Abstract
The Leu720–Leu764 region of the II–III loop of the dihydropyridine receptor is believed to be important for both orthograde and retrograde communications with the RyR (ryanodine receptor), but its actual role has not yet been resolved. Our recent studies suggest that voltage-dependent activation of the RyR channel is mediated by a pair of interacting N-terminal and central domains, designated as the ‘domain switch’. To investigate the effect of peptide C (a peptide corresponding to residues Glu724–Pro760) on domain- switch-mediated activation of the RyR, we measured Ca2+ release induced by DP (domain peptide) 1 or DP4 (which activates the RyR by mediation of the domain switch) and followed the Ca2+ release time course using a luminal Ca2+ probe (chlortetracycline) under Ca2+-clamped conditions. Peptide C produced a significant potentiation of the domain-switch-mediated Ca2+ release, provided that the Ca2+ concentration was sufficiently low (e.g. 0.1 μM) and the Ca2+ channel was only partially activated by the domain peptide. However, at micromolar Ca2+ concentrations, peptide C inhibits activation. Covalent cross-linking of fluorescently labelled peptide C to the RyR and screening of the fluorescently labelled tryptic fragments permitted us to localize the peptide-C-binding site to residues 450–1400, which may represent the primary region involved in physical coupling. Based on the above findings, we propose that the physiological role of residues Glu724–Pro760 is to facilitate depolarization-induced and domain-switch-mediated RyR activation at sub- or near-threshold concentrations of cytoplasmic Ca2+ and to suppress activation upon an increase of cytoplasmic Ca2+.
Keywords: dihydropyridine receptor (DHP receptor), domain–domain interaction, excitation–contraction coupling, peptide C, ryanodine receptor (RyR)
Abbreviations: BAPTA, bis-(o-aminophenoxy)ethane-N,N,N,N′-tetra-acetic acid; Caps, 3-(cyclohexylamino)propane-1-sulphonic acid; CTC, chlortetracycline; DHP, dihydropyridine; DP, domain peptide; e–c, excitation–contraction; MH, malignant hyperthermia; NHS-ASA, N-hydroxysuccinimidyl-4-azidosalicilic acid; RyR, ryanodine receptor; SR, sarcoplasmic reticulum
INTRODUCTION
An increasing body of evidence suggests that the regulation of the RyR (ryanodine receptor) Ca2+ channel is mediated by interactions between its N-terminal and central domains. These domains contain many MH (malignant hyperthermia) mutations associated with aberrant Ca2+ handling. A close contact between these domains (zipping) closes the channel, while removal of this contact (unzipping) produces channel activation [1,2]. We have designated these interacting domains as a ‘domain switch’, with the implication that the interacting regions may serve as a relay switch for the mechanism by which the activation signal applied to the RyR is transduced to channel opening. Importantly, it seems that depolarization-induced activation of the Ca2+ channel is mediated by this domain switch unzipping mechanism, as demonstrated by the distinct conformational responses of a fluorescence probe attached to the domain switch in the RyR, on depolarization of the T-tubule [3]. Several synthetic peptides, corresponding to portions of the domain switch, are found to produce domain unzipping and channel activation, suggesting that the DP (domain peptide)-induced Ca2+ release may serve as an in vitro model of e–c (excitation–contraction) coupling [3–5]. In our previous studies, we elucidated the correlation between domain unzipping and channel activation by chiefly using spectroscopic measurements and ryanodine binding assays [1]. However, only a limited amount of work on the kinetics of Ca2+ release has been done under physiologically relevant conditions using this peptide-induced e–c coupling model. In the present paper, we report the results obtained using a novel method that permitted us to follow the Ca2+ release kinetics in this in vitro e–c coupling model by clamping extra-vesicular Ca2+ at a physiological threshold concentration of Ca2+ (0.1 μM).
It is widely accepted that the II–III loop of the α1 subunit of the DHP (dihydropyridine) receptor is one of several key elements involved in structural and functional coupling between the DHP receptor and the RyR. There has been a considerable amount of dispute concerning the assignment of the II–III loop domain that is critical for the activation of e–c coupling, especially about the 671–690 region (the peptide A region). However, it is widely agreed that the Leu720–Leu764 region of the II–III loop is crucial for communication between the DHP receptor and the RyR1, chiefly based upon chimaera and mutation approaches, as: (a) replacement of the corresponding region of the cardiac loop with the skeletal sequence rescued skeletal-type e–c coupling (both orthograde [6] and retrograde [7] signalling); (b) coupling was abolished by deletion of residues 720–765 [8]; and (c) replacement of individual residues within this region (Ala739, Phe741, Pro742 and Asp744) with the corresponding cardiac residues resulted in significant reduction of e–c coupling [9]. Thus the Leu720–Leu764 region of the II–III loop seems to represent a critical determinant of skeletal type e–c coupling. However, these approaches have not permitted the questions as to why this region is critical and what is the actual function of this region to be answered.
In order to address these questions, we must establish an adequate approach that permits us to directly test several key issues, which could not be adequately addressed in the chimaera and mutation approaches. These issues include: (a) the interaction of the Leu720–Leu764 region of the II–III loop with the RyR, and (b) its effect on the physiological mechanism of activation of RyR Ca2+ channels. According to our recent studies of ryanodine binding and Ca2+ release, peptide C (a peptide corresponding to the Glu724–Pro760 region of the II–III loop [10]) alone produced little or no effect on the RyR1, but if added together with agonists (e.g. peptide A) it blocked agonist-dependent activation at micromolar concentrations of Ca2+; however, it showed additive activation effects at low concentrations of Ca2+ (e.g. <0.1 μM) [11]. According to recent studies of RyR1 channels incorporated into lipid bilayers [12–15], peptide C activates or inhibits the RyR1 Ca2+ channel depending on experimental conditions (e.g. the peptide concentration, the time of incubation and the presence of various effectors, such as Ca2+, Mg2+ or ATP). We have tested the most critical question regarding how well the isolated short peptide retains the physiological properties of its in vivo counterpart. As shown in our previous studies, pre-incubation of triad-enriched vesicles with peptide C resulted in an appreciable inhibition of SR (sarcoplasmic reticulum) Ca2+ release induced by chemical depolarization of the T-tubule moiety of the triad [16]. Similarly, pre-incubation of skinned muscle fibres with peptide C caused a significant inhibition of the depolarization-induced tension development in the skinned fibre [17]. These findings indicate that peptide C is competing with the physiological e–c coupling site of RyR where physiological interaction with the DHP receptor takes place. This suggests that peptide C can serve as a physiologically meaningful probe for the study of the II–III loop/RyR interaction. In order to investigate the effect of peptide C on the physiological mechanism of activation of RyR Ca2+ channels, in the present study we employed domain-peptide- and domain-switch-mediated channel activation as an in vitro model of e–c coupling, because T-tubule depolarization-induced Ca2+ release seems to be mediated by the domain switch (see above).
The main new finding in the present study is that peptide C produces significant augmentation of DP4-induced channel activation (Ca2+ release and ryanodine binding) at near- or sub-threshold Ca2+ concentrations, but at higher Ca2+ concentrations it suppresses channel activation. This suggests that one of the roles assigned to the in vivo counterpart of peptide C is to facilitate domain-switch-mediated RyR1 channel activation, at sub- or near-threshold concentrations of cytoplasmic Ca2+, and to suppress channel activation upon an increase of cytoplasmic Ca2+.
EXPERIMENTAL
Reagents
Unless otherwise stated, reagents were obtained from Sigma–Aldrich.
Membrane preparation
SR membrane vesicles were prepared from rabbit back and hind-leg skeletal muscles (Pel-Freez) by differential centrifugation of muscle homogenates as described in [18].
Peptide synthesis
Peptides were synthesized on an Applied Biosystems model 431A synthesizer, purified by reversed-phase HPLC and evaluated by MS. Peptide C corresponds to residues Glu724–Pro760 of the rabbit skeletal muscle DHP receptor α1 subunit. DP1 and DP4 correspond to residues Leu590–Cys609 and Leu2442–Pro2477 of rabbit skeletal RyR. DP4-mut contains the MH point mutation R2458C.
Ca2+-release assay
MgATP (0.4 mM) was added to a stirred solution containing SR vesicles (0.2 mg/ml protein), 0.15 M KCl and 20 mM Mops (pH 7.0). External Ca2+ was clamped at the required concentration using BAPTA [bis-(o-aminophenoxy)ethane-N,N,N,N′-tetra-acetic acid]-calcium buffers. The time course of Ca2+ uptake was monitored in a PerkinElmer LS55 luminescence spectrometer using CTC (chlortetracycline; 40 μM; excitation 392 nm, emission 536 nm; Fluka) as a luminal Ca2+ indicator. CTC exists as neutral and ionic forms, but only the neutral form crosses the membrane. Inside the SR, the anionic form complexes with Ca2+ (with a rate constant for association of 3.84×102 M−1 [19]) and associates with the membrane inner surface. After Ca2+ uptake had reached a plateau, the appropriate agonist was added, and the resultant Ca2+ release was monitored. Changes in luminal Ca2+ are expressed as changes in the intensity of CTC fluorescence (in arbitrary units). In control experiments, SR vesicles were equilibrated with various concentrations of Ca2+ (from 0.1 mM to 1.0 mM) by overnight incubation at 4 °C. Fluorescence intensity immediately after dilution with BAPTA/calcium buffer (0.1 μM free Ca2+) was then determined. The CTC fluorescence was a linear function of Ca2+ up to 0.5 mM, including the range of luminal Ca2+ concentrations achieved in subsequent active loading experiments (0.3–0.5 mM). A 100 unit change in the CTC fluorescence corresponded to a change in the luminal Ca2+ concentration of 0.15 mM.
[3H]Ryanodine-binding assay
SR vesicular aliquots (50 μg of protein) were incubated with 10 nM [3H]ryanodine (68.4 Ci/ml; PerkinElmer) and the desired concentration of DP4 for 90 min at 37 °C in 100 μl solution containing 150 mM KCl and 20 mM Mops (pH 7.0). BAPTA was used to buffer Ca2+ at 0.1 μM. The effect of peptide C (50 μM) on radioligand binding was determined under identical assay conditions. Specific binding and non-specific binding (in the presence of 10 μM ryanodine) were each determined in duplicate. Binding reactions were terminated by rapid filtration on to GF/B filters (Whatman). Bound [3H]ryanodine was then quantified using a Beckman LS 6500 liquid-scintillation counter.
Site-specific labelling with peptide C
Peptide C (0.5 mM) was reacted with an equimolar concentration of the succinimidyl ester of Alexa Fluor® 680 carboxylic acid (Molecular Probes) in 20 mM Hepes (pH 7.0) for 1 h at 22 °C, then with a 2–10-fold molar excess of NHS-ASA (N-hydroxysuccinimidyl-4-azidosalicilic acid; Pierce) for 2 h at 4 °C. Excess reagents were removed by centrifugation for 4 min at 2000 g in spin columns containing Sephadex® G15. Labelled peptide C (10 μM) was mixed with SR (2 mg/ml) in 150 mM KCl, 20 mM Mops (pH 7.2) and photolysed with long-wave UV light for 5 min.
Localization of the site of peptide C attachment within the RyR
Labelled SR was digested with calpain II (Calbiochem) and trypsin, and the resultant fragments were viewed at 700 nm using an Odyssey infrared imager (LI-COR) after SDS/6% PAGE. For Western blot analysis, fragments were transferred to Immobilon-P membranes (Millipore) at 90 V in 10% (v/v) methanol and 10 mM Caps [3-(cyclohexylamino)propane-1-sulphonic acid] (pH 11.0) at 4 °C. The blots were developed with anti-DP1–2 and anti-DP3 primary antibodies (raised against RyR residues 590–628 and 324–351 respectively; Bio-Synthesis) and peroxidase-conjugated secondary antibodies.
Statistical analysis
Results are expressed as means±S.E.M., where n is the number of experiments. Comparison of mean values was performed using paired Student's t tests; P<0.05 was considered significant.
RESULTS
The kinetics of Ca2+ release in the in vitro e–c coupling model
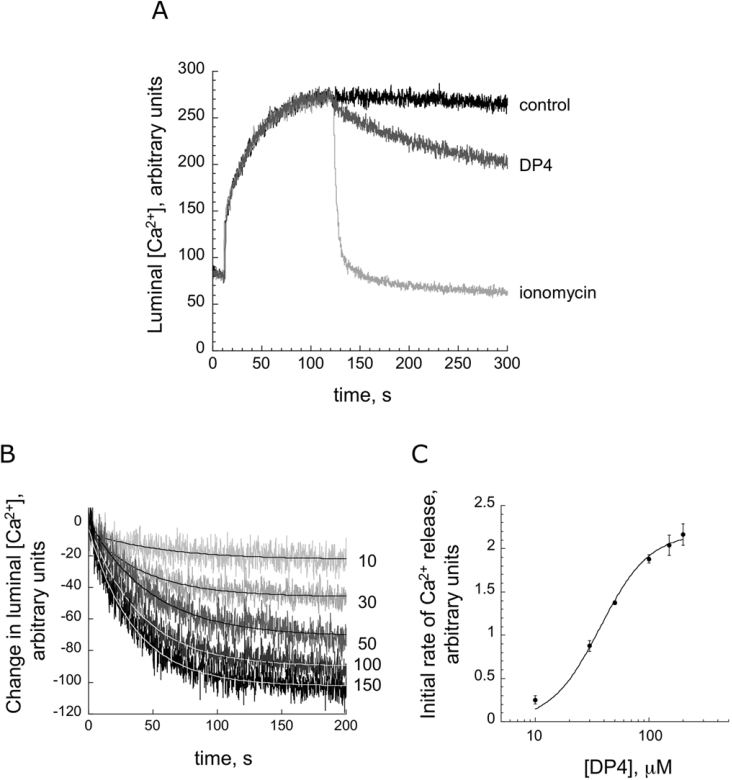
As described in the Introduction section, synthetic peptides corresponding to key regions of the domain switch cause domain unzipping and open the RyR1 Ca2+ channel. Evidence also suggests that T-tubule depolarization-induced Ca2+ release is mediated by the domain switch unzipping mechanism. This suggests that domain-peptide-induced Ca2+ release can be used as an in vitro model of e–c coupling. Previously, we carried out Ca2+ release measurements using an extra-vesicular Ca2+ probe, Fluo-3. However, using this approach it is not possible to clamp the extra-vesicular Ca2+ (corresponding to the cytoplasmic Ca2+) at a well-defined concentration. In order to follow the kinetics of Ca2+ release at a physiological threshold concentration of Ca2+, we adopted the Ca2+-release assay with CTC as a low-affinity luminal Ca2+ probe. CTC fluorescence has previously been demonstrated to provide a quantitative measure of Ca2+ concentration [19], and has been used to monitor SR Ca2+ release in skinned cardiac cells [20] or from SR vesicles that have been actively [21] or passively [22] loaded. This method allowed us to clamp the extra-vesicular Ca2+ (corresponding to cytoplasmic Ca2+ in vivo) at a fixed concentration throughout the experiment. Ca2+ loading was achieved by rapid ATP-dependent Ca2+ uptake. Figure 1(A) illustrates a representative time course of Ca2+ uptake and release, with extra-vesicular Ca2+ clamped at 0.1 μM using a BAPTA/calcium buffer. The fluorescence intensity of CTC at the starting point represents the concentration of luminal Ca2+ before Ca2+ uptake, as the extra-vesicular CTC has virtually no contribution to the fluorescence signal, due to the very low concentration of extra-vesicular Ca2+ and the low Ca2+ affinity of CTC. On addition of MgATP, the fluorescence intensity of CTC increased rapidly and reached a plateau, showing a time course essentially in mirror image to that of Ca2+ release monitored by an extra-vesicular Ca2+ probe such as Fluo-3. The addition of the Ca2+ ionophore ionomycin rapidly decreased the CTC fluorescence to a level even lower than the starting level, indicating that the luminal CTC followed the rapid decrease in the luminal Ca2+ concentration caused by the ionophore. Since the plateau of Ca2+ uptake is followed by a slow decay even without addition of release-inducing agents (control), in the following experiments we analysed Ca2+-release curves calculated from the difference between the control curve and the release curve. Figure 1(B) shows a family of time courses of Ca2+ release induced by various concentrations of DP4 and Figure 1(C) shows the initial rate of Ca2+ release as a function of DP4 concentration. The Ca2+-release time courses were fitted by the equation y=A·e−kt+yo, and the initial rate [(dy/dt)t->0=A·k] was calculated. The concentration-dependence of DP4 activation of Ca2+ release (Figure 1C) and that of DP4 activation of ryanodine binding activity (a chemical measure of channel activation), as shown previously [1], are very similar. As shown in our previous report [1] DP4-mut, in which the Arg residue of DP4 was replaced with Cys mimicking the R2458C MH mutation [23], has lost the ability to unzip the domain switch and activate the Ca2+ channel. Importantly, DP4-mut abolished DP4's original ability to induce Ca2+ release (cf. Figure 2B). This provides an excellent negative control to show that the activation seen with DP4 is physiologically significant and the effect is produced by a specific binding of the peptide.
Figure 1. Time course of Ca2+ release induced by various concentrations of DP4.
(A) Time course of Ca2+ flux (100 units of the CTC fluorescence change correspond to a 0.15 mM change in luminal Ca2+). Initial phase corresponds to active uptake of Ca2+ following addition of MgATP (at t=10 s). Fluorescence intensity subsequently decreased owing to passive Ca2+ leak into the BAPTA-buffered extra-vesicular solution (control trace). Ca2+ efflux is accelerated when RyR agonists are added (50 μM DP4 was added at t=90 s). The Ca2+ gradient is rapidly ablated by the addition of 1 μM ionomycin. (B) Time courses of Ca2+ released from SR vesicles at various DP4 concentrations. External Ca2+ was held at 0.1 μM. Following subtraction of control data, traces were fitted to equations of the form y=A·e−kt+yo, where A is the maximum amount of Ca2+ release, k is the rate constant of Ca2+ release and t is time. (C) Plot of A·k (initial rate of release) against DP4 concentration.
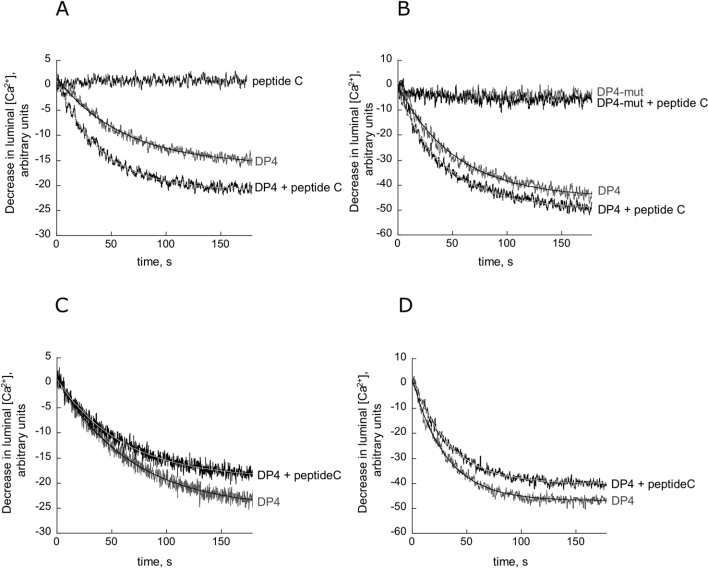
Figure 2. Augmentation of DP4-induced calcium release by peptide C.
At 0.1 μM Ca2+, RyR activation by 10 μM (A) and 30 μM (B) DP4 is accentuated in the presence of peptide C (50 μM). Averaged data and exponential fits for n=5 experiments are shown. Also shown is the lack of effect of 30 μM DP4-mut in the presence and absence of peptide C. The effect of peptide C alone is negligible. At 10 μM Ca2+, RyR activation by 10 μM (C) and 30 μM (D) DP4 is diminished in the presence of peptide C (50 μM). Averaged data and exponential fits for n=5 experiments are shown.
Effects of peptide C on the Ca2+ release in the in vitro e–c coupling model
In the experiment shown in Figures 2(A) and 2(B), we activated Ca2+ release with 10 and 30 μM DP4 at 0.1 μM Ca2+, and examined the effect of 50 μM peptide C. As seen in Table 1, peptide C increased the rate of DP4-induced Ca2+ release approx. 1.90 times at 10 μM DP4 and 1.23 times at 30 μM DP4 (P<0.05), consistent with the proposal that peptide C facilitates channel activation when the activation is only partial. The augmentation of Ca2+ release was also dependent on the peptide C concentration. The initial rates of release for 10 μM DP4 with 20, 50 and 80 μM peptide C are 0.392±0.112, 0.550±0.102 and 0.789±0.095 respectively (n=5). DP4-mut had almost no effect on Ca2+ release and DP4-mut remained inactive in the presence of 50 μM peptide C (Figure 2B). DP4-induced Ca2+-release experiments were also carried out at 10 μM Ca2+. At this Ca2+ concentration, 50 μM peptide C produced significant inhibition of the Ca2+ release induced by 10 and 30 μM DP4 (Figures 2C and 2D; Table 1), indicating that peptide C has Ca2+-dependent dual functions: activation at sub-micromolar Ca2+ concentrations and inhibition at micromolar Ca2+ concentrations. This also suggests that the observed effect is not due to a non-specific effect of the peptide.
Table 1. Initial rates of Ca2+ release from SR vesicles.
Ca2+ release was determined in the presence and absence of peptide C. Values are expressed as the means±S.E.M., n=5. Paired Student's t tests were employed to determine the statistical significance of the data (P value).
| 0.1 μM Ca2+ | 10 μM Ca2+ | ||||||
|---|---|---|---|---|---|---|---|
| Agonist | DP4 (10 μM) | DP4 (30 μM) | DP1 (30 μM) | Caffeine(1 mM) | Caffeine (10 mM) | DP4 (10 μM) | DP4 (30 μM) |
| Initial rate, 0 μM peptide C | 0.290±0.107 | 0.874±0.133 | 0.346±0.044 | 1.925±0.434 | 8.463±0.724 | 0.405±0.027 | 1.439±0.117 |
| Initial rate, 50 μM peptide C | 0.550±0.102 | 1.077±0.154 | 0.485±0.100 | 3.132±0.550 | 8.853±0.721 | 0.321±0.050 | 1.155±0.108 |
| Ratio (r50/r0) | 1.9 | 1.23 | 1.4 | 1.63 | 1.05 | 0.79 | 0.80 |
| P | 0.0032 | 0.0046 | 0.0212 | 0.0048 | 0.4180 | 0.0113 | 0.1059 |
The domain peptide DP1 (corresponding to RyR1 residues 590–609) also activates the Ca2+ channel by mediation of the domain switch, although its activation effect is weaker than that of DP4 [24]. Ca2+ release was induced by 30 μM DP1 in the absence or in the presence of 50 μM peptide C, and, as shown in Table 1, the initial rate of DP1-induced Ca2+ release was increased 1.4-fold (P<0.05) by peptide C. For comparison, we carried out similar experiments using caffeine. The rate of Ca2+ release induced by 1 mM caffeine is accelerated in the presence of peptide C (Table 1). In contrast, there was no significant potentiation by peptide C at 10 mM caffeine.
Ryanodine-binding assay of the effect of peptide C on DP4-induced activation of RyR1 Ca2+ channel
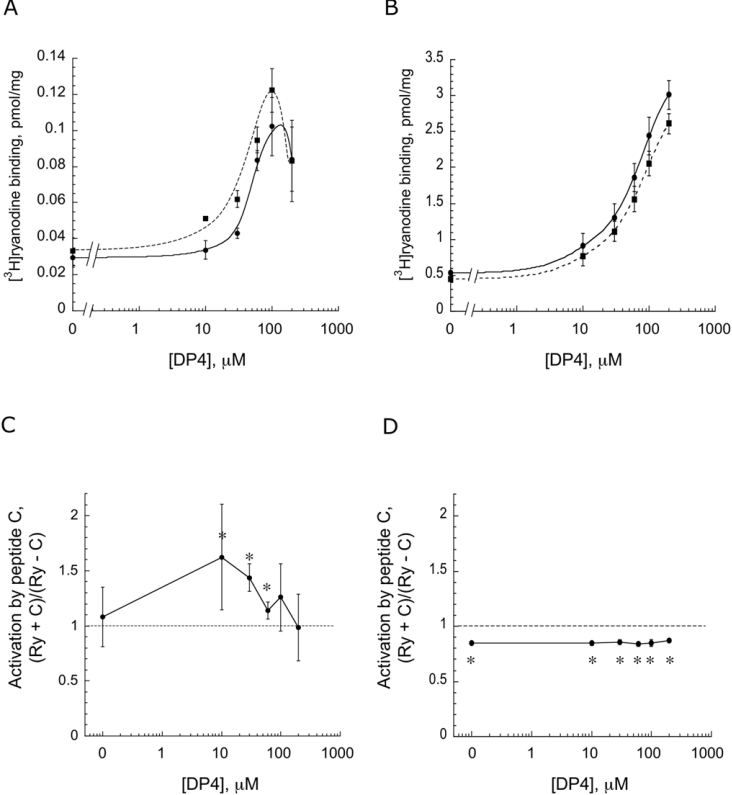
To confirm the Ca2+-dependent effect of peptide C, we carried out ryanodine-binding assays at 0.1 μM Ca2+ (Figures 3A and 3C) and at 10 μM Ca2+ (Figures 3B and 3D). In this experiment, RyR1 was activated with various concentrations of DP4 (as indicated on the abscissa) in the absence (control) or in the presence of 50 μM peptide C. The amount of ryanodine bound is plotted as a function of DP4 concentration in Figures 3(A) and 3(B), and the ratio of (ryanodine binding with peptide C)/(ryanodine binding of control) at each DP4 concentration is plotted in Figures 3(C) and 3(D). As can be seen from these Figures, peptide C produced significant potentiation of DP4-induced activation at 0.1 μM Ca2+. However, in agreement with the Ca2+-release data, it produced significant inhibition at 10 μM Ca2+. It is also clear from Figure 3(C) that the potentiating effect of peptide C (at 0.1 μM Ca2+) is statistically significant only in a range of sub-maximally activating concentrations of DP4: 10 μM (P=0.0092), 30 μM (P=0.006) and 60 μM (P=0.02). These results suggest that peptide C exerts its potentiating effect at sub-micromolar Ca2+ concentrations and under the conditions where RyR1 channels are only partially activated by DP4. In contrast with the restricted DP4 concentration-dependence of peptide C activation at sub-micromolar Ca2+ concentrations, the inhibition by peptide C at higher Ca2+ takes place to approximately the same extent regardless of the concentration of DP4.
Figure 3. Activation of [3H]ryanodine binding versus DP4 concentration.
[3H]Ryanodine-binding assays were performed in the absence or presence of 50 μM peptide C at various concentrations of DP4 in 0.1 μM Ca2+ (A) and 10 μM Ca2+ (B). Data points represent the means±S.E.M. of n=4 assays. Ratios of ryanodine binding with peptide C (Ry+C) to ryanodine binding of control (Ry-C) at each DP4 concentration are also shown (C, D).
Peptide C binding is specific to the RyR and its binding site is localized in a particular region of the primary structure
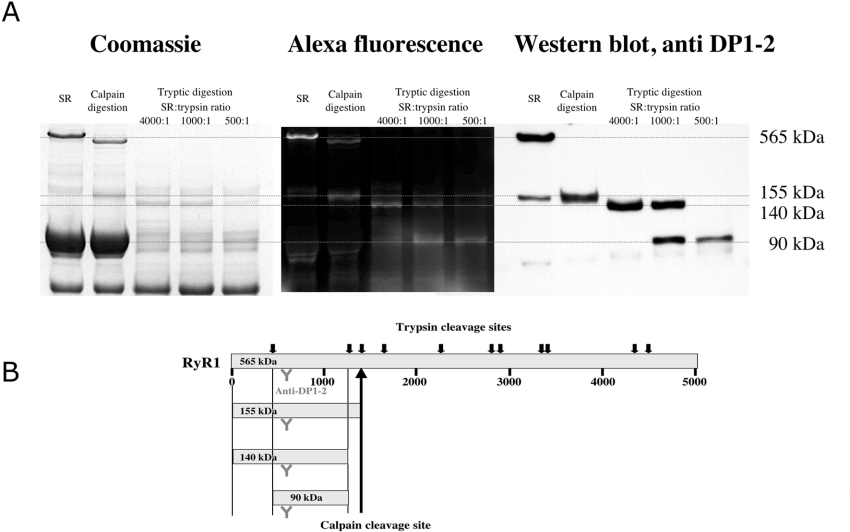
In order to evaluate the specificity of the peptide probe and to identify its binding region on the RyR1, we carried out the cross-linking studies shown in Figure 4. We previously showed that peptide-C-mediated site-specific MCA (methyl coumarin acetamido) labelling resulted in fluorescent labelling of the 150 kDa region from the N-terminus to the major calpain cleavage site at residue 1400 [11]. In the present study, in order to increase the intensity of fluorescence labelling, we synthesized a conjugate of peptide C with Alexa Fluor® 680 and chemically cross-linked the conjugate with the RyR1. Densitometry of the Commassie Blue-stained bands of undigested SR and the corresponding fluorescently labelled bands showed that the RyR1 constitutes approx. 10% of the protein above 70 kDa in size, whereas the fluorescence intensity of the labelled RyR1 comprises as much as 90% of the total fluorescence. This indicates that binding of peptide C was almost exclusively targeted to the RyR1 moiety of the SR membrane. We analysed the regions of RyR1 from which the fluorescently labelled tryptic fragments were derived using site-specific polyclonal antibodies. As shown in Figure 4, the major fluorescently labelled fragment that was detected following partial digestion was a 155 kDa band, which was recognized by anti-DP1–2 antibodies (raised against residues 590–628). Further cleavage produced a 140 kDa fluorescent band and then a 90 kDa fluorescent band, both of which were recognized by the anti-DP1–2 antibody. An anti-DP3 antibody (raised against residues 324–351) recognized the 140 kDa band, but not the 90 kDa band (results not shown, cf. [25]). These results suggest that the site of peptide C binding is localized in the region encompassing residues 450–1400, as shown in Figure 4(B). Since DP4 binds to a 51 kDa N-terminus containing region [25], the domain switch activator, DP4, and the domain switch modifier, peptide C, bind to consecutive segments of the 155 kDa N-terminal calpain fragment of RyR1.
Figure 4. Localization of peptide-C-binding site.
(A) Peptide-C-labelled RyR1 from SR was digested with calpain or trypsin. Tryptic fragments originating from the peptide-C-binding region of the RyR1 were identified with anti-DP1–2 antibodies. (B) Schematic diagram showing digestion pattern of RyR1.
DISCUSSION
Our recent studies [1–3] have provided evidence that interaction between the N-terminal and central domains of RyR controls opening and closing of the channel. Thus this interacting domain pair (designated as the domain switch) serves as a relay-switch-like mechanism in the process of transduction of the received activation signal to the channel opening. Several pieces of evidence also suggest that their abnormal interaction is the cause of the channel dysfunction evident in MH [2] and in cardiac myopathy [26]. Importantly, it seems that the voltage-dependent activation of the RyR1 Ca2+ channel is mediated by this domain switch. This is demonstrated by the fact that chemical depolarization of the T-tubule moiety of the triadic vesicles produced conformational changes in the domain switch as a prerequisite mechanism for SR Ca2+ release [3]. As shown in our previous reports, synthetic peptides, such as DP4 and DP1, that are based on portions of the domain switch activate the RyR Ca2+ channel by mediation of the domain switch [1,24]. The peptide probe approach affords considerable structural simplification and allows the use of well-defined conditions. Thus channel activation induced by these DPs may serve as a useful in vitro model to investigate the details of the domain-switch-mediated physiological route of channel activation, if the experiments are carried out under physiological conditions.
For this purpose, the kinetic assay of Ca2+ release from the isolated SR vesicles may be the most suitable approach, superior to other type of assays. We previously investigated DP-induced SR Ca2+ release using an extra-vesicular Ca2+ probe, but this conventional method did not permit strict control of the extra-vesicular Ca2+ (corresponding to the cytoplasmic Ca2+) at the desired level. In the present study, we could overcome this problem by employing the luminal Ca2+ probe CTC, which allowed us to clamp the extra-vesicular Ca2+ at the sub- or near-threshold concentration (0.1 μM), where the critical transition from relaxation to contraction takes place.
With this set up of our in vitro e–c coupling model, we investigated the effect of peptide C on the domain switch-mediated channel activation. The most important new aspect of the present study is the finding that peptide C produced a significant enhancement of the domain peptide-induced channel activation at optimal conditions; namely, at sub-micromolar Ca2+ concentrations and at sub-maximally activating concentrations of the DP. In such conditions, peptide C enhanced the DP-induced channel activation approx. 2-fold. Interestingly, at higher Ca2+ concentrations (e.g. 10 μM), peptide C produced significant suppression of the DP-induced channel activation. These results suggest that the actual function of peptide C is to facilitate domain-switch-mediated RyR1 channel activation at sub-maximally activated states, as well as at sub- or near-threshold concentrations of cytoplasmic Ca2+, and to suppress the channel opening when the channel is fully activated. The fact that peptide C works at the sub- or near-threshold Ca2+ indicates that peptide C may be involved in the pre-conditioning mechanism for the activation of e–c coupling. It is interesting to note that the Ca2+-dependent dual (activation and inhibition) functions of peptide C show a striking similarity to the Ca2+-dependent regulation of RyR1 channel by calmodulin [27,28]. This also suggests that the observed effect is not due to a non-specific effect that is the general concern in the peptide probe approach. Similarly, the fact that peptide C works only at lower concentrations of DP precludes the concern that the combined use of two peptides (DP and peptide C) might have produced artifactual effects.
According to the recent report of Haarmann et al. [13], in the presence of millimolar concentrations of Mg2+ and ATP (the conditions that both prevail in the in vivo SR Ca2+ release and are similar to those of the present Ca2+ release experiments), peptide C increased the concentration for a half-maximal inhibition of Mg2+, i.e. Mg2+ inhibition of RyR1 Ca2+ channels was partially relieved by peptide C. This is a significant finding in view of the earlier report of Lamb and Stephenson [29] that the e–c coupling signal received from the DHP receptor relieves the RyR from Mg2+ inhibition, allowing the channel to open. On the basis of the present finding that peptide C facilitates the domain-switch-mediated channel activation only under sub-optimal conditions for channel activation, it is reasonable that peptide-C-mediated potentiation also happens during partial inhibition of the domain switch.
The present fluorescence labelling study provided us with information as to the approximate location of the site where peptide C interacts with the RyR1. As shown in the present study, the shortest fluorescently labelled tryptic fragment identified so far is the 90 kDa fragment containing the epitope for the anti-DP1–2 antibody (the 590–628 sequence). This suggests that the major site of peptide C binding is located in the region corresponding to residues 450–1400 of RyR1. Since the N-terminal domain segment of the domain switch encompasses residues 30–615, there is a possibility that peptide C interacts directly with the domain switch at the distal portion of the N-terminal domain (cf. Figure 5). However, it seems more likely that the location of the peptide-C-binding site is outside of the domain switch than within it. If this is the case, the facilitation of the operation of the domain switch by peptide C, described in the present paper, is probably mediated by its interaction with a domain neighbouring the N-terminal domain of the domain switch. We tentatively propose that this peptide-C-binding region has a hinge-like function to control the operation of the domain switch.
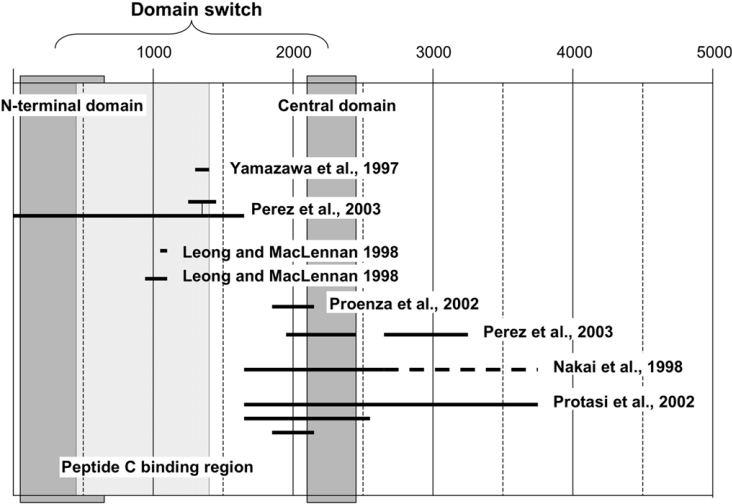
Figure 5. Diagram showing various regions of the RyR1 which have been suggested to be involved in physical interaction with the DHP receptor.
The peptide-C-binding region and the two domains constituting the domain switch (N-terminal and central domains) are also shown for reference.
Many researchers have sought to determine which portions of the RyR are involved in physical interaction with the DHP receptor, and, as summarized in Figure 5, multiple regions have been identified. Several of these are included in the N-terminal section (residues 450–1400) identified in the present study as the peptide-C-binding region. Early reports by Leong and MacLennan [30,31] suggested that the 922–1112 region of RyR1 binds to both II–III and III–IV loops of the DHP receptor. Residues 1303–1406 [32] and 1272–1455 [33], both of which contain the D2 (1342–1403) region, have been identified as critical for e–c coupling. According to a recent single particle image analysis of RyR1, the D2 region is localized in the part of the ‘clamp’ region designated as domain 6 [34], which appears to be located within the area where the image of the DHP receptor overlaps on the image of RyR1 [35], although this region has also recently been implicated in interactions between RyRs [36].
Several other regions have been proposed to be involved in coupling [37–40], and their distribution is more extensive, encompassing residue 1 to residue 3720 (Figure 5). This is commonly explained by the existence of multiple sites involved in the physical interaction between the DHP receptor and the RyR. Indeed, this is likely to be the case, since the II–III loop is not the only region of the DHP receptor thought to interact with the RyR [31,41,42]. However, there seem to be several intermediate steps in which the activation signal elicited by the DHP receptor/RyR interaction is translated into the channel opening. Therefore the domains, which are involved in the process of signal transduction rather than in the DHP receptor–RyR interaction as such, must be recognized as critical coupling domains, especially in many of those studies in which these domains were screened using RyR1–RyR2 or RyR1–RyR3 chimaera. According to our working hypothesis, binding of peptide C facilitates the activation process of the domain switch that is constituted of the N-terminal and central domains, in turn facilitating channel opening. Interestingly, most of the domains located in the central region of the RyR1 that are suggested to be critical for skeletal type e–c coupling at least partially overlap with the central domain of the domain switch (Figure 5). This would indicate that some of these postulated coupling domains are involved in the operation of the domain switch, which is one of the important steps in signal transduction. In further support of this view, the studies with RyR1/RyR3 chimera by Perez et al. [33] showed that insertion of the RyR1 sequence of the critical 1272–1455 region (containing the aforementioned D2 region) alone into the RyR3 background was not sufficient to confer e–c coupling, unless a long stretch (1–1680) was converted into the skeletal sequence. We should note that this stretch contains the N-terminal domain of the domain switch and the peptide-C-binding region identified in the present study (cf. Figure 5).
In agreement with the recent report that peptide C enhanced the response of RyR1 Ca2+ channels to caffeine [43], we found that peptide C enhanced not only DP4-induced Ca2+ release, but also caffeine-induced Ca2+ release. However, in contrast with the finding of Gallant et al. [43] that peptide C produced its maximal effect at a maximally activating concentration of caffeine, we have shown in the present study that peptide C produces its activation effect only at sub-maximally activating concentrations of caffeine, as in the case of peptide C activation of DP4-induced Ca2+ release (cf. Table 1). This agonist concentration-dependence, common to the two different types of agonists (DP4, the domain-switch-mediated channel activator, and caffeine that potentiates Ca2+-dependent activation), suggests that the domain-switch-mediated activation and Ca2+-dependent activation are not independent, but co-ordinated processes. The most critical question in a peptide probe study is how well the isolated peptide retains the physiological properties of its in vivo counterpart. As shown in our previous reports, pre-incubation of triad-enriched vesicles with peptide C resulted in an appreciable inhibition of SR Ca2+ release induced by chemical depolarization of the T-tubule moiety of the triad [16] and, similarly, pre-incubation of skinned muscle fibres with peptide C caused a significant inhibition of depolarization-induced tension development [17]. This indicates that peptide C can competitively bind at the physiological DHP receptor–RyR interaction site. Thus it is not unreasonable to assume that the Leu720–Leu764 region of the II–III loop binds to the region of RyR1 identified in the present study and has the same type of functions as deduced from the present study with peptide C.
In summary, we examined the effect of peptide C on Ca2+ release from the SR and ryanodine binding, two measures of Ca2+ channel function. Although peptide C by itself had little effect on channel activation, it produced a significant increase in the extent of channel activation by peptides DP4 and DP1, which are known to activate RyR1 channels by mediation of the domain switch. Peptide C activation was pronounced at low Ca2+ concentrations (0.1 μM), and at lower concentrations of the agonist, i.e. when the activation was only partial. Our results suggest that the binding of peptide C to the RyR1 (to a 450–1400 residue region, which may represent the primary region involved in physical coupling) facilitates the domain-switch-mediated channel activation under conditions when the channel is sub-maximally activated, and suppresses the channel opening when the channel is maximally activated. We propose that similar functions can be assigned to the corresponding region of the II–III loop. If so, the reason for the peptide C region of the loop being critical for e–c coupling is that the loop–RyR1 interaction is required for an effective operation of the domain switch (or effective transmission of the activating signal to the channel) at sub- or near-threshold concentrations of Ca2+, and for its deceleration at above-threshold concentrations of Ca2+.
Acknowledgments
We thank senior scientists Dr Renne C. Lu and Dr Paul Leavis, and resarch assistants David Schrier and Elizabeth Gowell (Boston Biomedical Research Institute, Watertown, MA, U.S.A.) for synthesis and purification of the peptides. This work was supported by National Institutes of Health Grants AR 16922 from NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases) and HL072841 from NHLBI (National Heart, Lung, and Blood Institute).
References
- 1.Yamamoto T., El-Hayek R., Ikemoto N. Postulated role of interdomain interaction within the ryanodine receptor in Ca2+ channel regulation. J. Biol. Chem. 2000;275:11618–11625. doi: 10.1074/jbc.275.16.11618. [DOI] [PubMed] [Google Scholar]
- 2.Ikemoto N., Yamamoto T. Regulation of calcium release by interdomain interaction within ryanodine receptors. Front. Biosci. 2002;7:671–683. doi: 10.2741/A803. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto T., Ikemoto N. Spectroscopic monitoring of local conformational changes during the intramolecular domain-domain interaction of the ryanodine receptor. Biochemistry. 2002;41:1492–1501. doi: 10.1021/bi015581z. [DOI] [PubMed] [Google Scholar]
- 4.Lamb G. D., Posterino G. S., Yamamoto T., Ikemoto N. Effects of a domain peptide of the ryanodine receptor on Ca2+ release in skinned skeletal muscle fibres. Am. J. Physiol. Cell Physiol. 2001;281:C207–C214. doi: 10.1152/ajpcell.2001.281.1.C207. [DOI] [PubMed] [Google Scholar]
- 5.Shtifman A., Ward C. W., Yamamoto T., Wang J., Olbinski B., Valdivia H. H., Ikemoto N., Schneider M. F. Interdomain interactions within ryanodine receptors regulate Ca2+ spark frequency in skeletal muscle. J. Gen. Physiol. 2002;119:15–32. doi: 10.1085/jgp.119.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakai J., Tanabe T., Konno T., Adams B., Beam K. G. Localization in the II–III loop of the dihydropyridine receptor of a sequence critical for excitation-contraction coupling. J. Biol. Chem. 1998;273:24983–24986. doi: 10.1074/jbc.273.39.24983. [DOI] [PubMed] [Google Scholar]
- 7.Grabner M., Dirksen R. T., Suda N., Beam K. G. The II–III loop of the skeletal muscle dihydropyridine receptor is responsible for the bi-directional coupling with the ryanodine receptor. J. Biol. Chem. 1999;274:21913–21919. doi: 10.1074/jbc.274.31.21913. [DOI] [PubMed] [Google Scholar]
- 8.Ahern C. A., Bhattacharya D., Mortenson L., Coronado R. A component of excitation-contraction coupling triggered in the absence of the T671–L690 and L720–Q765 regions of the II–III loop of the dihydropyridine receptor α1s pore subunit. Biophys. J. 2001;81:3294–3307. doi: 10.1016/S0006-3495(01)75963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kugler G., Weiss R. G., Flucher B. E., Grabner M. Structural requirements of the dihydropyridine receptor α1S II–III loop for skeletal-type excitation-contraction coupling. J. Biol. Chem. 2004;279:4721–4728. doi: 10.1074/jbc.M307538200. [DOI] [PubMed] [Google Scholar]
- 10.El-Hayek R., Antoniu B., Wang J., Hamilton S. L., Ikemoto N. Identification of calcium release-triggering and blocking regions of the II–III loop of the skeletal muscle ryanodine receptor. J. Biol. Chem. 1995;270:22116–22118. doi: 10.1074/jbc.270.38.22116. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T., Rodriguez J., Ikemoto N. Ca2+-dependent dual functions of peptide C. J. Biol. Chem. 2002;277:993–1001. doi: 10.1074/jbc.M105837200. [DOI] [PubMed] [Google Scholar]
- 12.Stange M., Tripathy A., Meissner G. Two domains in dihydropyridine receptor activate the skeletal muscle Ca2+ release channel. Biophys. J. 2001;81:1419–1429. doi: 10.1016/S0006-3495(01)75797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haarmann C. S., Green D., Casarotto M. G., Laver D. R., Dulhunty A. F. The random-coil ‘C’ fragment of the dihydropyridine receptor II–III loop can activate or inhibit skeletal ryanodine receptors. Biochem. J. 2003;372:305–316. doi: 10.1042/BJ20021763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haarmann C. S., Dulhunty A. F., Laver D. R. Regulation of skeletal ryanodine receptors by dihydropyridine receptor II–III loop C-region peptides: relief of Mg2+ inhibition. Biochem. J. 2005;387:429–436. doi: 10.1042/BJ20040786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dulhunty A. F., Karunasekara Y., Curtis S. M., Harvey P. J., Board P. G., Casarotto M. G. Role of some unconserved residues in the “C” region of the skeletal DHPR II–III loop. Front. Biosci. 2005;10:1368–1381. doi: 10.2741/1626. [DOI] [PubMed] [Google Scholar]
- 16.Saiki Y., El-Hayek R., Ikemoto N. Involvement of the Glu724–Pro760 region of the dihydropyridine receptor II–III loop in skeletal muscle-type excitation-contraction coupling. J. Biol. Chem. 1999;274:7825–7832. doi: 10.1074/jbc.274.12.7825. [DOI] [PubMed] [Google Scholar]
- 17.Lamb G. D., El-Hayek R., Ikemoto N., Stephenson D. G. Effects of dihydropyridine receptor II–III loop peptides on Ca2+ release in skinned skeletal muscle fibres. Am. J. Physiol. Cell Physiol. 2004;279:C891–C905. doi: 10.1152/ajpcell.2000.279.4.C891. [DOI] [PubMed] [Google Scholar]
- 18.Ikemoto N., Kim D. H., Antoniu B. Measurement of calcium release in isolated membrane systems: coupling between the transverse tubule and sarcoplasmic reticulum. Methods Enzymol. 1988;157:469–480. doi: 10.1016/0076-6879(88)57096-9. [DOI] [PubMed] [Google Scholar]
- 19.Dixon D., Brandt N., Haynes D. H. Chlortetracycline fluorescence is a quantitative measure of the free internal Ca2+ concentration achieved by active transport. J. Biol. Chem. 1984;259:13737–13741. [PubMed] [Google Scholar]
- 20.Fabiato A., Fabiato F. Use of chlorotetracycline fluorescence to demonstrate Ca2+-induced release of Ca2+ from the sarcoplasmic reticulum of skinned cardiac cells. Nature (London) 1979;281:146–148. doi: 10.1038/281146a0. [DOI] [PubMed] [Google Scholar]
- 21.Dehpour A. R., Mousavizadeh K., Gerayesh-Nejad S. Calcium release by diltiazem from isolated sarcoplasmic reticulum of rabbit skeletal muscle. Gen. Pharmacol. 1998;31:463–468. doi: 10.1016/s0306-3623(98)00009-3. [DOI] [PubMed] [Google Scholar]
- 22.Nagasaki K., Kasai M. Fast release of calcium from sarcoplasmic reticulum vesicles monitored by chlortetracycline fluorescence. J. Biochem. 1983;94:1101–1109. doi: 10.1093/oxfordjournals.jbchem.a134453. [DOI] [PubMed] [Google Scholar]
- 23.Manning B. M., Quane K. A., Lynch P. J., Urwyler A., Tegazzin V., Krivosic-Horber R., Censier K., Comi G., Adnet P., Wolz W., et al. Novel mutations at a CpG dinucleotide in the ryanodine receptor in malignant hyperthermia. Hum. Mutat. 1998;11:45–50. doi: 10.1002/(SICI)1098-1004(1998)11:1<45::AID-HUMU7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 24.El-Hayek R., Saiki Y., Yamamoto T., Ikemoto N. A postulated role of the near amino-terminal domain of the ryanodine receptor in the regulation of the sarcoplasmic reticulum Ca2+ channel. J. Biol. Chem. 1999;274:33341–33347. doi: 10.1074/jbc.274.47.33341. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi S., Bannister M. L., Gangopadhyay J. P., Hamada T., Parness J., Ikemoto N. Dantrolene stabilizes domain interactions within the ryanodine receptor. J. Biol. Chem. 2005;280:6580–6587. doi: 10.1074/jbc.M408375200. [DOI] [PubMed] [Google Scholar]
- 26.Oda T., Yano M., Yamamoto T., Tokuhisa T., Okuda S., Doi M., Ohkusa T., Ikeda Y., Kobayashi S., Ikemoto N., Matsuzaki M. Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure. Circulation. 2005;111:3400–3410. doi: 10.1161/CIRCULATIONAHA.104.507921. [DOI] [PubMed] [Google Scholar]
- 27.Tripathy A., Xu L., Mann G., Meissner G. Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor) Biophys. J. 1995;69:106–119. doi: 10.1016/S0006-3495(95)79880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodney G. G., Williams B. Y., Strasburg G. M., Beckingham K., Hamilton S. L. Regulation of RyR1 activity by Ca2+ and calmodulin. Biochemistry. 2000;39:7807–7812. doi: 10.1021/bi0005660. [DOI] [PubMed] [Google Scholar]
- 29.Lamb G. D., Stephenson D. G. Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. J. Physiol. 1991;434:507–528. doi: 10.1113/jphysiol.1991.sp018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leong P., MacLennan D. H. A 37-amino acid sequence in the skeletal muscle ryanodine receptor interacts with the cytoplasmic loop between domains II and III in the skeletal muscle dihydropyridine receptor. J. Biol. Chem. 1998;273:7791–7794. doi: 10.1074/jbc.273.14.7791. [DOI] [PubMed] [Google Scholar]
- 31.Leong P., MacLennan D. H. The cytoplasmic loops between domains II and III and domains III and IV in the skeletal muscle dihydropyridine receptor bind to a contiguous site in the skeletal muscle ryanodine receptor. J. Biol. Chem. 1998;273:29958–29964. doi: 10.1074/jbc.273.45.29958. [DOI] [PubMed] [Google Scholar]
- 32.Yamazawa T., Takeshima H., Shimuta M., Iino M. A region of the ryanodine receptor critical for excitation-contraction coupling in skeletal muscle. J. Biol. Chem. 1997;272:8161–8164. doi: 10.1074/jbc.272.13.8161. [DOI] [PubMed] [Google Scholar]
- 33.Perez C. F., Mukherjee S., Allen P. D. Amino acids 1–1,680 of ryanodine receptor type 1 hold critical determinants of skeletal type for excitation-contraction coupling. J. Biol. Chem. 2003;278:39644–39652. doi: 10.1074/jbc.M305160200. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z., Zhang J., Wang R., Wayne Chen S. R., Wagenknecht T. Location of divergent region 2 on the three-dimensional structure of cardiac muscle ryanodine receptor/calcium release channel. J. Mol. Biol. 2004;338:533–545. doi: 10.1016/j.jmb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Serysheva I. I. Structural insights into excitation-contraction coupling by electron cryomicroscopy. Biochemistry (Moscow) 2004;69:1226–1232. doi: 10.1007/s10541-005-0068-5. [DOI] [PubMed] [Google Scholar]
- 36.Yin C. C., Blayney L. M., Lai F. A. Physical coupling between ryanodine receptor-calcium release channels. J. Mol. Biol. 2005;349:538–546. doi: 10.1016/j.jmb.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Nakai J., Sekiguchi N., Rando T. A., Allen P. D., Beam K. G. Two regions of the ryanodine receptor involved in coupling with L-type Ca2+ channels. J. Biol. Chem. 1998;273:13403–13406. doi: 10.1074/jbc.273.22.13403. [DOI] [PubMed] [Google Scholar]
- 38.Proenza C., O'Brien J., Nakai J., Mukherjee S., Allen P. D., Beam K. G. Identification of a region of RyR1 that participates in allosteric coupling with the α1S (CaV1.1) II–III loop. J. Biol. Chem. 2002;277:6530–6535. doi: 10.1074/jbc.M106471200. [DOI] [PubMed] [Google Scholar]
- 39.Protasi F., Paolini C., Nakai J., Beam K. G., Franzini-Armstrong C., Allen P. D. Multiple regions of RyR1 mediate functional and structural interactions with α1S-dihydropyridine receptors in skeletal muscle. Biophys. J. 2002;83:3230–3244. doi: 10.1016/S0006-3495(02)75325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez C. F., Voss A., Pessah I. N., Allen P. D. RyR1/RyR3 chimeras reveal that multiple domains of RyR1 are involved in skeletal-type e–c coupling. Biophys. J. 2003;84:2655–2663. doi: 10.1016/S0006-3495(03)75071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slavik K. J., Wang J. P., Aghdasi B., Zhang J. Z., Mandel F., Malouf N., Hamilton S. L. A carboxy-terminal peptide of the α1-subunit of the dihydropyridine receptor inhibits Ca2+-release channels. Am. J. Physiol. 1997;272:C1475–C1481. doi: 10.1152/ajpcell.1997.272.5.C1475. [DOI] [PubMed] [Google Scholar]
- 42.Beurg M., Ahern C. A., Vallejo P., Conklin M. W., Powers P. A., Gregg R. G., Coronado R. Involvement of the carboxy-terminus region of the dihydropyridine receptor β1a subunit in excitation-contraction coupling of skeletal muscle. Biophys. J. 1999;77:2953–2967. doi: 10.1016/S0006-3495(99)77128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallant E. M., Hart J., Eager K., Curtis S., Dulhunty A. F. Caffeine sensitivity of native RyR channels from normal and malignant hyperthermic pigs: effects of a DHPR II–III loop peptide. Am. J. Physiol. Cell Physiol. 2004;286:C821–C830. doi: 10.1152/ajpcell.00311.2003. [DOI] [PubMed] [Google Scholar]