Abstract
UGE (UDP-Glc 4-epimerase or UDP-Gal 4-epimerase; EC 5.1.3.2) catalyses the interconversion of UDP-Gal and UDP-Glc. Both nucleotide sugars act as activated sugar donors for the biosynthesis of cell wall polysaccharides such as cellulose, xyloglucans, (1,3;1,4)-β-D-glucan and pectins, together with other biologically significant compounds including glycoproteins and glycolipids. Three members of the HvUGE (barley UGE) gene family, designated HvUGE1, HvUGE2 and HvUGE3, have been characterized. Q-PCR (quantitative real-time PCR) showed that HvUGE1 mRNA was most abundant in leaf tips and mature roots, but its expression levels were relatively low in basal leaves and root tips. The HvUGE2 gene was transcribed at significant levels in all organs examined, while HvUGE3 mRNA levels were very low in all the organs. Heterologous expression of a near full-length cDNA confirmed that HvUGE1 encodes a functional UGE. A non-covalently bound NAD+ was released from the enzyme after denaturing with aqueous ethanol and was identified by its spectrophotometric properties and by electrospray ionization MS. The Km values were 40 μM for UDP-Gal and 55 μM for UDP-Glc. HvUGE also catalyses the interconversion of UDP-GalNAc and UDP-GlcNAc, although it is not known if this has any biological significance. A three-dimensional model of the HvUGE revealed that its overall structural fold is highly conserved compared with the human UGE and provides a structural rationale for its ability to bind UDP-GlcNAc.
Keywords: barley, gene expression, nucleotide sugar, plant cell wall, UDP-D-glucose 4-epimerase (UGE), UDP-GlcNAc
Abbreviations: EPS I, exopolysaccharide I; EST, expressed sequence tag; UGE, UDP-Glc 4-epimerase or UDP-Gal 4-epimerase; HvUGE, barley UGE; IPTG, isopropyl β-D-thiogalactoside; Ni-NTA, Ni2+-nitrilotriacetate; Q-PCR, quantitative real-time PCR; QTL, quantitative trait locus; R.M.S.D., root mean square deviation; UXS, UDP-GlcA decarboxylase; HvUGAE, barley UDP-GlcA 4-epimerase; HvUGDH, barley UDP-Glc dehydrogenase
INTRODUCTION
UGE (UDP-Glc 4-epimerase or UDP-Gal 4-epimerase; EC 5.1.3.2) catalyses the interconversion of UDP-Glc and UDP-Gal and, in plants, may also function in the so-called salvage pathways for removing galactose [1]. UDP-Glc occupies a central position in the synthesis of plant cell wall polysaccharides, as the key primary nucleotide sugar in nucleotide sugar interconversion pathways. It acts as the donor of glucosyl residues during the biosynthesis of cellulose, xyloglucans, (1,3;1,4)-β-D-glucans, glycoproteins and other oligosaccharides and metabolites. UDP-Gal is a substrate for the biosynthesis of arabinogalactan-proteins, pectic polysaccharides, xyloglucans, galactomannans, galactolipids, complex N-glycans and glycosyl sterols [1]. UDP-Glc can be oxidized by UDP-Glc dehydrogenase to generate UDP-GlcA [1,2], which is converted into UDP-GalA by UDP-GlcA 4-epimerase. UXS (UDP-GlcA decarboxylase), which converts UDP-GlcA into UDP-Xyl in an irreversible decarboxylation reaction [3], is a particularly important enzyme because it forms nucleotide sugars that carry pentosyl residues. The UDP-Xyl is epimerized to UDP-Ara, and both of these act as sugar donors in the synthesis of important pentose-containing polysaccharides such as heteroxylans and xyloglucans. Regulation of carbon flow through the nucleotide sugar interconversion pathways is probably regulated at several levels. Transcriptional activities of the various genes will clearly affect the relative abundance of key enzymes in the interconversion pathways, and substrate-level regulation of individual enzymes can occur through such processes as feedback inhibition of UXS activity by UDP-Xyl [1–3]. Whether or not the nucleotide sugar interconversion pathways are connected, either actively or passively, with the flux of carbon into the different wall polysaccharides remains to be demonstrated [2].
In the present study, we have focused on the HvUGE [barley (Hordeum vulgare L.) UGE] gene family. In bacteria, the reaction catalysed by UGE requires a non-covalently bound NAD+ cofactor [4]. It has been shown using X-ray crystallography that substrate binding leads to a conformational change in the enzyme, which transfers a hydride moiety from the axially orientated C-4 atom of UDP-Gal to the bound NAD+ and produces a keto-sugar intermediate [4]. The hydride from the NADH is transferred back to the keto-sugar intermediate, but approaches from a different direction so that the hydroxy group on C-4 is re-formed in the equatorial orientation, to generate UDP-Glc. The UGE from Escherichia coli is a homodimeric enzyme with a molecular mass of 39 kDa for each subunit, both of which contain a non-covalently bound NAD+. Removal of the NAD+ inhibits the enzyme activity [5]. Plant UGEs are also assumed to contain bound NAD+, because the addition of exogenous NAD+ to the reaction mixture does not stimulate enzyme activity for the recombinant Arabidopsis thaliana UGE expressed in E. coli [6]. However, there has been no direct demonstration of the nature of the association between the enzyme and NAD+ in plant UGEs.
Several UGE genes have been cloned from plants, including pea [7], A. thaliana [6] and the endospermous legume guar [8]. Unlike UXS [3], UDP-GlcA 4-epimerase [9,10] and UDP-Xyl epimerase [11], all of which include type II membrane-bound isoforms with terminal membrane anchors, plant UGE genes cloned so far encode enzymes that lack transmembrane motifs and are presumably located in the cytosol.
Although it is assumed that UGE plays an important role in galactose metabolism and in the biosynthesis of galactose-containing polysaccharides, the extent and breadth of its physiological functions are unclear in plants. Mutation of the corresponding genes through transposon insertions causes a loss of the pathogenicity for Erwinia amylovora, the causative agent of ‘fire blight’ in rosaceous plants [12]. In Rhizobium meliloti, the formation of nodules on legumes is also affected by mutation of the exoB gene, a homologue of the E. coli UGE, and the mutations result in an inability of the plant to synthesize the galactose-containing capsular polysaccharides EPS I (exopolysaccharide I) and EPS II [13]. In A. thaliana, a mutation in one of the five UGE genes results in abnormal root growth in a mutant (rhd1) [14], which is attributed to abnormal biosynthesis of xyloglucans and arabinogalactans in root epidermal cells, as well as to the down-regulation of some arabinogalactan-proteins. Ethylene suppresses all rhd1 phenotypes by restoring the biosynthesis of galactose-containing xyloglucans and arabinogalactans. However, ethylene does not up-regulate expression of other UGE isoforms [2,14]. A possible function of UGE in plant root growth has also been reported in rice, where drought stress induces increased expression of a UGE gene, which maps to a root thickness QTL (quantitative trait locus) region [15].
Here, we have examined the barley HvUGE gene family through analyses of extensive barley EST (expressed sequence tag) databases. Three members of the HvUGE gene family have been cloned and their sequences were used to design gene-specific primers for Q-PCR (quantitative real-time PCR) for comparisons of relative transcriptional activities of individual members of the gene family in a range of barley organs. One near full-length cDNA has been expressed in E. coli to confirm that HvUGE activity is associated with the protein product of the cDNA. The expressed enzyme was characterized biochemically and also examined for the presence of a bound NAD+. A three-dimensional model of HvUGE1 was also constructed using human UDP-Gal epimerase as a template, to establish a structural basis for the enzyme's substrate specificity. The results from enzymatic assays and the three-dimensional modelling have demonstrated that the HvUGE also catalyses the reversible interconversion of UDP-GlcNAc and UDP-GalNAc. Thus the enzyme might have several functions in plants, including the synthesis of both polysaccharides and glycoproteins.
EXPERIMENTAL
Materials
UDP-Glc, galactose dehydrogenase, NADH and NAD+ were purchased from Sigma–Aldrich (Australia). Superscript II RNase H reverse transcriptase, Gateway protein expression system and TRIzol® reagent were from Invitrogen Australia (Mount Waverley, Victoria, Australia).
Isolating HvUGE cDNAs
After searching barley EST databases, which contain approx. 360000 entries, with a sequence of the A. thaliana UGE gene (accession number Z54214), approx. 90 cDNA clones were identified. Analysis of these clones with ContigExpress software (Informax, Frederick, ML, U.S.A.) generated three consensus sequences. Three gene-specific oligonucleotide primers (ATGGTTTCTGCTGTTCTTC, CCGCTCCGCTCGCTCGCTCCCT and ATGGCGATCGGCGGCGTT) were used with an oligo-dT primer to amplify barley leaf cDNA by PCR. Three barley cDNAs were obtained and were designated HvUGE1, HvUGE2 and HvUGE3.
cDNA preparations and Q-PCR analysis of transcript levels
Total RNA preparation, cDNA synthesis and Q-PCR followed the same procedures described by Zhang et al. [3]. The preparation of barley leaf and root segments was done as described by Burton et al. [16]. The primer pairs and optimal acquisition temperatures for Q-PCR are shown in Table 1.
Table 1. Q-PCR primer sequences, product sizes and optimal acquisition temperatures.
Barley GAPDH (glyceraldehyde-3-phosphate dehydrogenase), HSP70 (heat-shock protein 70), α-tubulin, cyclophilin and CesA 1 (cellulose synthase 1) were used as control genes. T, the melting temperature.
| Gene | Forward primer | Reverse primer | Size (bp) | T (°C) |
|---|---|---|---|---|
| GAPDH | GTGAGGCTGGTGCTGATTACG | TGGTGCAGCTAGCATTTGAGAC | 198 | 80 |
| HSP70 | CGACCAGGGCAACCGCACCAC | ACGGTGTTGATGGGGTTCATG | 108 | 83 |
| α-Tubulin | AGTGTCCTGTCCACCCACTC | AGCATGAAGTGGATCCTTGG | 248 | 80 |
| Cyclophilin | CCTGTCGTGTCGTCGGTCTAAA | ACGCAGATCCAGCAGCCTAAAG | 122 | 79 |
| CesA 1 | TGTGGCATCAACTGCTAGGAAA | CGTACAAAGTGCCTCATAGGAAA | 267 | 75 |
| HvUGE1 | CGCAGAGACAATGAAGGAGGAAGA | CAAGGCAAGGGAACCAACCTTAT | 176 | 75 |
| HvUGE2 | GATCGGTCCGTGTGGACTCACC | GCTGCTAGGCAATGCAACTCAA | 268 | 77 |
| HvUGE3 | TCGTCGTGGCGTCTCTTCTG | ATGCGTGGAGCCCATACTGAC | 241 | 78 |
Heterologous expression
The HvUGE1 cDNA was cloned into E. coli using the Gateway protein expression system following the same procedures as those described for HvUXS1 expression [3], after the near full-length barley HvUGE1 cDNA was amplified by PCR using the primer pair GGGGACAAGTTTGTACAAAAAAGCAGGCTTCGAAGGAGATAGAACCATGGGTCATCACCATCACCATCACCAGATGGTTTCTGCTGTTCTTCGTACGA/GGGGACCACTTTGTACAAGAAAGCTGGGTCCTATCAGTGGCCGTT- GTCGCTGGATCCG. The underlined nucleotides indicate gene-specific regions. Recombinant protein was induced with 0.5 mM IPTG (isopropyl β-D-thiogalactoside) and purified using Ni-NTA (Ni2+-nitrilotriacetate) resin. Expressed proteins were analysed by SDS/PAGE on 12% (w/v) denaturing gels as described previously [3]. The N-terminal amino acid sequence of the recombinant protein was determined using a Hewlett–Packard G1000A automated amino acid sequencer (Hewlett–Packard, Palo Alto, CA, U.S.A.).
Enzyme assay and NAD+ determination
Procedures similar to those used for HvUXS1 [3] were used for the demonstration of non-covalently bound NAD+ in the recombinant HvUGE1 enzyme, including HPLC and MS analyses. HvUGE1 activity was assayed in a reaction mixture (75 μl) containing 20 mM sodium phosphate (pH 8.0), 2 mM NAD+, 0.05 mM UDP-Gal or UDP-Glc and purified recombinant HvUGE1 (12 ng of protein). The assay was performed at 25 °C for 10 min unless otherwise specified and terminated by quickly transferring the reaction tube to a boiling-water bath or by addition of 4 vol. of 100% ethanol. The reaction mixture was dried and analysed by HPLC on a Hewlett–Packard 1090 LC. UDP-Glc and UDP-Gal were separated using a reversed phase column (Hypersil ODS, 5 μm, 2.1 mm×250 mm; Agilent Technologies, Wilmington, DE, U.S.A.) and eluted isocratically with 0.1 M triethylamine acetate and 0.1 mM EDTA (pH 6.8) at a flow rate of 0.2 ml/min. UDP-GlcNAc and UDP-GalNAc were separated on a Prevail Carbohydrate ES column (150 mm×4.6 mm, 5 μm; Alltech, Deerfield, IL, U.S.A.) using a gradient of 25 mM NaB4O7/25 mM NaClO4 (0–15 min) to 75 mM NaB4O7/75 mM NaClO4 (15–25 min) at 0.6 ml/min. The elution was monitored at 262 nm. Various concentrations of UDP-Glc, UDP-Gal (6–250 μM) and UDP and UMP (0–125 μM) were used for determinations of kinetic constants, estimated from the Lineweaver–Burk (Km, kcat) and Dixon (Ki) plots. Care was taken to ensure that initial reaction rates were measured. Substrate consumption never exceeded 10% of initial concentrations. For assessment of the equilibrium constants (Keq), the reaction mixture (75 μl) contained the standard reaction ingredients specified above except that 360 ng of protein of the recombinant HvUGE1 (instead of 12 ng) was added, and the reactions were sampled at 5, 15, 40 and 60 min time intervals.
Construction of a three-dimensional model for HvUGE1
A three-dimensional molecular model of HvUGE1 was constructed using the Modeler 7v7 program [17,18], which uses the satisfaction of empirical spatial restraints and statistical analysis of the relationships between pairs of homologous structures in order to produce a protein fold. The CHARMM22 force-field energy function enforcing proper stereochemistry was implemented during energy minimization of models to produce an objective function, which represents a combined term with the spatial restraints. The first step of the experimental approach was the identification of a known three-dimensional structure (template) related to the target HvUGE1 sequence. The modelling uses coordinates of the template protein as a basis for further modelling. To identify the most suitable template for HvUGE1, searches through the Structure Prediction Meta-Server ([19]; http://bioinfo.pl/meta/pdb-test.pl), including SeqAlert (Bioinformatics and Biological Computing, Weizmann Institute of Science, Rehovot, Israel), Protein Data Bank (http://www.rcsb.org/pdb/) and 3D-PSSM Server (Imperial College of Science, Technology and Medicine, London, U.K.) were performed. The best template was found to be the human UGE with the PDB entries 1ek5, 1ek6 and 1hzj, which represent binary complexes of human UGE with NAD+, NAD+ plus UDP-Glc, and NAD+ plus UDP-GlcNAc respectively. The second step in modelling process was the alignment of the template with the target sequence, using the T-COFFEE server. The alignment contained four 1–6-amino-acid-long gap insertions and deletions (results not shown). The alignment was further checked manually using hydrophobic cluster analysis to maintain integrity of hydrophobic cluster patterns and the distribution of secondary structural elements. The positional sequence identity and similarity scores were calculated by the Bestfit program from the University of Wisconsin GCG software package [20], with the implemented gap penalty function and dynamic programming algorithm of Smith and Waterman [21]. The identity and similarity scores between the human UGE and HvUGE enzymes were 60.5 and 73.2% respectively. In the final step, the structurally aligned HvUGE1 and human UGE sequences containing the ligands NAD+, NAD+ plus UDP-Glc, and NAD+ plus UDP-GlcNAc were used as input parameters to build three-dimensional molecular models on a Silicon Graphics model O2 workstation, running IRIX 6.5. The final three-dimensional molecular model of HvUGE1 was selected from 40 models; the model with the lowest value of the Modeller objective function was chosen for further refinement. This was done by energy minimization using the GROMOS96 implementation in Swiss-PdbViewer [22] and SYBYL 6.9.1 [23].
The stereochemical quality and overall G-factors of the final HvUGE1 model were calculated with PROCHECK [24]. Z-score values were calculated by ProsaIIv3; the plots were smoothed using a window size of 50 amino acid residues. The routine ‘compare.top’ implemented in the Modeller 7v7 [17] was used to determine the R.M.S.D. (root mean square deviation) values in the Cα positions and distance R.M.S.D. values in atom distances between the two three-dimensional structures. The molecular graphics were generated with PyMol graphical software (http://www.pymol.org).
The cDNA sequences determined here for the HvUGE1, HvUGE2 and HvUGE3 genes have been lodged in the GenBank® and EMBL databases under the accession numbers AY943955, AY943956 and AY943954 respectively.
RESULTS
HvUGE cDNA clones
Analysis of barley EST sequences revealed three consensus sequences. PCR amplification from barley leaf cDNA using primers designed from the consensus sequences produced three HvUGE cDNA clones. The cDNA sequences were 1317, 1642 and 1475 bp in length and were designated HvUGE1, HvUGE2 and HvUGE3 respectively. The open reading frames of HvUGE1, HvUGE2 and HvUGE3 encoded proteins consisting of 353, 374 and 370 amino acid residues respectively. HvUGE proteins showed moderate sequence identities (54–72%) with human and A. thaliana UGEs at the amino acid level (Figure 1, Table 2), but a much higher sequence identity with the orthologous UGE from rice (>79%; Table 2). They all contained GXXGXXG NAD+-binding motifs and conserved Ser, Tyr and Lys amino acid residues that are believed to be located in the catalytic site (Figure 1).
Figure 1. Alignment of amino acid sequences of UGEs.
Amino acid sequences of UGEs from barley (HvUGE1, HvUGE2 and HvUGE3), A. thaliana (AtUGE1, Swiss-Prot Data Bank entry Q42605), rice (TIGR locus: Os05g51670) and human (PDB entry 1hzj) were aligned using the GeneDoc program (www.psc.edu/biomed/genedoc). The conserved motifs GXXGXXG (NAD+-binding) and catalytic amino acid residues Ser and Tyr-Xaa-Xaa-Xaa-Lys of the active site are underlined.
Table 2. Comparison of sequence identities.
Amino acid sequence identities (%) of HvUGE1, HvUGE2 and HvUGE3 are compared with barley UDP-Xyl synthases (HvUXS1–HvUXS4) [3], barley UDP-Xyl 4-epimerases (HvUXE1–HvUXE3), HvUGDH, HvUGAE (Q. Zhang and G. B. Fincher, unpublished work), rice UGEs (TIGR Data Bank loci Os05g51670, Os09g15420, Os09g35800 and Os08g28730), a human UGE (PDB entry 1hzj) and A. thaliana UGE1 (Swiss-Prot Data Bank entry Q42605).
| Sequence identity (%) | |||
|---|---|---|---|
| HvUGE1 | HvUGE2 | HvUGE3 | |
| HvUGE1 | 100 | ||
| HvUGE2 | 74 | 100 | |
| HvUGE3 | 63 | 60 | 100 |
| HvUXS1 | 26 | 27 | 20 |
| HvUXS2 | 28 | 27 | 26 |
| HvUXS3 | 28 | 29 | 27 |
| HvUXS4 | 29 | 28 | 24 |
| HvUXE1 | 41 | 41 | 37 |
| HvUXE2 | 42 | 42 | 40 |
| HvUXE3 | 40 | 39 | 39 |
| HvUGDH | 21 | 17 | 21 |
| HvUGlcAE | 25 | 27 | 21 |
| Rice Os05g51670 | 90 | 76 | 63 |
| Rice Os09g15420 | 74 | 87 | 60 |
| Rice Os09g35800 | 61 | 62 | 79 |
| Rice Os08g28730 | 76 | 82 | 63 |
| Human UGE | 59 | 55 | 54 |
| A. thaliana UGE1 | 66 | 61 | 72 |
The sequence identities between HvUGE1, HvUGE2 and HvUGE3 were between 60 and 74% at the amino acid level (Table 2). The UGEs were clearly distinguishable from other barley nucleotide sugar interconverting enzymes such as the HvUXSs [3], HvUGDH (barley UDP-Glc dehydrogenase) and HvUGAE (barley UDP-GlcA 4-epimerase), with which there was less than 30% sequence identity at the amino acid level (Table 2). However, the HvUGE shared more than 37% sequence identity with the UDP-Xyl epimerase.
Biochemical properties of HvUGE1
To confirm that the cloned HvUGE cDNAs encoded enzymes with UGE activity, the HvUGE1 cDNA was selected for heterologous expression in E. coli. The selection was based on observations that HvUGE1 mRNA was relatively abundant in many barley organs. A recombinant protein induced with 0.5 mM IPTG had a molecular mass of 39 kDa (Figure 2), as predicted from the HvUGE1 cDNA. N-terminal amino acid sequencing showed that the recombinant protein contained the amino acid residues MVSAVLRTILVT after the His tag, and this sequence was as expected from the HvUGE1 cDNA sequence (Figure 1).
Figure 2. SDS/PAGE of barley HvUGE1 expressed in E. coli.
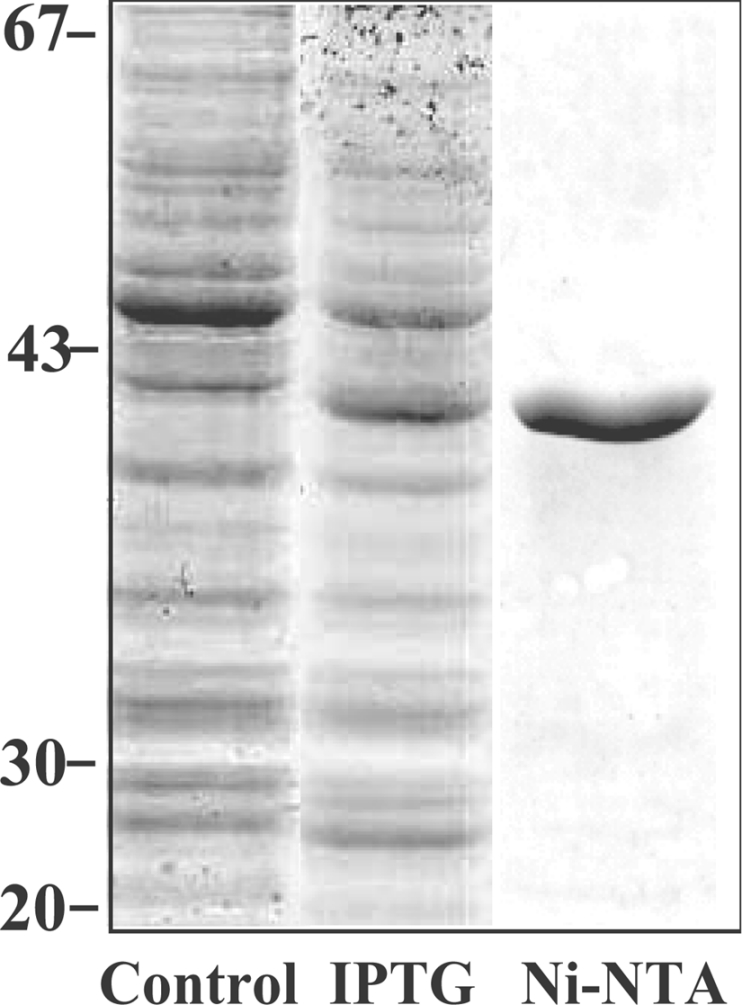
The barley HvUGE1 cDNA was expressed in E. coli and proteins were separated by SDS/PAGE. Control, cell extracts without addition of IPTG; IPTG, protein expression following induction with 0.5 mM IPTG; Ni-NTA, the purified fraction following Ni-NTA affinity chromatography. Protein molecular mass standards (in kDa) are indicated on the left.
The recombinant protein catalysed the interconversion of UDP-Glc and UDP-Gal. It displayed a broad pH optimum in the range pH 8–9.5. The bivalent cations Mg2+ and Ca2+ neither stimulated nor inhibited the activities of the recombinant HvUGE1 (results not shown). As expected from these results, the addition of 2 mM EDTA had no effect on enzyme activity (results not shown).
The recombinant HvUGE1 had Km values of 40 μM for UDP-Gal and 55 μM for UDP-Glc, and kcat values of 21 s−1 for UDP-Gal and 11 s−1 for UDP-Glc (Table 3). The efficiency of the reaction catalysed by HvUGE1 was approx. 2.5-fold higher for UDP-Gal than for UDP-Glc (Table 3). Standard deviations were generally less than 15%. After the reaction reached equilibrium, UDP-Gal and UDP-Glc concentrations were approx. 23 and 77% of total nucleotide sugars in the reaction system respectively. The Keq obtained from the ratio of substrate to product was 0.30, while that calculated from kcat/Km values for the forward and reverse reactions was 0.40. These values can be compared with a Keq of 0.38 reported by Piller et al. [25].
Table 3. Kinetic constants of HvUGE1.
The values are means for three experiments and the standard deviations are indicated for UDP-Gal and UDP-Glc. The values for UDP-GalNAc and UDP-Glc are estimates only because of difficulties in the HPLC separation of the two compounds.
| Substrates | Km (μM) | kcat (s−1) | kcat/Km (s−1·μM−1) |
|---|---|---|---|
| UDP-Gal | 40±4 | 21±3 | 550±80 |
| UDP-Glc | 55±1 | 11±2 | 220±30 |
| UDP-GalNAc | 160 | 0.12 | 0.8 |
| UDP-GlcNAc | 200 | 0.07 | 0.4 |
The heterologously expressed HvUGE1 also catalysed the interconversion of UDP-GalNAc and UDP-GlcNAc. The final equilibrium proportions were 26 and 74% of UDP-GalNAc and UDP-GlcNAc respectively, and Keq values were therefore very similar to those calculated for the UDP-Glc/UDP-Gal reaction (Table 3). The Km values for UDP-GalNAc and UDP-GlcNAc were 160 and 200 μM respectively (Table 3). The ratios of catalytic efficiencies (kcat/Km) for the substrate pairs UDP-Glc/UDP-GlcNAc and UDP-Gal/UDP-GalNAc were 550 and 688 respectively, and these values indicated that the enzyme is 500–600 times more efficient with the UDP-Glc and UDP-Gal substrates.
UDP and UMP strongly inhibited the interconversion of UDP-Gal and UDP-Glc, with Ki values of 36 and 32 μM respectively. A similar Ki value (20 μM) has been reported for UGE from porcine submaxillary glands [25]. Glucose and galactose had little effect on the reaction rate of the barley enzyme, such that Ki values for these sugars could not be calculated. The barley enzyme appears to have very low levels of activity on UDP-Xyl (results not shown), but this observation has not been examined in detail.
Characterization of bound NAD+
Addition of exogenous NAD+, NADH, NADP+ or NADPH to the reaction mixture did not significantly stimulate the catalytic rate (results not shown), although it is known that NAD+ plays a critical role in the reaction mechanism [4,26]. To confirm that the heterologously expressed enzyme contained bound NAD+, the recombinant HvUGE1 was treated with aq. 80% (v/v) ethanol. An ethanol-soluble compound was released, and was shown to have an HPLC retention time and an absorbance spectrum similar to standard NAD+ (results not shown). When the ethanol extractable component was incubated with galactose dehydrogenase in the presence of galactose, a compound that had the same HPLC profile and absorbance spectrum of standard NADH was produced (results not shown). It was therefore concluded that the extracted compound from the recombinant HvUGE1 was NAD+, which had been non-covalently associated with the enzyme before ethanol extraction. Purification of the expressed HvUGE1 on the Ni-NTA resin had no effect on the bound NAD+. Analysis of the 80% ethanol-soluble component by electrospray ionization MS showed that the component had a mass of 664 Da, which is the mass of standard NAD+ (results not shown). The molar ratio of NAD+ in the recombinant HvUGE1 protein was estimated to be 0.85. It is likely therefore that each protein molecule contains one molecule of NAD+.
Transcript levels of HvUGE genes in different organs
Transcript levels of the three individual HvUGE genes were determined by Q-PCR using gene-specific primers designed from 3′-untranslated sequences. The HvUGE1 mRNA was most abundant in leaf tip and mature roots, where HvUGE1 mRNA levels were 300- and 10-fold higher than the HvUGE1 mRNA levels in basal leaves and root tips respectively (Figure 3). In contrast, HvUGE1 mRNA levels in developing grains, coleoptiles and stems were at least 15-fold lower than the HvUGE1 mRNA level in leaf tips. The HvUGE2 gene was transcribed in all organs examined at approximately similar levels, but the mRNA abundance was only approx. 15% of HvUGE1 mRNA levels in leaf tips. Levels of HvUGE2 mRNA were higher than the levels of HvUGE1 mRNA in leaf bases, root tips, early developing grains, coleoptiles and stems (Figure 3). Levels of HvUGE3 mRNA were lowest in all organs and generally accounted for less than 5% of HvUGE1 mRNA levels in leaf tips.
Figure 3. Normalized expression levels of HvUGE genes in different barley organs.
Levels of mRNA are presented as the number of copies per μg of total RNA after normalization. Organs were as described by Burton et al. [16] and included leaf tips, leaf bases, root tip and the maturation zone (m/zone) of roots, flowers just before anthesis (FL early), flowers at anthesis (FL anthesis), developing grain 3 and 13 days post-anthesis (Grain early and Grain mid), 3-day-old coleoptiles (Col 3 day) and the stem of mature plants just below the emerging ear (Stem). The results shown are means for four separate evaluations and S.D.s are indicated.
Transcript levels of HvUGE genes in young leaves, coleoptiles and roots
In young barley leaves, expression of the HvUGE1 gene was highest in leaf tips, but rapidly decreased to very low levels in the basal region of the leaves. The HvUGE1 mRNA level was approx. 100-fold lower in leaf bases than in leaf tips (Figure 4A). Both HvUGE2 and HvUGE3 mRNA levels were low in all leaf segments.
Figure 4. Normalized expression levels of HvUGE genes in young barley leaves, roots and coleoptiles.
Barley leaves were sectioned into five segments and roots were cut into four segments as described by Burton et al. [16]. Units and statistical treatments were as described in the legend to Figure 3.
In young barley roots, mRNA levels of HvUGE1 were very high in the maturation zone compared with other regions of the root (Figure 4B). The HvUGE1 transcript levels were approx. 10-fold higher in maturation zones than in root tips. Similar levels of HvUGE2 transcripts were detected in all root segments, but they were at least 5-fold lower than the levels of HvUGE1 mRNA in the maturation zone. The HvUGE2 mRNA level was 2.5-fold higher than HvUGE1 transcript level in root tips. Transcripts of the HvUGE3 gene were not detectable in the root segments (Figure 4B). Comparison of mRNA levels between leaves and roots shows that the expression levels were 4-fold higher in the maturation zones of roots than in leaf tips (Figures 4A and 4B).
In coleoptiles, levels of HvUGE1 mRNA were low in day 1 and day 3 coleoptiles (Figure 4C), but increased approx. 28-fold in day 5 coleoptiles. In contrast, the level of HvUGE2 mRNA remained essentially unchanged from day 1 to day 5. The HvUGE3 mRNA was undetectable in day 1 and day 3 coleoptiles, but showed a 20-fold increase by day 5. Overall, the level of HvUGE mRNA was over 10-fold lower in coleoptiles than in mature roots.
Molecular modelling of HvUGE1
The human UGE structure (PDB entry 1hzj), which represents a mixed α/β protein fold based on the CATH (Class, Architecture, Topology and Homologous superfamily) classification [27], served as an excellent structural template for modelling the HvUGE1. The solved crystal structure of the human UGE also included the binding positions of NAD+ and UDP-GlcNAc and the template therefore represented a ternary complex of the human UGE enzyme, with bound substrate and cofactor [28]. The stereochemical quality and overall G-factors of the final HvUGE1 enzyme/substrate/cofactor model [29] showed that none of the amino acid residues in the modelled structure were positioned in disallowed regions, and the overall G-factors were 0.18 and 0.16 for the template and modelled three-dimensional structures respectively. The Z-score values, which reflect the combined statistical potential energy of the modelled HvUGE1 and template three-dimensional structures and the estimate compatibility of the sequence with an overall fold and conservation of the key functional and structural residues, were −10.96 and −10.07 for 345 and 343 residues of the template and modelled structures respectively. The R.M.S.D. values in the Cα positions and distance R.M.S.D. values in atom distances between the two three-dimensional structures were 0.951 and 1.903 Å respectively (1 Å=0.1 nm) for 343 amino acid residues. The modelled HvUGE1 structure was further evaluated using the pG server [18]; the pG score of 1.0 again indicated that the HvUGE1 model could be classified as highly reliable.
The overall three-dimensional shape and the distribution of the secondary structure elements of the HvUGE1 were highly conserved in comparison with the human UGE crystal structure (results not shown), and the overall geometry of the active site was also conserved. However, there were differences in insertions and deletions of loops that connect the individual secondary structure elements in the HvUGE1, although these insertions and deletions were always positioned at the surface of the enzyme (results not shown). Insertions and deletions of 1, 2, 3 and 6 amino acid residues were located in the loop regions at amino acid positions 152, 57, 261 and 43 respectively. The two highly conserved, key catalytic amino acid residues in the human UGE, namely Ser132 and Tyr157, are 4.7 Å apart in the human enzyme. In the HvUGE1 enzyme, these residues corresponded to Ser132 and Tyr156, which were 4.8 Å apart. The relatively close proximity of these two key residues is a characteristic feature for UGEs [28].
The molecular modelling of the HvUGE1 also allowed the locations of the NAD+ cofactor and the UDP-GlcNAc substrate in the active site region to be defined (Figure 5A). The reason for the choice of UDP-GlcNAc as a nucleotide sugar substrate donor for the molecular modelling was to investigate if the HvUGE1 enzyme could actually accommodate UDP-GlcNAc in its active site [28]. The modelling clearly showed that there were no inter-atomic clashes among amino acid residues that participate in binding of the cofactor NAD+ and the substrate UDP-GlcNAc (Figure 5B). The triplet of catalytic amino acid residues Ser, Tyr and Lys was positioned at the interface between bound NAD+ and UDP-GlcNAc in both human UGE and HvUGE (results not shown). Analysis of the model of the barley enzyme showed that only a few favourable hydrogen-bonding interactions were formed among Tyr225, Asp305, Arg302, Asn206 and Asn186, and the corresponding groups of the UDP moiety of UDP-GlcNAc. From these interactions, the shortest hydrogen bond of 2.7 Å was made between Asp305 and the C-4′ OH group of the ribosyl moiety of UDP-GlcNAc. It was also apparent from the model that the N-acetyl group at the C-2′ atom of UDP-GlcNAc was not bonded to amino acid residues that were positioned nearby. Instead, a hydrogen bond of 3.3 Å was formed between Ser132 and the C-4′ OH group of the GlcNAc moiety of UDP-GlcNAc. However, it must be emphasized that the distances between the active site residues of the modelled HvUGE1 structure and UDP-GlcNAc should be treated as estimates only, because of interpretative problems associated with molecular modelling. It was therefore rationalized in silico that the HvUGE1 enzyme would be able to accommodate UDP-GlcNAc in its active site, and hence would have the potential to epimerize this nucleotide sugar.
Figure 5. Molecular model of the HvUGE1.
(A) Stereo view of the ribbon representation of the HvUGE1 model shows secondary structural elements of the enzyme folded into a mixed α/β protein fold. The R.M.S.D. value between the template structure (PDB entry 1hzj) and the modelled HvUGE1 is 0.951 Å over 343 amino acid residues. The N- and C-termini of the enzyme are shown in the lower and upper parts of the panel respectively. Spheres in atomic colours represent van der Waals radii of bound UDP-GlcNAc and NAD+ ligands. (B) Stereo representation of the molecular surface area depicts the active site region with bound NAD+ and UDP-GlcNAc. The molecular surface was generated with a probe radius of 1.4 Å. The Figure was prepared with PyMol.
DISCUSSION
Database searches and Q-PCR analyses indicate that the UGE enzymes of barley are encoded by a small gene family of at least three members. In rice, there are at least four UGE genes, while in A. thaliana the UGE family consists of five individual genes [1,2]. Near full-length cDNAs encoding the three barley isoenzymes, designated HvUGE1, HvUGE2 and HvUGE3, were amplified by PCR from cDNA preparations from young barley leaves and characterized. The HvUGE1 cDNA was expressed in E. coli to confirm the substrate specificity of the encoded enzyme and to define its kinetic and chemical properties.
The UGEs belong to a superfamily of dehydrogenases/reductases [1]. They contain an absolutely conserved GXXGXXG NAD+-binding motif and a triplet of amino acid residues, Ser, Tyr and Lys, that is positioned at the interface between NAD+ and UDP-GlcNAc in the crystal structure of the human UGE [28]. The three HvUGE isoenzymes also have the conserved NAD+-binding motif and the triplet of catalytic site amino acid residues (Figure 1). The barley enzymes contain 353–370 amino acid residues and therefore have molecular masses similar to those for the human and A. thaliana UGE enzymes (Figure 1). The HvUGEs lack any obvious organellar targeting signals or transmembrane helices (Figure 1), and are therefore predicted to be located in the cytosol. Similarly, all five isoforms of AtUGE from A. thaliana lack membrane-binding motifs and are believed to be cytosolic enzymes [2]. This is in contrast with other nucleotide sugar interconverting enzymes such as UDP-Xyl synthases, for which both cytosolic and membrane-bound isoforms are found [3,30].
The cytosolic location for plant UGEs is consistent with the observation that most of the cellular UDP-Glc and UDP-Gal, as measured by [31P]NMR, is located in the cytosol [31]. However, UDP-Glc and UDP-Gal are also used for biosynthesis of non-cellulosic polysaccharide and glycoproteins in the Golgi apparatus [2,32] and specific transporters are required for transferring nucleotide sugars from the cytosol to the lumen of the Golgi cisternae. A nucleotide sugar transporter gene has been cloned in A. thaliana, and the gene product transports both UDP-Gal and UDP-Glc into the Golgi [33]. A near full-length cDNA sequence encoding a putative barley UDP-Gal transporter with 79% amino acid sequence identity to the A. thaliana UDP-Gal transporter has also been cloned (Q. Zhang and G. B. Fincher, unpublished work).
Following heterologous expression of the barley HvUGE1 cDNA and affinity purification of the expressed protein, UGE activity was confirmed. The expressed enzyme exhibited biochemical properties that were similar to those of UGE enzymes from other plants, insofar as it was not activated by Mg+ or Ca+ ions and it had a broad pH optimum of 8.0–9.5 [34]. Furthermore, it was shown that the expressed HvUGE1 enzyme contained a non-covalently bound NAD+ cofactor, as suggested by proposed reaction mechanisms and inhibition studies, but not previously demonstrated in plant UGEs [1,2]. Certain nucleotide sugar interconverting enzymes can be activated or inhibited by cofactors or products. Thus a UDP-D-apiose/UDP-Xyl synthase from A. thaliana is activated by NAD+ and inhibited by NADH [35]. UDP-Glc dehydrogenases from soya bean and sugarcane are subject to feedback inhibition by UDP-Xyl [36,37], as are the UDP-Xyl synthases from A. thaliana and barley [3,6]. However, activity of the HvUGE1 enzyme is not affected by the cofactor NAD+, consistent with the conclusion that this cofactor is bound within the enzyme molecule, and we could find no kinetic evidence for product inhibition of reactions catalysed by HvUGE1.
The expressed HvUGE1 enzyme showed a relatively high affinity for its UDP-Gal and UDP-Glc substrates compared with bacterial and human UGEs. Thus the Km values for HvUGE1 were 40 and 55 μM, depending on the substrate, while those for human and bacterial UGEs are generally approx. 200 μM [38]. It is worth noting that the concentration of UDP-Glc in endosperm cells from Lolium multiflorum is approx. 30 μM [39]. The kinetic analysis also revealed that the HvUGE1 has a higher catalytic efficiency for UDP-Gal (kcat/Km∼550 s−1·μM−1) than UDP-Glc (kcat/Km∼220 s−1·μM−1). Thus HvUGE1 could play a role in removing galactose, which can have inhibitory effects on cell growth [40,41]. In the recycling process, free galactose is phosphorylated to galactose 1-phosphate by galactose kinase and subsequently converted into UDP-Gal by UDP-Gal pyrophosphorylase [42].
The heterologous expression of HvUGE1 enabled the substrate specificity of the enzyme to be examined in more detail. Three groups of UGEs with slightly different substrate specificities have been identified [28,43–45]. The first group is represented by bacterial UGEs, which only catalyse the interconversion of UDP-Glc and UDP-Gal [43]. The second group is represented by human and some bacterial UGEs, which epimerize not only UDP-Gal and UDP-Glc, but also UDP-GalNAc and UDP-GlcNAc [28,44]. A third group of UGEs, found in Pseudomonas aeruginosa, epimerizes only UDP-GalNAc and UDP-GlcNAc [45]. In the present study, it has been shown that the expressed HvUGE1 enzyme catalyses not only the interconversion of UDP-Gal and UDP-Glc, but also that of UDP-GalNAc and UDP-GlcNAc. The catalytic efficiencies with the latter two substrates are much lower than those for UDP-Gal and UDP-Glc (Table 3).
The broader substrate specificity of the HvUGE1 can be rationalized through molecular modelling (Figure 5). The models of the barley enzyme confirmed that UDP-GalNAc could serve as a substrate, because there were no inter-atomic clashes between the acetyl group on the C-2′ atom of the UDP-GalNAc molecule and neighbouring amino acid residues of the enzyme or the NAD+ cofactor. Indeed, there were no steric clashes predicted for any atom of the UDP-GlcNAc substrate. Furthermore, the juxtaposition of NAD+ cofactor and C-4′ of the UDP-GalNAc substrate can be clearly seen (Figure 5). The results from the molecular modelling indicated that at least five critical hydrogen bonds were formed between the UDP moiety of UDP-GlcNAc and the enzyme, while only one such bond was detected between the GlcNAc moiety of UDP-GlcNAc and the enzyme. The latter interaction is also critical for binding of the Glc moiety of UDP-Glc to the human UGE [46]. When comparing binding modes of UDP-Glc and UDP-GlcNAC in the human UGE crystal structures, it is apparent that substantial rotations, of approx. 90°, of the Glc and GlcNAc moieties relative to UDP occur [28,38], while the hydrogen bonds between Glc and GlcNAc of the nucleotide sugar substrates and the corresponding amino acid residues are maintained. It is expected that similar rotations could take place in the barley enzyme, because secondary structural elements of HvUGE1 and amino acid residues that participate in the formation of critical interactions among cofactors and substrates are conserved in both structures. We could therefore conclude that UDP-GlcNAc could be accommodated in the active site of the barley enzyme and that the UDP component of UDP-GlcNAc is expected to be bound more tightly than the GlcNAc moiety.
Despite the relatively low catalytic efficiency of the reaction, the ability of the HvUGE1 to interconvert UDP-GalNAc and UDP-GlcNAc raises more general questions about the functions of UGEs in plants. Although GlcNAc will be a common constituent of plant glycoproteins, GalNAc has also been detected in plant polymers [47,48]. Thus it is possible but not proven that plant UGEs might participate in the biosynthesis of glycolipids or glycoproteins.
A combined analysis of the kinetic, substrate specificity and structural modelling data provided some insight into enzyme–substrate binding reactions. Thus the similarities of the Km value for UDP-Glc (55 μM) and the Ki values for UDP and UMP (36 and 32 μM respectively) indicated that the UDP region of the nucleotide sugar is likely to be a key determinant of initial substrate binding to the enzyme. The inability of Glc to inhibit the reaction to any significant extent would be consistent with this conclusion. This is not to say that the sugar region of the substrate is not involved in binding once the enzyme–substrate complex is formed. Structural information from the human UGE [28,30] clearly shows interactions between the enzyme and the sugar moiety of the substrate, but the observation that five hydrogen bonds are formed between the enzyme and the UDP moiety of UDP-GlcNAc, while only one hydrogen bond is formed between the sugar moiety and the enzyme, is consistent with the importance of the UDP moiety in substrate binding, as indicated by our kinetic data. If it is assumed that Km (k−1+k2)/k1 is indicative of Ks (k−1/k1, when k2≪k−1), it could be concluded that the N-acetyl group at the C-2′ carbon of the UDP-GlcNAc substrate has a greater effect on catalytic rate than on substrate binding.
It is worth noting that the concentration of UDP in potato tubers is approx. 3 μM [49], which is approx. 10 times lower than the Ki value of HvUGE1 for UDP, and UDP is therefore unlikely to directly affect activity of UGE enzymes under physiological conditions.
The availability of the three HvUGE cDNA sequences enabled the design of specific PCR primers for the quantitative analysis of relative mRNA levels in a range of barley organs (Figures 3 and 4). In many organs, HvUGE1 mRNA is more abundant than mRNAs encoding the other two isoenzymes, although HvUGE2 mRNA predominates in some organs (Figure 3). In mature vegetative organs such as leaf tip and the maturation zone of roots, HvUGE1 mRNA levels are relatively high compared with other organs, but in developing and dividing organs including basal leaves, root tips and young coleoptiles, the levels of HvUGE1 mRNA were lower (Figures 3 and 4).
The precise physiological functions of UGEs in plants remain unclear. In A. thaliana, expression of UGE genes is relatively high in roots and a mutation of the AtUGE4 gene, which is orthologous to the barley HvUGE1 gene, results in abnormal root hair formation [50]. In rice, expression of a UGE gene is induced by drought treatment and this gene has been mapped to a root thickness QTL region [15]. In pea roots, UGE gene expression increases 5-fold in 3-week-old plants, compared with 4-day-old seedlings [7]. These observations suggest that the UGE enzymes play a role in plant root development and/or growth.
It has also been suggested that nucleotide sugar interconversions and hence the composition of the nucleotide sugar pool in plant cells might reflect the compositions of cell walls in those cells and might also represent a point of regulation of wall composition [3]. It is not possible at this stage to determine whether or not levels of HvUGE mRNA or enzyme might be correlated with wall composition or with regulatory control mechanisms, but the cloning of genes encoding the key enzymes involved in nucleotide sugar interconversions in plants [1,2], together with emerging techniques for metabolite profiling and for detecting individual wall polysaccharides in vitro, has provided the tools and resources necessary for a detailed analysis of links between nucleotide sugar pools and cell wall biosynthesis.
Acknowledgments
We thank Rachel Burton (School of Agriculture and Wine, University of Adelaide, Glen Osmond, SA, Australia) for providing organ mRNA samples and for helpful discussions. We are also grateful to Deb Mohnen from the Complex Carbohydrate Research Center at the University of Georgia (Athens, GA, U.S.A.) for providing the UDP-Xyl. This work was funded by grants from the Grains Research and Development Corporation (to G. B. F.) and the Australian Research Council (to G. B. F. and M. H.).
References
- 1.Reiter W. D., Vanzin G. F. Molecular genetics of nucleotide sugar interconversion pathways in plants. Plant Mol. Biol. 2001;47:95–113. [PubMed] [Google Scholar]
- 2.Seifert G. J. Nucleotide sugar interconversions and cell wall biosynthesis: how to bring the inside to the outside. Curr. Opin. Plant Biol. 2004;7:277–284. doi: 10.1016/j.pbi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q., Shirley N., Lahnstein J., Fincher G. B. Characterization and expression patterns of UDP-D-glucuronate decarboxylase genes in barley. Plant Physiol. 2005;138:131–141. doi: 10.1104/pp.104.057869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer A. J., Rayment I., Frey P. A., Holden H. M. The molecular structure of UDP-galactose 4-epimerase from Escherichia coli determined at 2.5 Å resolution. Proteins. 1992;12:372–381. doi: 10.1002/prot.340120409. [DOI] [PubMed] [Google Scholar]
- 5.Barat B., Bhattacharyya D. UDP-galactose 4-epimerase from Escherichia coli: formation of catalytic site during reversible folding. Arch. Biochem. Biophys. 2001;391:188–196. doi: 10.1006/abbi.2001.2380. [DOI] [PubMed] [Google Scholar]
- 6.Dörmann P., Benning C. Functional expression of uridine 5′-diphospho-glucose 4-epimerase (EC 5.1.3.2) from Arabidopsis thaliana in Saccharomyces cerevisiae and Escherichia coli. Arch. Biochem. Biophys. 1996;327:27–34. doi: 10.1006/abbi.1996.0088. [DOI] [PubMed] [Google Scholar]
- 7.Lake M. R., Williamson C. L., Slocum R. D. Molecular cloning and characterization of a UDP-glucose 4-epimerase gene (gaZE) and its expression in pea tissues. Plant Physiol. Biochem. 1998;36:555–562. [Google Scholar]
- 8.Joersbo M., Pedersen S. G., Nielsen J. E., Marcussen J., Brunstedt J. Isolation and expression of two cDNA clones encoding UDP-glucose epimerase expressed in developing seeds of the endospermous legume guar. Plant Sci. 1999;142:147–153. [Google Scholar]
- 9.Gu X., Bar-Peled M. The biosynthesis of UDP-galacturonic acid in plants. Functional cloning and characterization of Arabidopsis UDP-D-glucuronic acid 4-epimerase. Plant Physiol. 2004;136:4256–4264. doi: 10.1104/pp.104.052365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mølhøj M., Verma R., Reiter W. D. The biosynthesis of D-galacturonate in plants. Functional cloning and characterization of a membrane-anchored UDP-D-glucuronate 4-epimerase from Arabidopsis. Plant Physiol. 2004;135:1221–1230. doi: 10.1104/pp.104.043745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burget E. G., Verma R., Molhoj M., Reiter W. D. The biosynthesis of L-arabinose in plants: molecular cloning and characterization of a Golgi-localized UDP-D-xylose 4-epimerase encoded by the MUR4 gene of Arabidopsis. Plant Cell. 2003;15:523–531. doi: 10.1105/tpc.008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metzger M., Bellemann P., Bugert P., Geider K. Genetics of galactose metabolism of Erwinia amylovora and its influence on polysaccharide synthesis and virulence of the fire blight pathogen. J. Bacteriol. 1994;176:450–459. doi: 10.1128/jb.176.2.450-459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buendia A. M., Enenkel B., Koplin R., Niehaus K., Arnold W., Puhler A. The Rhizobium meliloti exoZl exoB fragment of megaplasmid 2: ExoB functions as a UDP-glucose 4-epimerase and ExoZ shows homology to NodX of Rhizobium leguminosarum biovar viciae strain TOM. Mol. Microbiol. 1991;5:1519–1530. doi: 10.1111/j.1365-2958.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 14.Seifert G. J., Barber C., Wells B., Dolan L., Roberts K. Galactose biosynthesis in Arabidopsis: genetic evidence for substrate channeling from UDP-D-galactose into cell wall polymers. Curr. Biol. 2002;12:1840–1845. doi: 10.1016/s0960-9822(02)01260-5. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen T. T., Klueva N., Chamareck V., Aarti A., Magpantay G., Millena A. C., Pathan M. S., Nguyen H. T. Saturation mapping of QTL regions and identification of putative candidate genes for drought tolerance in rice. Mol. Genet. Genomics. 2004;272:35–46. doi: 10.1007/s00438-004-1025-5. [DOI] [PubMed] [Google Scholar]
- 16.Burton R. A., Shirley N. J., King B. J., Harvey A. J., Fincher G. B. The CesA gene family of barley. Quantitative analysis of transcripts reveals two groups of co-expressed genes. Plant Physiol. 2004;134:224–236. doi: 10.1104/pp.103.032904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sali A., Blundell T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez R., Sali A. Large-scale protein structure modeling of the Saccharomyces cerevisiae genome. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13597–13602. doi: 10.1073/pnas.95.23.13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginalski K., Elofsson A., Fischer D., Rychlewski L. 3D-Jury: a simple approach to improve protein structure predictions. Bioinformatics. 2003;19:1015–1018. doi: 10.1093/bioinformatics/btg124. [DOI] [PubMed] [Google Scholar]
- 20.Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith T. F., Waterman M. S. Identification of common molecular subsequences. J. Mol. Biol. 1981;147:195–197. doi: 10.1016/0022-2836(81)90087-5. [DOI] [PubMed] [Google Scholar]
- 22.Guex N., Peitsch M. C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 23.Clark M., Cramer R. D., Van Opdenbosch N. Validation of the general purpose Tripos 5.2 force field. J. Comp. Chem. 1989;10:982–1012. [Google Scholar]
- 24.Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. [Google Scholar]
- 25.Piller F., Hanlon M. H., Hill R. L. Co-purification and characterization of UDP-glucose 4-epimerase and UDP-N-acetylglucosamine 4-epimerase from porcine submaxillary glands. J. Biol. Chem. 1983;258:10774–10778. [PubMed] [Google Scholar]
- 26.Shaw M. P., Bond C. S., Roper J. R., Gourley D. G., Ferguson M. A., Hunter W. N. High-resolution crystal structure of Trypanosoma brucei UDP-galactose 4′-epimerase: a potential target for structure-based development of novel trypanocides. Mol. Biochem. Parasitol. 2003;126:173–180. doi: 10.1016/s0166-6851(02)00243-8. [DOI] [PubMed] [Google Scholar]
- 27.Orengo C. A., Michie A. D., Jones S., Jones D. T., Swindells M. B., Thornton J. M. CATH – a hierarchic classification of protein domain structures. Structure. 1997;5:1093–1108. doi: 10.1016/s0969-2126(97)00260-8. [DOI] [PubMed] [Google Scholar]
- 28.Thoden J. B., Wohlers T. M., Fridovich-Keil J. L., Holden H. M. Human UDP-galactose 4-epimerase. Accommodation of UDP-N-acetylglucosamine within the active site. J. Biol. Chem. 2001;276:15131–15136. doi: 10.1074/jbc.M100220200. [DOI] [PubMed] [Google Scholar]
- 29.Engh R. A., Huber R. Accurate bond and angle parameters for X-ray protein structure refinement. Acta Crystallogr. A. 1991;47:392–400. [Google Scholar]
- 30.Pattathil S., Harper A. D., Bar-Peled M. Biosynthesis of UDP-xylose: characterization of membrane-bound AtUxs2. Planta. 2005;221:538–548. doi: 10.1007/s00425-004-1471-7. [DOI] [PubMed] [Google Scholar]
- 31.Bligny R., Gardestrom P., Roby C., Douce R. 31P NMR studies of spinach leaves and their chloroplasts. J. Biol. Chem. 1990;265:1319–1326. [PubMed] [Google Scholar]
- 32.Moore P. J., Swords K. M., Lynch M. A., Staehelin L. A. Spatial organization of the assembly pathways of glycoproteins and complex polysaccharides in the Golgi apparatus of plants. J. Cell Biol. 1991;112:589–602. doi: 10.1083/jcb.112.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norambuena L., Marchant L., Berninsone P., Hirschberg C. B., Silva H., Orellana A. Transport of UDP-galactose in plants. Identification and functional characterization of AtUTr1, an Arabidopsis thaliana UDP-galactose/UDP-glucose transporter. J. Biol. Chem. 2002;277:32923–32929. doi: 10.1074/jbc.M204081200. [DOI] [PubMed] [Google Scholar]
- 34.Fan D. F., Feingold D. S. Nucleoside diphosphate sugar 4-epimerases. I. Uridine diphosphate glucose 4-epimerase of wheat germ. Plant Physiol. 1969;44:599–604. doi: 10.1104/pp.44.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molhoj M., Verma R., Reiter W. D. The biosynthesis of the branched-chain sugar D-apiose in plants: functional cloning and characterization of a UDP-D-apiose/UDP-D-xylose synthase from Arabidopsis. Plant J. 2003;35:693–703. doi: 10.1046/j.1365-313x.2003.01841.x. [DOI] [PubMed] [Google Scholar]
- 36.Hinterberg B., Klos C., Tenhaken R. Recombinant UDP-glucose dehydrogenase from soybean. Plant Physiol. Biochem. 2002;40:1011–1017. [Google Scholar]
- 37.Turner W., Botha F. C. Purification and kinetic properties of UDP-glucose dehydrogenase from sugarcane. Arch. Biochem. Biophys. 2002;407:209–216. doi: 10.1016/s0003-9861(02)00500-3. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y., Thoden J. B., Kim J., Berger E., Gulick A. M., Ruzicka F. J., Holden H. M., Frey P. A. Mechanistic roles of tyrosine 149 and serine 124 in UDP-galactose 4-epimerase from Escherichia coli. Biochemistry. 1997;36:10675–10684. doi: 10.1021/bi970430a. [DOI] [PubMed] [Google Scholar]
- 39.Smith M. M., Stone B. A. β-Glucan synthesis by cell-free extracts from Lolium multiflorum endosperm. Biochim. Biophys. Acta. 1973;313:72–94. doi: 10.1016/0304-4165(73)90189-x. [DOI] [PubMed] [Google Scholar]
- 40.Roberts R. M., Heishman A., Wicklin C. Growth inhibition and metabolite pool levels in plant tissues fed D-glucosamine and D-galactose. Plant Physiol. 1971;48:36–42. doi: 10.1104/pp.48.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto R., Masuda Y. Galactose inhibition of auxin-induced cell elongation in oat coleoptile segments. Physiol. Plant. 1984;61:321–326. [Google Scholar]
- 42.Feingold D. S., Avigad G. Sugar nucleotide transformations in plants. In: Stumpf P. K., Conn E. E., editors. The Biochemistry of Plants: A Comprehensive Treatise. New York: Academic Press; 1980. pp. 101–170. [Google Scholar]
- 43.Thoden J. B., Henderson J. M., Fridovich-Keil J. L., Holden H. M. Structural analysis of the Y299C mutant of Escherichia coli UDP-galactose 4-epimerase. Teaching an old dog new tricks. J. Biol. Chem. 2002;277:27528–27534. doi: 10.1074/jbc.M204413200. [DOI] [PubMed] [Google Scholar]
- 44.Soldo B., Scotti C., Karamata D., Lazarevic V. The Bacillus subtilis Gne (GneA, GalE) protein can catalyse UDP-glucose as well as UDP-N-acetylglucosamine 4-epimerisation. Gene. 2003;319:65–69. doi: 10.1016/s0378-1119(03)00793-5. [DOI] [PubMed] [Google Scholar]
- 45.Ishiyama N., Creuzenet C., Lam J. S., Berghuis A. M. Crystal structure of WbpP, a genuine UDP-N-acetylglucosamine 4-epimerase from Pseudomonas aeruginosa: substrate specificity in UDP-hexose 4-epimerases. J. Biol. Chem. 2004;279:22635–22642. doi: 10.1074/jbc.M401642200. [DOI] [PubMed] [Google Scholar]
- 46.Thoden J. B., Wohlers T. M., Fridovich-Keil J. L., Holden H. M. Crystallographic evidence for Tyr157 functioning as the active site base in human UDP-galactose 4-epimerase. Biochemistry. 2000;39:5691–5701. doi: 10.1021/bi000215l. [DOI] [PubMed] [Google Scholar]
- 47.Hanisch F. G. O-glycosylation of the mucin type. Biol. Chem. 2001;382:143–149. doi: 10.1515/BC.2001.022. [DOI] [PubMed] [Google Scholar]
- 48.Masuda Y., Ohnuma S., Kawagoe M., Sugiyama T. A glycoside of Nicotiana tabacum affects mouse dopaminergic behavior. Methods Find. Exp. Clin. Pharmacol. 2003;25:41–43. doi: 10.1358/mf.2003.25.1.772546. [DOI] [PubMed] [Google Scholar]
- 49.Geigenberger P., Fernie A. R., Gibon Y., Christ M., Stitt M. Metabolic activity decreased as an adaptive response to low internal oxygen in growing potato tubers. Biol. Chem. 2000;381:723–740. doi: 10.1515/BC.2000.093. [DOI] [PubMed] [Google Scholar]
- 50.Schiefelbein J. W., Somerville C. Genetic control of root hair development in Arabidopsis thaliana. Plant Cell. 1990;2:235–243. doi: 10.1105/tpc.2.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]






