium. The cells were pelleted by brief centrifugation and lipids were extracted, purified and analysed as described previously [25]. The same protocol was applied to AFT-024 cells treated previously with 10 μM PDMP for 72 h and maintained in the presence of PDMP in order to obtain the conditioned medium.
Labelling of co-cultures
Co-cultures were submitted to different metabolic labelling protocols. (i) COC group: AFT-024 and FDC-P1 cells were incubated with 0.5 μCi/ml [14C]galactose for the last 12 h of 48 h co-cultures. (ii) ST group: semi-confluent monolayers of AFT-024 cells were incubated with 0.5 μCi/ml [14C]galactose for 12 h, washed three times with CMF-BSS and FDC-P1 cells were inoculated and co-cultured for the following 48 h. (iii) PDMP group: AFT-024 cells were incubated with 0.5 μCi/ml [14C]galactose for 12 h, washed three times with CMF-BSS and FDC-P1 cells treated previously with 10 μM PDMP for 6 h were inoculated on to the semi-confluent monolayers of AFT-024 cells and co-cultured for the following 48 h in the presence of PDMP. (iv) PREC group: FDC-P1 cells were incubated with 0.5 μCi/ml [14C]galactose for 12 h, washed three times with CMF-BSS, inoculated on to the semi-confluent monolayers of AFT-024 cells and cocultured for the following 48 h. For harvesting FDC-P1 cells from co-cultures, the medium was collected and the culture plates were washed three times with PBS at room temperature, under gentle stirring. FDC-P1 cells were harvested from washes. Under these conditions, a fraction of adherent FDC-P1 cells remained attached to the AFT-024 cell stroma.
Exosome preparations
Exosomes were collected from the medium of the ST and PDMP groups described above. Supernatants were centrifuged at 300 g for 5 min and at 1200 g for 20 min in order to eliminate cells and debris, followed by a centrifugation for 1.5 h at 70000 g. Two fractions were obtained: a high-density (pellet) and a low-density (hypodense) fraction. The exosomes concentrated in the pellet were washed in a large volume of PBS and centrifuged at 70000 g for 1.5 h [28]. The lipids were extracted, purified and analysed as described previously [28].
RESULTS
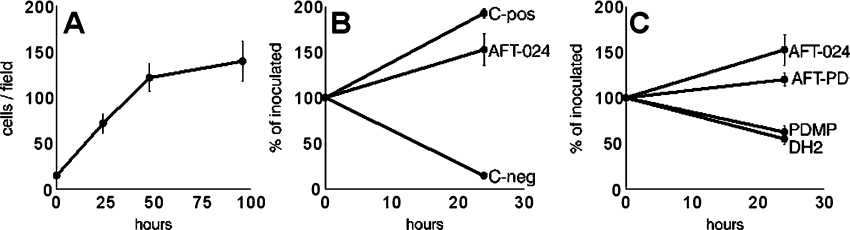
FDC-P1 cells proliferated both in co-culture with AFT-024 stroma (Figures 1A and 2) and in the presence of the AFT-024 cell-culture supernatant (Figure 1B). This is in accordance with our previous study [29] showing that AFT-024 cells, although lacking IL-3, produced and secreted high amounts GM-CSF, as well as heparan-sulphate-bearing proteoglycans that interacted with GM-CSF. These stroma cells could thus be used for studies on the GM-CSF-dependent myeloid cell proliferation. Inhibition of ganglioside synthesis in the stroma layer with PDMP significantly decreased the myelosupportive capacity in both experimental conditions (Figures 1C and 2), indicating that the synthesis and release of gangliosides into the supernatants were required for FDC-P1 cell proliferation. To exclude a direct toxic effect of PDMP on FDC-P1 cells, supernatant was collected from standard AFT-024 cell cultures and PDMP was added prior to its addition to FDC-P1 cells. Results indicated a small decrease in FDC-P1 proliferation, but this direct effect of PDMP was much lower than the inhibition observed when gangliosides shed by stroma cells was reduced due to PDMP treatment (Figure 1C).
Figure 1. Myelosupportive activity of AFT-24 cells for survival and proliferation of FDC-P1 cells.
(A) FDC-P1 cell proliferation in co-culture with AFT-024 cells. (B) FDC-P1 cell proliferation in the presence of the supernatant of AFT-024 cells, of the supernatant of WeHi-3B cells as the positive control (C-pos), and in the RPMI 1640 culture medium supplemented with 10% (v/v) FBS as the negative control (C-neg). (C) Inhibition of the FDC-P1 cell proliferation in the presence of AFT-024 cell supernatant harvested from cultures in which the synthesis of gangliosides was inhibited by PDMP, standard AFT-024 cell supernatant in the presence of 10 μM PDMP added at the beginning of culture (AFT-PD) or AFT-024 cell supernatant in the presence of DH2 (a monoclonal antibody to GM3) compared with the proliferation in the presence of the standard AFT-024 cell supernatants (AFT-024).
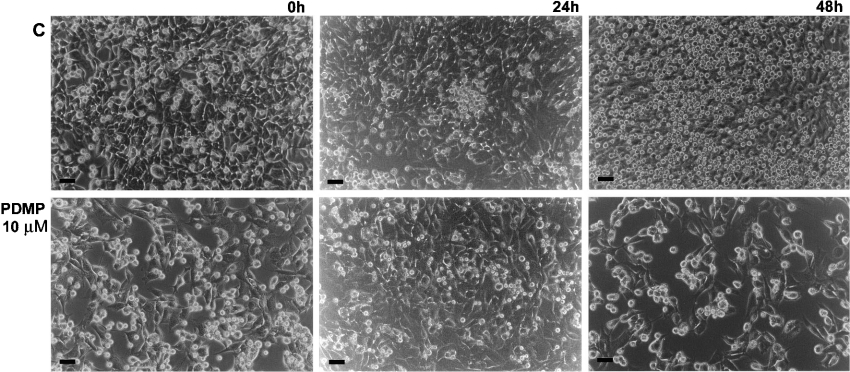
Figure 2. Inhibition of FDC-P1 cell survival and proliferation in co-culture with AFT-024 cells in which ganglioside synthesis was inhibited by PDMP.
FDC-P1 cells washed previously with CMF-BSS were inoculated on to the semi-confluent monolayers of AFT-024 cells at 1×105 cells/well and maintained for 0, 24 and 48 h. C, co-cultures maintained in standard conditions. PDMP, co-cultures maintained with 10 μM PDMP after inoculation of FDC-P1 cells upon AFT-024 semi-confluent cell monolayer treated previously with the inhibitor for 48 h. Phase-contrast microscopy of the cells is shown. Scale bar, 20 μm.
We had suspected previously that sialic-acid-containing molecules played an important role in modulating the cross-talk between stromal and myeloid cells, since capping of negatively charged molecules, sensitive to sialidase digestion, occurred in both cells at the site of interaction [21]. For this reason, we next analysed ganglioside synthesis in AFT-024 and FDC-P1 cells (Figures 3A and 3B, and Table 1). AFT-024 accumulated essentially GM3 and, to a lower extent, GD1a and GM1. This is similar to other myelosupportive stromata that we have studied: the S17 cell line derived from the bone marrow (F. C. R. Guma, unpublished work) and the liver connective tissue cells involved in inflammatory fibro-granulomatous reactions that have a tissue origin similar to AFT-024 cells (C. M. B. Andrade, unpublished work). Conversely, FDC-P1 cells accumulated only a low amount of GM3, which was metabolized further to several gangliosides of the a and b series. GM1 was the major ganglioside accumulated by FDC-P1 cells. All major gangliosides ran as doublets, in accordance with the fact that sphingolipids have differences in ceramide structures [30,31].
Figure 3. Analysis of glycosphingolipids in AFT-024 and FDC-P1 cells.
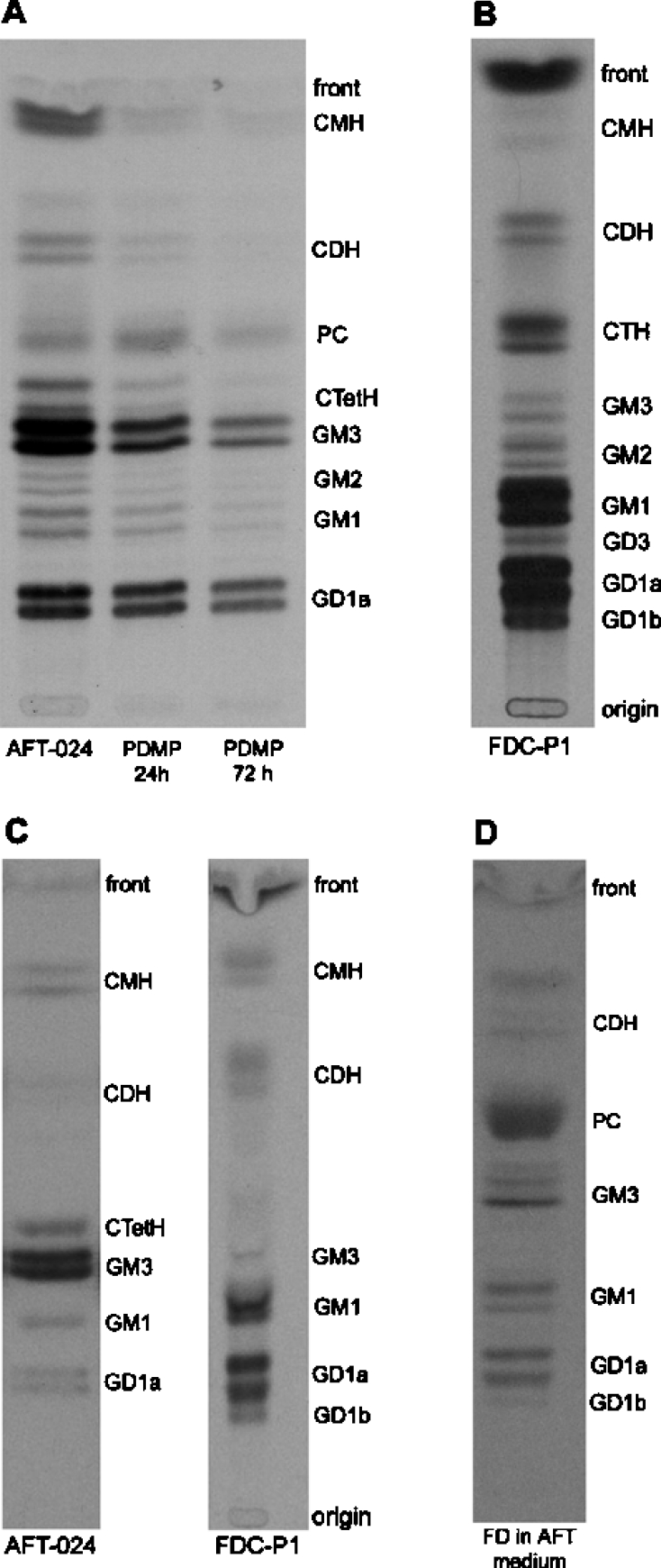
(A) Synthesis of glycosphingolipids in AFT-024 cells. Cell cultures were incubated for 12 h with [14C]galactose and for the last 12 h of treatment with 10 μM PDMP for 24 or 72 h. (B) Synthesis of glycosphingolipids in FDC-P1 cells. (C) Glycosphingolipids shed by AFT-024 and FDC-P1 cells. Cell cultures were incubated 12 h with [14C]galactose and the lipids were shed into the medium for 48 h. (D) FDC-P1 uptake of glycosphingolipids from AFT-024 cell-conditioned medium. AFT-024 cultures were incubated for 12 h with [14C]galactose and the lipids were shed into the medium for 48 h. FDC-P1 cells washed previously with CMF-BSS were then inoculated in RPMI supplemented with 10% (v/v) FBS and 50% (v/v) of medium conditioned by AFT-024 cells. Lipids were extracted, purified, analysed by HPTLC and visualized by fluorography. The radioactive bands correspond to the lipids indicated. CTetH: ceramide tetrahexoside; CTH, ceramide trihexoside; CDH, ceramide dihexoside; CMH, ceramide monohexoside; PC, phosphatidylcoline. Gangliosides are named according to Svennerholm [47].
Table 1. Densitometric analysis of metabolically labelled lipids from AFT-024 and FDCP-1 cells.
Cell cultures were incubated for 12 h with [14C]galactose and lipids were extracted, purified, analysed by HPTLC and visualized by fluorography. Results are expressed as a percentage of the total radioactivity incorporated. GD1b, GD1a, GM1, GM2, GD3 and GM3 are gangliosides named according to Svennerholm [47]; CtetH, ceramide tetrahexoside; CTH, ceramide trihexoside; CDH, ceramide dihexoside; CMH, ceramide monohexoside; PC, phosphatidylcholine; n.d, not detected.
| Proportion of glyolipids (% of total radioactivity) | ||
|---|---|---|
| Lipids | AFT-024 | FDC-P1 |
| GD1b | n.d. | 6.9 |
| GD1a | 20.6 | 26.7 |
| GD3 | n.d. | 2.4 |
| GM1 | 3.6 | 24.2 |
| GM2 | 0.9 | 3.8 |
| GM3 | 52.8 | 2.6 |
| CtetH | 7.0 | n.d. |
| PC | 2.5 | n.d. |
| CTH | n.d. | 9.7 |
| CDH | 3.0 | 4.0 |
| CMH | 8.1 | n.d. |
The required presence of gangliosides in the stroma-mediated support of myelopoiesis was shown by PDMP-dependent inhibition of their synthesis. Exposure of AFT-024 cells to 10 μM PDMP for 3 days led to a noticeable decrease in ganglioside synthesis (57 and 82% in 24 and 72 h respectively) and in their precursors lactosylceramide [CDH (ceramide dihexoside)] and glucosylceramide [CMH (ceramide monohexoside)] (Figure 3A). We questioned whether the major ganglioside of the stroma, GM3, was specifically involved in this interaction. In the presence of DH2 (a monoclonal antibody against GM3), inhibition of FDC-P1 cell proliferation was equivalent to that elicited by the overall inhibition of ganglioside synthesis, indicating the crucial role of GM3 in the myelosupportive function of the stroma (Figure 1C). Additionally, we performed experiments to discard a direct inhibition of cell proliferation by DH2. FDC-P1 cells were incubated in the standard culture medium containing DH2 at the same concentration as used in the experiment in Figure 1(C). No effect of DH2 on FDC-P1 cell proliferation was found, clearly excluding a direct effect of DH2 (results not shown).
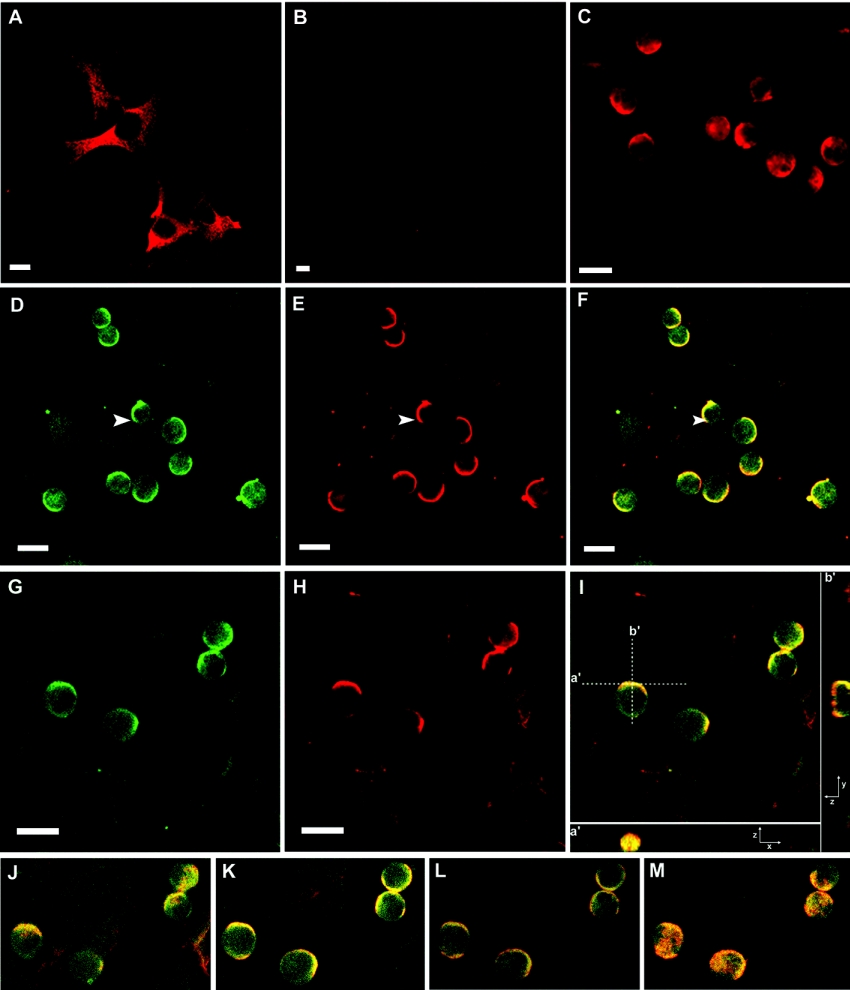
Since the response to GM-CSF stimulation requires its interaction with the FDC-P1 membrane through binding to the GM-CSF-R, we investigated the spatial distribution of gangliosides and GM-CSF-Rα on FDC-P1 cells (Figure 4). In accordance with the TLC analysis of metabolic incorporation of [14C]galactose (Figures 3A and 3B, and Table 1), AFT-024 cells displayed a strong labelling with DH2 (monoclonal anti-GM3 antibody) in the perinuclear region and a faint labelling of cell membranes (Figure 4A). Conversely, the GM3 labelling of FDC-P1 cells was barely detectable (Figure 4B). However, when co-cultures were studied, an intense labelling of GM3 was observed on the membranes of FDC-P1 cells, but not in the perinuclear region. Instead, labelling disclosed a full capping on one side of the cell only (Figure 4C). A similar labelling is shown in Figures 4(D) and 4(G) and it can be compared with the labelling of the GM-CSF-Rα (Figures 4E and 4H). The merged images disclosed that GM-CSF-Rα-stained areas co-distributed almost completely with GM3 staining, despite extensive areas of GM3 staining outside of areas of GM-CSF-Rα staining (Figures 4F and 4I). In many cases, the capping was observed at the contact regions both between two FDC-P1 cells and between stroma and FDC-P1 cells (Figures 4Ia′, 4Ib′ and 4J–4M).
Figure 4. Subcellular location of GM3 and GM-CSF-Rα in cell cultures and co-culture systems.
Cells were immunostained for GM3 with DH2 and immunostained for GM-CSF-Rα with a polyclonal anti-(GM-CSF-Rα) antibody. (A) AFT-024 cells immunostained for GM3. (B) FDC-P1 cells immunostained for GM3. (C) Co-culture immunostained for GM3. (D and G) Co-cultures immunostained for GM3 with monoclonal antibody DH2. (E and H) Co-cultures immunostained for GM-CSF-Rα. (F) Merged image from (D) and (E). (I) Merged image of (G) and (H). Single confocal sections (x, y) of 0.7 μm were taken parallel to the coverslip, except for (I) in which a series of z sections were collected and displayed using the ortho-mode in LSM5 Pascal software provided with the microscope. A single x, y section of the stack is shown in (I). An x, z section (a′) is shown at the bottom of (I) and a y, z section (b′) at the right of (I). (J–M) are single x, y sections from the same z stack of (I) (from bottom to top). The arrows indicate capping of GM3 (D), GM-CSF-Rα (E) and co-capping of GM3 and GM-CSF-Rα (F). Scale bars, 10 μm.
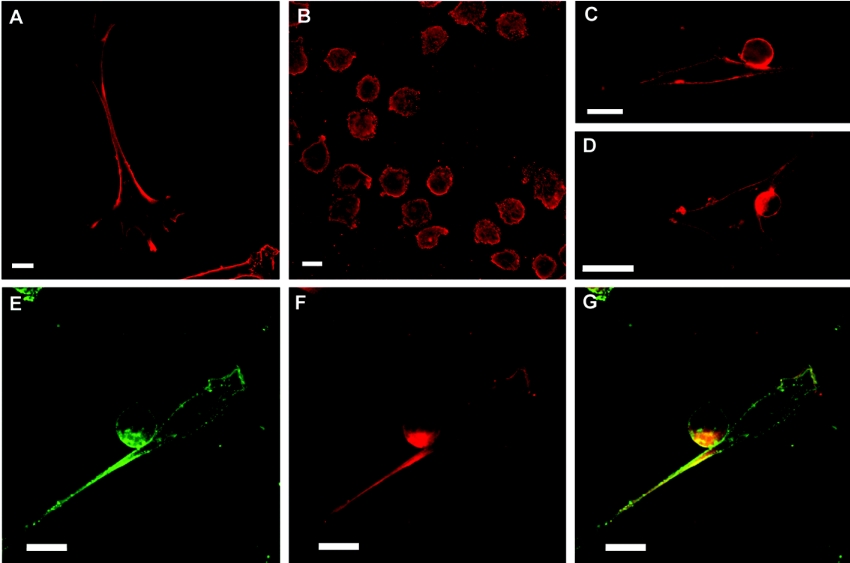
Taking into consideration that GM1 is the major ganglioside produced by FDC-P1 cells and to a lesser extent in AFT-024 cells (Figures 3A and 3B, and Table 1), the subcellular distribution of this lipid was analysed and compared with the distribution of GM3. GM1 was present homogeneously on the membranes of both the stroma and myeloid cells (Figures 5A and 5B respectively). When co-cultures were studied, the pattern of GM1 distribution was similar to GM3. A significant capping of GM1 was observed at the contact region between AFT-024 and FDC-P1 cells (Figures 5C and 5D). However, the analysis of GM-CSF-Rα distribution disclosed only a partial co-distribution of the receptor staining with GM1 staining, mainly at the contact region (Figures 5E–5G).
Figure 5. Subcellular location of GM1 and GM-CSF-Rα in cell cultures and co-culture systems.
GM1 was examined by incubation of cholera toxin, followed by a monoclonal anti-(cholera toxin) antibody or by the fluorescence of FITC-conjugated cholera toxin. Cells were immunostained for GM-CSF-Rα with a polyclonal anti-(GM-CSF-Rα) antibody. (A) AFT-024 cells immunostained for GM1. (B) FDC-P1 cells immunostained for GM1. (C–E) Co-cultures immunostained for GM1. (F) Co-cultures immunostained for GM-CSF-Rα. (G) Merged images from (E) and (F). Single confocal sections of 0.7 μm were taken parallel to the coverslip. Scale bars, 10 μm.
The increased presence of GM3 on FDC-P1 cells in co-culture with AFT-024 raised the question of the mechanism of this apparent contribution of the stroma to the ganglioside composition of the myeloid cell membrane. In order to provide direct evidence of the functional intercellular transfer of sphingolipids in the model of mylosupportive stroma, shedding of radiolabelled gangliosides from AFT-024 and FDC-P1 cells was monitored (Figure 3C). The major ganglioside shed by the stroma cells was GM3, whereas FDC-P1 cells shed essentially GM1 and GD1a. The gangliosides harvested from the culture medium represented essentially the pattern of their synthesis in the two cell types, with a relatively low presence of GD1a in the supernatant compared with its presence in AFT-024 cells. When unlabelled FDC-P1 cells were incubated with the supernatant harvested from the AFT-024 cell culture treated with [14C]galactose in which GM3 was the major component (Figure 3B), a number of labelled compounds were detected (Figure 3D). The pattern suggests that the GM3 captured from the medium was metabolized further in a similar manner to the metabolism of endogenous GM3 in FDC-P1 cells (Figure 3B). When the same experiment was done with conditioned medium obtained from AFT-024 cells metabolically labelled and treated with PDMP, the presence of the same ganglioside species in FDC-P1 cells was observed, although the incorporated radioactivity was lower than in control cells (results not shown).
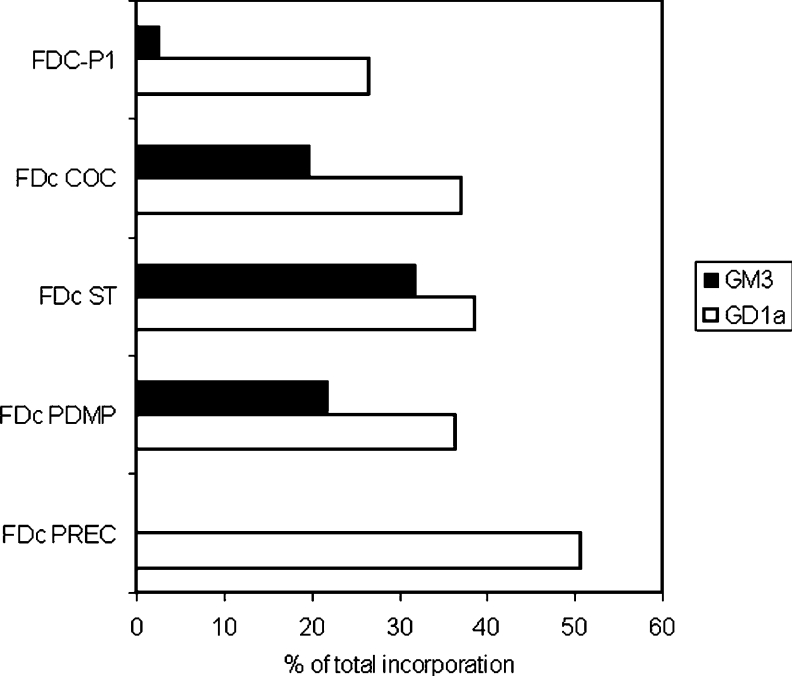
Quantification of the densitometric analysis of GM3 and GD1a (Figure 6) in FDC-P1 cells co-cultured with AFT-024 cells showed an increased quantity of labelled GM3 (COC group=20%) when compared with FDC-P1 cells cultivated alone (2.6%). The increased amount of GM3 labelled in FDC-P1 cells isolated from co-cultures was clearly detectable when only stroma cells were labelled (ST group=32%). These results indicate the transfer of GM3 from AFT-024 cells to FDC-P1 cells. To exclude the possibility of the GM3 synthesis by FDC-P1 cells from metabolically labelled ganglioside precursors captured from AFT-024 cells, the de novo synthesis of FDC-P1 glycosphingolipids was inhibited by PDMP before inoculation on to the AFT-024 cellular monolayer (PDMP group) labelled previously. Under this condition, an increased fraction of GM3 (22%) was found in FDC-P1 cells isolated from co-culture, indicating again the transfer of GM3. Moreover, when only FDC-P1 cells were metabolically labelled before inoculation on to stroma cells (PREC group), GM3 was not detected in FDC-P1 cells isolated from co-cultures. The presence of GD1a in ST and PDMP groups confirms the further metabolism of the captured GM3. Taken together, our results stand in contrast with the data reported for fibroblasts, neurons and neuroblastoma cells that incorporated 1-[3H]sphingosine and synthesized labelled sphingolipids [32]. In this study, the released sphingolipids were taken up by cells, but they segregated to lysosomes and were entirely catabolized, indicating that, in this case, trafficking of sphingolipids to and from the extracellular environment does not allow the modification of cell membrane composition [32].
Figure 6. Analysis of FDC-P1 uptake of gangliosides GM3 and GD1a from AFT-024-cell-conditioned medium.
Results are expressed as the percentage of total radioactivity incorporated. FDc corresponds to myeloid progenitors isolated after co-culture submitted to different metabolic labelling protocols as described in the Experimental section. COC, metabolic labelling during the last 12 h of 48 h of co-culture; ST, metabolic labelling only of stromal cells prior to co-culture for 48 h; PDMP, metabolic labelling only of stromal cells and treatment of FDC-P1 cells with 10 μM PDMP prior to co-culture for 48 h in the presence of 10 μM PDMP; PREC, metabolic labelling only of myeloid precursor cells prior to co-culture for 48 h.
The results shown in Figures 4 and 5 are in agreement with our previous observation of extensive capping of raft-like structures on membranes of the stroma in contact with FDC-P1 cells [21]. Taken together, these data suggest formation of specific regions of the membranes enriched in proteoglycans, glycosphingolipids and growth factor receptors at the interface between the stroma and target cells. This model is compatible with a non-random transfer of membrane compounds between the adjacent stroma and the myeloid cells that represents a new model of co-operation between the myelosupportive stromata and the target cells. We questioned further whether the intercellular transfer of membrane regions with sorted compounds, which potentially represent operational molecular units, could be achieved by the release and subsequent uptake of exosomes. The exosomal fraction was isolated by ultracentrifugation, as described previously by Skokos et al. [28]. We found that 40% of radiolabelled gangliosides released into the supernatant in a co-culture containing AFT-024 and FDC-P1 cells were found in the exosome fraction.
DISCUSSION
The concept of tissue stromata has gained increasing interest in view of the extending use of cell therapies, based upon introduction of exogenous stem or progenitor cells into the tissues that require repair or regeneration. It is expected that the introduced cells can receive sufficient information from the stroma in order to proliferate within the physiological limits and to differentiate into the required cell types in the injured or degenerated tissues. Cells have also to re-establish appropriate spatial tissue organization. Tissue stromata have been studied extensively in the context of providing the required cytokines and chemokines, as well as mediators of homoeostasis or inflammatory reactions and repair. They are also known to provide extracellular matrix that provides spatial tissue support. The present study indicates that, in addition, stroma cells may also supply membrane elements to the target cells, which can considerably modulate their ability to respond to given cytokines by segregating the receptors and enhancing or inhibiting intracellular signalling cascades.
We have demonstrated in the present and in parallel studies (C. M. B. Andrade and F. C. R. Guma, unpublished work) that GM3 is the major ganglioside in different myelosupportive stroma cells, such as AFT-024, S17 and GR cells, but it is only a minor ganglioside in the myeloid precursor FDC-P1 cells, although it is required for their optimal response to GM-CSF. In the present study, we describe the transfer of GM3 from stroma to FDC-P1 cells, the capping of GM3 at the contact regions between the two cell types and the co-localization of GM-CSF-Rα with GM3 on the myeloid cells. The GM-CSF-Rα co-localizes only partially with ganglioside GM1, a minor constituent of stroma cells, although the capping of GM1 by both cells at the contact regions was detectable, suggesting that GM3 and GM1 are located in different membrane regions or in different rafts, as shown previously in other studies [33].
The biological significance of gangliosides shedding from normal and tumour cells and their effect on other cells has been well documented [27,34–36]. Gangliosides are shed from the cell plasma membrane as monomers, micelles or membrane vesicles enriched in caveolin-1 [37,38]. It has been also reported that exosomes, small vesicles formed from the fusion of multivesicular endosomes with the cell surface, could be transferred from professional to non-professional antigen-presenting cells for effective T-cell stimulation [39,40]. In this model, exosomes carry a number of immunoregulatory molecules and may be considered as immunologically sophisticated vectors for various antigens [41]. In the present study, we have shown that AFT-024 cells transfer GM3 to FDC-P1 cells. This transfer can be done by a direct contact between the cells or by uptake of GM3 shed to the extracellular environment. A random shedding and uptake of gangliosides is less probable, in view of their specific localization and metabolism on the target cells. The proliferation of FDC-P1 cells in the supernatant of AFT-024 cells was dependent on GM3 and we found that gangliosides were shed in the exosomal fractions. Therefore the exosomes might be an alternative way for secretion and cell uptake of GM3 now described in our experimental model.
Interaction of gangliosides with growth factor receptors has been studied extensively [42,43]. In many cases, glycosphingolipids were reported to have an inhibitory activity on signalling, decreasing the response to cytokines such as EGF (epidermal growth factor), PDGF (platelet-derived growth factor) or FGF (fibroblast growth factor). In contrast, in our model of stroma-dependent myelopoiesis, gangliosides and, in particular, GM3 are required for optimal growth factor signalling. We have already proposed that this is due to generation of a particular negatively charged intercellular space in which the interaction among the growth factor, heparan sulphate proteoglycans and the corresponding receptor are most active [20,21]. We can now propose the following scenario. The physical contact between stroma and myeloid progenitors elicits extensive capping on both cells and the concentration of selected sets of membrane molecules at the cell interfaces. Stroma cells provide growth factors, which are required for survival and proliferation of myeloid cells. They also supply gangliosides, which are transferred from stroma to target cells, generating on the latter specific membrane domains with molecular complexes that include growth factor receptors surrounded by the associated molecules. It is known that such a formation of lipid rafts containing membrane receptors is often required, particularly in cases where the functional receptor is composed of many interacting units as, for example, the T-lymphocyte cell receptor [44].
Three mechanistic models for modulation of growth factor and the corresponding receptor activity by gangliosides have been proposed: (i) ganglioside–ligand interaction, (ii) ganglioside regulation of receptor dimerization, and (iii) ganglioside modulation of receptor activity and its subcellular localization [43]. The modulation of receptor activity involves regulation of kinase activity, such as PKC (protein kinase C) [45] and Src kinases [46]. In this sense, our results suggest that modulation of GM-CSF-R interaction with the ligand, through formation of macromolecular complexes involving GM3, may be determinant for signal transduction through phosphorylation cascades. Further studies will be required to determine whether GM3 directly activates the GM-CSF interaction with its receptor or indirectly involves the activity of co-activators or inhibitors segregated in the same membrane microdomains.
Acknowledgments
We are grateful to Tatiana Coelho-Sampaio for critical review of the manuscript prior to submission. We also thank C. Mas for excellent assistance with confocal microscopy and image analysis. This work was supported in part by grants from SECyt (Secretaria de Ciencia y Tecnología)-Universidad Nacional de Córdoba, International Society for Neurochemistry (Special ISN One-Time Fund), PEI (Proyecto de Estímulo a la Investigación) of CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas; grant no. 6462), Fundación Antorchas (grant no. 14116-112) and from Agencia Nacional de Promoción Cientifica y Tecnológica (FONCYT; grant no. 01-13522) to J. L. D.; Programa de Centros Asociados de Posgrado Brasil/Argentina [CAPES-SPU (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Secretaría de Politicas Universitárias); grant no. 001/02) to J. L. D. and F. C. R. G.; and CNPq (Conselho Nacional De Desenvolvimento Científico e Tecnológico) and FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro) grants to R. B.
References
- 1.Allen T. D., Dexter T. M., Simmons P. J. Marrow biology and stem cells. In: Dexter T. M., Garland J. M., Testa N. G., editors. Colony Stimulating Factors. New York: Marcel Dekker; 1990. pp. 1–38. [Google Scholar]
- 2.Borojevic R., El-Cheikh M. C., Alvarez-Silva M., Almeida K. G., Dutra H. S. Cells Hepatic Sinusoid, vol, 4. Leiden, The Netherlands: Kupfer Cell Fundation; 1993. Hepatic myelopoiesis associated with inflammation: liver connective tissue cells form a myelopoietic stroma; pp. 130–133. [Google Scholar]
- 3.Zon L. I. Developmental biology of hematopoiesis. Blood. 1995;86:2876–2891. [PubMed] [Google Scholar]
- 4.Heyworth C. M., Ponting I. L. O., Dexter T. M. The response of hematopoietic cells to growth factors: developmental implications of synergic interactions. J. Cell Sci. 1988;91:239–247. doi: 10.1242/jcs.91.2.239. [DOI] [PubMed] [Google Scholar]
- 5.Dexter T. M., Coutinho L. H., Spooncer E., Heyworth C. M., Daniel C. P., Schirot R., Chang J., Allen T. D. Molecular control of hematopoiesis. Ciba Found. Symp. 1990;148:76–86. doi: 10.1002/9780470513880.ch6. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho M. A., Arcanjo K., Silva L. C. F., Borojevic R. The capacity of connective tissue stromas to sustain myelopoiesis depends both upon the growth factors and the local intercellular environment. Biol. Cell. 2000;92:605–614. doi: 10.1016/s0248-4900(01)01113-3. [DOI] [PubMed] [Google Scholar]
- 7.Arai K., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Ann. Ver. Bioch. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- 8.Taipaile J., Keski-Oja J. Growth factors in the extracellular matrix. FASEB J. 1997;11:51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 9.Giebel B., Corbeil D., Beckmann J., Höhn J., Giesen K., Fischer J., Kögler G., Wernet P. Segregation of lipid raft markers including CD133 in polarized human hematopoietic stem and progenitor cells. Blood. 2004;104:2332–2338. doi: 10.1182/blood-2004-02-0511. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton J. A. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 11.Holgate S. T. Epithelial damage and response. Clin. Exp. Allergy. 2000;30:37–41. doi: 10.1046/j.1365-2222.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- 12.Franzen R., Bouhy D., Schoenen J. Nervous system injury: focus on the inflammatory cytokine ‘granulocyte-macrophage colony stimulating factor’. Neurosci. Lett. 2004;361:76–78. doi: 10.1016/j.neulet.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Desmoulière A., Tuchweber B., Gabbiani G. Role of myofibroblast differentiation during liver fibrosis. J. Hepatol. 1995;22:61–64. [PubMed] [Google Scholar]
- 14.Mann A., Breuhahn K., Schirmacher P., Blessing M. Keratinocyte-derived granulocyte-macrophage colony stimulating factor accelerates wound healing: Stimulation of keratinocyte proliferation, granulation tissue formation, and vascularization. J. Invest. Dermatol. 2001;117:1382–1390. doi: 10.1046/j.0022-202x.2001.01600.x. [DOI] [PubMed] [Google Scholar]
- 15.Bretscher V., Andreutti D., Neuville P., Martin M., Martin F., Lefebvre O., Gilles C., Benzonana G., Gabbiani G. GM-CSF expression by tumor cells correlates with aggressivity and with stroma reaction formation. J. Submicrosc. Cytol. Pathol. 2000;32:525–533. [PubMed] [Google Scholar]
- 16.Dexter T. M., Testa N. G. Differentiation and proliferation of hemopoietic cells in culture. Methods Cell Biol. 1976;14:387–405. doi: 10.1016/s0091-679x(08)60498-7. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez-Silva M., Silva L. C., Borojevic R. Cell membrane-associated proteoglycans mediate extramedullar myeloid proliferation in granulomatous inflammatory reactions to schistosome eggs. J. Cell Sci. 1993;104:477–484. doi: 10.1242/jcs.104.2.477. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez-Silva M., Borojevic R. GM-CSF and IL-3 activities in schistosomal liver granulomas are controlled by stroma-associated heparan-sulfate proteoglycans. J. Leukocyte Biol. 1996;59:435–441. doi: 10.1002/jlb.59.3.435. [DOI] [PubMed] [Google Scholar]
- 19.Modrowski D., Basle M., Lomri A., Marie P. J. Syndecan-2 is involved in the mitogenic activity and signaling of granulocyte-macrophage colony-stimulating factor in osteoblasts. J. Biol. Chem. 2000;275:9178–9185. doi: 10.1074/jbc.275.13.9178. [DOI] [PubMed] [Google Scholar]
- 20.Wettreich A., Sebollela A., Carvalho M. A., Azevedo S. P., Borojevic R., Ferreira S. T., Coelho-Sampaio T. Acidic pH modulates the interaction between human granulocyte-macrophage colony-stimulating factor and glycosaminoglycans. J. Biol. Chem. 1999;274:31468–31475. doi: 10.1074/jbc.274.44.31468. [DOI] [PubMed] [Google Scholar]
- 21.Borojevic R., Carvalho M. A., Corrêa-Junior J. D., Arcanjo K., Gomes L., Joazeiro P. P., Balduino A., Wettreich A., Coelho-Sampaio T. Stroma-mediated granulocye-macrophage colony-stimulating factor (GM-CSF) control of myelopoiesis: spatial organisation of intercellular interactions. Cell Tissue Res. 2003;313:55–62. doi: 10.1007/s00441-003-0726-0. [DOI] [PubMed] [Google Scholar]
- 22.Zurita A. R., Maccioni H. J., Daniotti J. L. Modulation of epidermal growth factor receptor phosphorylation by endogenously expressed gangliosides. Biochem. J. 2001;355:465–472. doi: 10.1042/0264-6021:3550465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams M. A., McCluer R. H. The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J. Neurochem. 1980;35:266–269. doi: 10.1111/j.1471-4159.1980.tb12515.x. [DOI] [PubMed] [Google Scholar]
- 24.Nores G. A., Mizutamari R., Kremer M. Chromatographic tank designed to obtain highly reproducible high-performance thin-layer chromatograms of gangliosides and neutral glycosphingolipids. J. Chromatogr., A. 1994;686:155–157. [Google Scholar]
- 25.Crespo P. M., Zurita A. R., Giraudo C. G., Maccioni H. J. F., Daniotii J. L. Ganglioside glycosyltransferases and newly synthesized gangliosides are excluded from detergent-insoluble complexes of Golgi membranes. Biochem. J. 2004;377:561–568. doi: 10.1042/BJ20031016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosales-Fritz V. M., Daniotti J. L., Maccioni H. J. Chinese hamster ovary cells lacking GM1 and GD1a synthesize gangliosides upon transfection with human GM2 synthase. Biochim. Biophys. Acta. 1997;1354:153–158. doi: 10.1016/s0167-4781(97)00117-6. [DOI] [PubMed] [Google Scholar]
- 27.Sietsma H., Nijhof W., Dontje B., Vellenga E., Kamps W. A., Kok J. W. Inhibition of hemopoiesis in vitro by neuroblastoma-derived gangliosides. Cancer Res. 1998;58:4840–4844. [PubMed] [Google Scholar]
- 28.Skokos D., Le Panse S., Villa I., Rousselle J. C., Peronet R., David B., Namane A., Mécheri S. Mast cell-cependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J. Immunol. 2001;166:868–876. doi: 10.4049/jimmunol.166.2.868. [DOI] [PubMed] [Google Scholar]
- 29.Arcanjo K., Belo G., Folco C., Werneck C. C., Borojevic R., Silva L. C. F. Biochemical characterization of heparan sulfate derived from murine hemopoietic stromal cell lines: a bone marrow-derived cell line S17 and a fetal liver-derived cell line AFT024. J. Cell. Biochem. 2002;87:160–172. doi: 10.1002/jcb.10293. [DOI] [PubMed] [Google Scholar]
- 30.Ziulkoski A. L., Zimmer A. R., Zanettini J. S., Trugo L. C., Guma F. C. R. Synthesis and transport of different sphingomyelin species in rat Sertoli cells. Mol. Cell. Biochem. 2001;219:57–64. doi: 10.1023/a:1011039630613. [DOI] [PubMed] [Google Scholar]
- 31.Andrade C. M. B., Trindade V. M. T., Cardoso C. C. A., Ziulkoski A. L., Trugo L. C., Guaragna R. M., Borojevic R., Guma F. C. R. Changes of sphingolipid species in the phenotype conversion from myofibroblasts to lipocytes in hepatic stellate cells. J. Cell. Biochem. 2003;88:533–544. doi: 10.1002/jcb.10373. [DOI] [PubMed] [Google Scholar]
- 32.Chigorno V., Giannotta C., Ottico E., Sciannamblo M., Mikulak J., Prinetti A., Sonnino S. Sphingolipid uptake by cultured cells: complex aggregates of cell sphingolipids with serum proteins and lipoproteins are rapidly catabolized. J. Biol. Chem. 2005;280:2668–2675. doi: 10.1074/jbc.M407749200. [DOI] [PubMed] [Google Scholar]
- 33.Gómez-Moutón C., Abad J. L., Mira E., Lacalle R. A., Gallardo E., Jiménez-Baranda S., Illa I., Bernad A., Mañes S., Martínez-A C. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9642–9647. doi: 10.1073/pnas.171160298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Li R., Ladisch S. Exogenous ganglioside GD1a enhances epidermal growth factor receptor binding and dimerization. J. Biol. Chem. 2004;279:36481–36489. doi: 10.1074/jbc.M402880200. [DOI] [PubMed] [Google Scholar]
- 35.McKallip R., Li R., Ladisch S. Tumor gangliosides inhibit the tumor-specific immune response. J. Immunol. 1999;163:3718–3726. [PubMed] [Google Scholar]
- 36.Brodsky V., Zvezdina N., Nechaeva N., Novikova T., Gvasava I., Fateeva V., Gracheva H. Loss of hepatocyte co-operative activity after inhibition of ganglioside GM1 synthesis and shedding. Cell Biol. Int. 2003;27:935–942. doi: 10.1016/j.cellbi.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Kong Y., Li R., Ladisch S. Natural forms of shed tumor gangliosides. Biochim. Biophys. Acta. 1998;1394:43–56. doi: 10.1016/s0005-2760(98)00096-4. [DOI] [PubMed] [Google Scholar]
- 38.Dolo V., Li R., Dillinger M., Flati S., Manela J., Taylor B. J., Pavan A., Ladisch S. Enrichment and localization of ganglioside GD3 and caveolin-1 in shed tumor cell membrane vesicles. Biochim. Biophys. Acta. 2000;1486:265–274. doi: 10.1016/s1388-1981(00)00063-9. [DOI] [PubMed] [Google Scholar]
- 39.de Gassart A., Géminard C., Février B., Raposo G., Vidal M. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102:4336–4344. doi: 10.1182/blood-2003-03-0871. [DOI] [PubMed] [Google Scholar]
- 40.Denzer K., Kleijmeer M. J., Heijnen H. F., Stoorvogel W., Geuze H. J. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 41.Dimitris S., Hany G. B., Michèle R., Salah M. Immunoregulatory properties of mast cell-derived exosomes. Mol. Immunol. 2001;38:1359–1362. doi: 10.1016/s0161-5890(02)00088-3. [DOI] [PubMed] [Google Scholar]
- 42.Hakomori S. The glycosynapse. Proc. Natl. Acad. Sci. U.S.A. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miljan E. A., Bremer E. G. Regulation of growth factor receptors by gangliosides. SciSTKE 2002. 2002:RE15. doi: 10.1126/stke.2002.160.re15. [DOI] [PubMed] [Google Scholar]
- 44.Razzaq T. M., Ozegbe P., Jury E. C., Sembi P., Blackwell N. M., Kabouridis P. S. Regulation of T-cell receptor signalling by membrane microdomains. Immunology. 2004;113:413–426. doi: 10.1111/j.1365-2567.2004.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sorice M., Longo A., Garofalo T., Mattei V., Misasi R., Pavan A. Role of GM3-enriched microdomains in signal transduction regulation in T lymphocytes. Glycoconj. J. 2004;20:63–70. doi: 10.1023/B:GLYC.0000018018.29488.c6. [DOI] [PubMed] [Google Scholar]
- 46.Wang X., Sun P., Paller A. S. Ganglioside GM3 blocks the activation of epidermal growth factor receptor induced by integrin at specific tyrosine sites. J. Biol. Chem. 2003;278:48770–48778. doi: 10.1074/jbc.M308818200. [DOI] [PubMed] [Google Scholar]
- 47.Svennerholm L. Chromatographic separation of human brain gangliosides. J. Neurochem. 1963;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]