Abstract
Certain selenoproteins such as GPX-1 (glutathione peroxidase-1) and TrxR1 (thioredoxin reductase-1) possess important antioxidant defence functions in vascular endothelial cells. Reduced selenoprotein activity during dietary selenium (Se) deficiency can result in a compensatory increase of other non-Se-dependent antioxidants, such as HO-1 (haem oxygenase-1) that may help to counteract the damaging effects of oxidant stress. However, the role of individual selenoproteins in regulating vascular-derived protective gene responses such as HO-1 is less understood. Using an oxidant stress model based on Se deficiency in BAECs (bovine aortic endothelial cells), we sought to determine whether TrxR1 activity may contribute to the differential regulation of HO-1 expression as a function of altered redox environment. Se-sufficient BAECs up-regulated HO-1 expression following stimulation with the pro-oxidant, 15-HPETE (15-hydroperoxyeicosatetraenoic acid), and levels of this antioxidant inversely correlated with EC apoptosis. While Se-deficient BAECs exhibited higher basal levels of HO-1, it was not up-regulated upon 15-HPETE treatment, which resulted in significantly higher levels of pro-apoptotic markers. Subsequent results showed that HO-1 induction depended on the activity of TrxR1, as proved with chemical inhibitor studies and direct inhibition with TrxR1 siRNA. Finally, restoring intracellular levels of the reduced substrate Trx (thioredoxin) in Sedeficient BAECs was sufficient to increase HO-1 activation following 15-HPETE stimulation. These data provide evidence for the involvement of the Trx/TrxR system, in the regulation of HO-1 expression in BAECs during pro-oxidant challenge.
Keywords: haem oxygenase-1, oxidant stress, selenium, thioredoxin, thioredoxin reductase
Abbreviations: AA, arachidonic acid; BAEC, bovine aortic endothelial cell; COX, cyclo-oxygenase; DCFH-DA, 2′,7′-dichlorofluorescein diacetate; DNCB, 2,4-dinitrochlorobenzene; DTNB, 5,5-dithiobis-(2-nitrobenzoic acid); EC, endothelial cell; FAHP, fatty acid hydroperoxide; FBS, foetal bovine serum; GPX, glutathione peroxidase; 15-HETE, 15-hydroxyeicosatetraenoic acid; HO-1, haem oxygenase-1; 15-HPETE, 15-hydroperoxyeicosatetraenoic acid; HRP, horseradish peroxidase; LDL, low-density lipoprotein; LOX, lipoxygenase; QC RT–PCR, quantitative competitive reverse transcription–PCR; ROS, reactive oxygen species; siRNA, short interfering RNA; SnPPIX, tin protoporphyrin; SP-HPLC, straight-phase HPLC; TBS, Tris-buffered saline; Trx, thioredoxin; rTrx, recombinant human Trx; TrxR, thioredoxin reductase
INTRODUCTION
A deficiency of the micronutrient selenium (Se) in the vascular microenvironment induces oxidant stress and is associated with the progression of atherosclerosis [1]. The outcome of clinical trials investigating the relationship between Se supplementation and the prevention of cardiovascular disease remains controversial and difficult to interpret owing to the lack of mechanistic data on this micronutrient [2]. One beneficial outcome of Se supplementation in ECs (endothelial cells) is the increased activities of the Se-dependent antioxidants, GPX (glutathione peroxidase) and TrxR (thioredoxin reductase), which regulate oxidant stress through several mechanisms. In addition to the ability to scavenge ROS (reactive oxygen species), GPX and TrxR control the redox status of their substrates, glutathione and Trx (thioredoxin) [3]. Once reduced, glutathione and Trx can scavenge ROS and control cellular responses by reducing critical cysteine residues in redox-sensitive proteins. Additionally, it is well documented that selenoproteins regulate the activity of AA (arachidonic acid) metabolism, in particular the COX (cyclo-oxygenase) and LOX (lipoxygenase) pathways. For instance, ROS can either stimulate or inhibit enzyme activity, depending on the specific isoform and magnitude of ROS accumulation [4]. Selenoproteins also regulate the specificity of the oxygenation products produced by COX and LOX enzymes, including the reduction of the primary 15-LOX-1 metabolite, 15-HPETE (15-hydroperoxyeicosatetraenoic acid), into its reduced form, 15-HETE (15-hydroxyeicosatetraenoic acid), thus inhibiting the accumulation of the primary product [5,6]. The hydroperoxy metabolite, 15-HPETE, acts as a reactive pro-oxidant species in many cell types and is specifically pro-apoptotic in ECs [7,8]. Finally, in addition to their multiple roles in regulating the cellular redox environment, recent evidence suggests that selenoproteins play a role in regulating the expression of alternative non-Se-dependent stress-response enzymes such as HO-1 (haem oxygenase-1) [9].
The antioxidant HO-1 catalyses the conversion of the pro-oxidant molecule haem into the products biliverdin, iron and CO. HO-1 is expressed ubiquitously in many cell types, and transcription is activated by numerous pro-oxidant molecules, including haem, heavy metals, oxidized LDL (low-density lipoprotein), proinflammatory cytokines and ROS [10–13]. Based on its antioxidant properties, it is not surprising that expression of HO-1 prevents diseases associated with oxidant stress, including ischaemia–reperfusion, graft rejection and atherosclerosis [14,15]. In fact, studies performed in vivo confirm a therapeutic as well as preventative role for HO-1 in the development of atherosclerosis. Chemical induction of HO-1 produces smaller atherosclerotic lesions in LDL-receptor-deficient mice, whereas inhibition of HO-1 exacerbates lesion size compared with controls [16,17]. Targeted overexpression of HO-1 in the blood vessels of apolipoprotein B-deficient mice delays the progression towards clinical manifestations of atherosclerosis [18]. While the significance of HO-1 in protection against these diseases is well-established, the regulation of its expression in vascular cells such as ECs during times of oxidant stress is not clearly defined.
Recent studies demonstrate a correlation between selenoprotein activity and HO-1 expression [19], although the exact mechanism(s) involved in this relationship remains to be determined. In vivo studies of Se deficiency demonstrate a compensatory increase in HO-1 expression in rat hepatocytes, indicating a significant impact of non-Se-dependent antioxidant regulation at the site of abundant selenoprotein synthesis [19]. However, these same studies show that HO-1 levels are unaltered in other organs, suggesting that regulation of non-Se-dependent defences at these sites is different from that in the liver. Other studies demonstrate a specific role for selenoproteins in the regulation of HO-1 during oxidant stress and implicate TrxR as the primary selenoprotein involved in that regulation [19,20]. Conflicting results exist with these studies which suggest both a positive and negative effect of TrxR activity on HO-1 expression. In one instance, an increase in TrxR activity decreases ROS, a potential source of stimulus for HO-1 gene transcription in hepatocytes [19,20]. Alternatively, elevated TrxR activity results in an increase in Trxred (reduced Trx), which has been suggested to increase HO-1 expression through the reduction of redox-sensitive signalling proteins upstream of HO-1 gene transcription in macrophages [19,20]. In order to clarify further the association between selenoproteins and HO-1 expression in the vascular endothelium, the present study used an oxidant stress model with primary BAECs (bovine aortic ECs) deficient in Se that allows for the evaluation of compensatory antioxidant mechanisms in the absence of Se-dependent antioxidants. The present paper demonstrates that there is differential regulation of HO-1 expression following pro-oxidant exposure depending on the Se status of EC. Through the specific inhibition of TrxR1 with siRNA (short interfering RNA), we demonstrate that this selenoprotein positively regulates the level of HO-1 expression through the control of intracellular redox status.
EXPERIMENTAL
Reagents
The following reagents were purchased from Sigma Chemical Co.: insulin, heparin, transferrin, PMSF, aprotinin, AA, sodium selenite, calcium ionophore A23187, DTNB [5,5-dithiobis-(2-nitrobenzoic acid)], DNCB (2,4-dinitrochlorobenzene) and recombinant TrxR. Antibiotics and antimycotics, L-glutamine, trypsin/EDTA, Hepes buffer, TRIzol® were obtained from Invitrogen. Ham's F-12K (Kaighn's nutrient Mixture F-12) was obtained from Irvine Scientific. Hyclone Laboratory supplied FBS (foetal bovine serum) with reduced Se levels as requested (ranging from 200 to 250 nM final concentration). Hoechst 33342 and DCFH-DA (2′,7′-dichlorofluorescein diacetate) were purchased from Molecular Probes. ApoONE homologous caspase 3/7 assay and Dual-Luciferase® Reporter Assay System was obtained from Promega. SnPPIX (tin protoporphyrin) was obtained from Tocris Chemical Co. rTrx (recombinant human Trx) was purchased from American Diagnostica and was stored under reduced conditions. HO-1 4.0 kb promoter luciferase construct was provided by Dr Xi-Lin Chen (AtheroGenics Inc., Alpharetta, GA, U.S.A.). Anti-HO-1 monoclonal antibody was purchased from Stressgen, anti-Trx antibody was purchased from BD Pharmingen and anti-actin was purchased from Chemicon. Anti-TrxR antibody was obtained from Upstate and detects the N-terminal region of TrxR. M-PER® (mammalian protein extraction reagent) and secondary mouse IgG conjugated to HRP (horseradish peroxidase) were from Pierce. The siRNA plasmid vector, pSuppressor-Neo, was obtained from Imgenex. Oxis Research supplied the GSH/GSSG-412™ kit.
Preparation of 15-LOX-1 metabolites
Soya bean lipoxygenase (40 mg) was added to 600 ml of oxygen-saturated 150 mM potassium phosphate buffer, pH 8.7. The reaction was initiated by the addition of 1.5 ml of 64 mM AA and then was incubated under O2 stream for 1.5 min at 37 °C, with vigorous shaking. The reaction was terminated by adjusting the pH to 2.0 with the addition of 10 ml of 6 M HCl, and was extracted immediately with 800 ml of hexane/diethyl ether (1:1, v/v). The organic extract was dried by rotary-evaporation at 30 °C under vacuum. Crude 15-HPETE was dissolved in 2 ml of HPA-30 (hexane/propan-2-ol/acetic acid, 969:30:1, by vol.) and subjected to SP-HPLC (straight-phase HPLC) purification. HPLC was performed on a Beckman Gold System with a Phenomenex semi preparation column (10 μm, 10 mm×250 mm, silica) using an isocratic mobile phase of HPA-30 at a flow rate of 4 ml/min. Absorbance of the eluate was monitored at 235 nm. The 15-HPETE was eluted from the column at approx. 7–8 min and was rotary-evaporated and resuspended in 2 ml of ethanol. To obtain 15-HETE, 1500 AU (units of absorbance at 235 nm) of 15-HPETE was reduced with 100 mg of NaBH4 in 60 ml of methanol at 0 °C for 1 min. Following acidification with 1 ml of 6 M HCl and dilution with 150 ml of water, 15-HETE was extracted and purified by SP-HPLC as described above, except using HPA-15 solvent (hexane/propan-2-ol/acetic acid, 984:15:1, by vol.) instead of HPA-30. Radiolabelled [1-14C]15-HPETE was synthesized using [1-14C]AA and soya bean LOX as described above.
Cell culture
Primary BAECs were isolated from the bovine aorta through a collagenase digestion method as described previously [21]. The ECs were cloned to a single-cell level using the limiting dilution method and were maintained until confluent in Ham's F-12K medium with 10% (v/v) FBS supplemented with L-glutamine, Hepes buffer, insulin, transferrin and heparin. After three to four passages, serum levels were reduced to 5% and contained a basal Se concentration of 10 nM for Se-deficient medium (−Se). Additional sodium selenite (10 ng/ml) was added to Se-sufficient medium (+Se) to make a final Se concentration of 70 nM and a 7-fold increase in Se concentration. Se status was confirmed by the level of GPX activity and TrxR activity as described previously [21,22]. Briefly, GPX activity was measured via the generation of NADPH in the presence of glutathione and glutathione reductase [21,22]. Activity of TrxR was measured by the reduction of thiol residues in the substrate insulin in the presence of rTrx and NADPH [21,22]. Trx activity was measured by the reduction of thiol residues in the insulin substrate in the presence of recombinant TrxR and NADPH as described previously [23]. Intracellular ROS production was measured by relative fluorescence of peroxide-sensitive DCFH-DA probe as described in [21]. Levels of GSH and GSSG were measured using the GSH/GSSG-412 kit according to the manufacturer's instructions. Before stimulation with 15-HPETE, 15-HETE or AA, BAECs were cultured in serum-free F-12K medium. For rTrx and DNCB experiments, 10 μg/ml rTrx or 10 nM DNCB was added to cultures 3 h before stimulation.
QC RT–PCR (quantitative competitive reverse transcription–PCR)
Total RNA was isolated with TRIzol® reagent according to the manufacturer's instructions, and 100 ng of sample was used in all reactions. Construction of mRNA internal standards was performed according to the procedures described previously [24]. QC RT–PCR was then performed using the following primers to measure HO-1 mRNA levels: forward 5′-TCTAGACTAGCTGGATGTTGA-3′ and reverse 5′-TAGGACCCCGTACGACTTA-AG-3′. For confirmation of the siRNA system, TrxR1 products were measured using the following primers that were directed against the C-terminal region of bovine TrxR1: forward, 5′-TG-AAATGGGGAAAAACAAAAGATG-3′, and reverse, 5′-CACG-GCCTAGATATAACGCACAGT-3′. The reactions were initially heated at 94 °C for 3 min followed by 30 cycles of 10 s at 94 °C, 30 s at 52 °C for HO-1 (58 °C for TrxR1) and 45 s at 72 °C. PCR products were separated on a 2% agarose gel, and densitometry was performed to quantify amounts of HO-1 and TrxR1 mRNA relative to the levels of internal standards.
Western blot
Whole-cell lysates were harvested in M-PER® and were centrifuged at 10000 g for 10 min at 4 °C to remove membrane fractions. Equal amounts of protein (20–30 μg) were resolved by SDS/10–15% PAGE and transferred on to a nitrocellulose membrane. The membrane was blocked in 5% (w/v) dried milk powder in TBS (Tris-buffered saline) with (v/v) 0.01% Tween-20 at room temperature (25 °C) and was incubated with anti-(human HO-1) (1:1000 dilution), anti-(human Trx) (1:2000 dilution) or anti-(human TrxR1) (1:500 dilution) overnight in 5% (w/v) BSA in TBS with 0.01% (v/v) Tween-20. Secondary antibody against HRP-conjugated mouse IgG (1:5000 dilution) was then added to the membrane for 1 h at room temperature, washed and exposed to HRP substrate (Pierce) and visualized by chemiluminescence on autoradiography film. Membrane was stripped and re-blotted under similar conditions with anti-(human actin) (Chemicon, 1:5000) followed by anti-(mouse IgG)–HRP secondary antibody (1:5000 dilution).
Luciferase analysis
Primary +Se and −Se BAECs were transfected using a calcium phosphate precipitation method as described previously [25]. Briefly, 4×104 cells were plated on a 24-well plate, and DNA/calcium phosphate precipitates containing 0.4 μg of reporter plasmid (HO-1 4.0 kb promoter-pGL3) and 0.02 μg of Renilla control plasmid (pRL-TK) were added to each well. After 4 h, cells were subjected to 45 s of osmotic shock in serum-free medium containing 20% (v/v) glycerol and then cultured in 5% +Se or −Se medium. After 44 h, cells were harvested in 100 μl of Passive Lysis Buffer, and luciferase activity was measured using the Dual-Luciferase® Reporter Assay System according to the manufacturer's instructions. The ratio of firefly/Renilla luciferase activity was calculated and reported as fold increase over +Se control cells.
Apoptosis assays
For caspase 3/7 assay, 104 ECs were seeded in a 96-well plate in +Se or −Se medium as described above. Following overnight culture, HO-1 inhibitor, SnPPIX, was added in serum-free medium for 15 h, followed by 6 h of stimulation with 30 μM 15-HETE or 15-HPETE. Caspase 3/7 activation was measured according to the manufacturer's instructions. ApoONE homologous caspase 3/7 reagent (25 μl) was added to each well and mixed for 4 h at room temperature. Following incubation, samples were analysed for rhodamine-110 fluorescence. Results are reported as fold increase in caspase 3/7 activity over +Se unstimulated. For Hoechst 33342 staining, approx. 5×104 BAECs were seeded in a six-well plate in −Se or +Se medium and treated with SnPPIX as described above. Following a 2 h stimulation with 15-HPETE, ECs were fixed with 4% (w/v) paraformaldehyde in PBS overnight at 4 °C, stained at room temperature for 10 min with 10 μg/ml Hoechst 33342 in PBS and washed with distilled water. Approx. 200 ECs per well, in triplicate per treatment, were manually scored for apoptosis based on the presence or absence of nucleic acid condensation.
TrxR siRNA
The pSuppressorNeo siRNA plasmid vector was used to construct a 21 bp head-to-head hairpin loop of bovine TrxR1 (GenBank® accession number NM_174625, residues 303–324) that was confirmed to be unique to bovine TrxR1. The optimal TrxR1 sequence was tcga-ggaggcagccaaatatgacaa-gagtactg-ttgtcatatttggctgcctcc-ttttt where the corresponding sense and antisense selenoprotein sequences are in bold letters, stem loop sequences are in italics, and the cloning overhangs (XhoI) are underlined. This region corresponds to a 5′ region of the bovine TrxR gene located approx. 30 bp downstream of the transcription start site that is present in all known splice variants of TrxR1 identified in other species [26,27]. For the construct, two complementary oligonucleotides were synthesized and annealed to generate double-stranded DNA. For the control siRNA vector, a scrambled sequence that does not contain any significant similarity in bovine or human gene sequences was developed alongside. The strands were ligated into the linearized pSuppressorNeo vector, transformed into competent Escherichia coli, and the RNA interference insert was sequenced to confirm proper insertion. Plasmid was prepared by caesium chloride gradient centrifugation, and was stably transfected into BAECs using the calcium phosphate method as described above and reported previously [25]. At 48 h after transfection, stable BAECs were selected in G418 10% (v/v) FBS medium containing Se. At 2 weeks following antibiotic selection, a decrease in TrxR1 activity was confirmed with TrxR1 QC RT–PCR, Western blot and activity assays as described above.
RESULTS
Confirmation of oxidant stress during Se deficiency
Se-sufficient (+Se) and -deficient (−Se) BAECs were established through in vitro culture in the presence or absence of sodium selenite supplementation for two to three passages as described in the Experimental section and demonstrated previously [21]. The level of oxidant stress was monitored by measuring the activity of the predominant EC-derived selenoproteins, GPX and TrxR, as well as the elevation of ROS (Table 1) [28,29]. During oxidant stress, there also are alterations in AA metabolism, including the accumulation of downstream metabolites [5,6]. Biochemical research shows that, under normal cellular redox conditions, the 15-LOX-1 metabolite, 15-HPETE, is converted into its reduced form, 15-HETE, by the selenoproteins, GPX-1 and TrxR1 [5,6]. Therefore Se deficiency decreases the conversion of 15-HPETE into 15-HETE, causing a significant accumulation of this pro-oxidant in these cells. As shown in Table 1, there was elevated 15-HETE in +Se BAECs and increased 15-HPETE in −Se BAECs, demonstrating altered LOX metabolism during oxidant stress in BAECs.
Table 1. Effects of Se deficiency on oxidant stress.
BAECs were cultured in F-12K medium supplemented with 5% (v/v) FBS in the presence and absence of Se (10 ng/ml). GPX activity in nmol of NADPH oxidized/min. TxrR activity in A412 units×1000/min per mg of protein. Intracellular ROS production was measured by relative fluorescence of peroxide-sensitive DCFH-DA probe. 15-LOX-1 (15-HETE and 15-HPETE) metabolites in pmol/106 BAECs per h following stimulation with 30 μM AA and 2 μM calcium ionophore A23187. Results are means±S.E.M. for three individual experiemts. *, P<0.05 compared with corresponding value in +Se group (paired Student's t test).
| Activity | ||
|---|---|---|
| Assay | +Se | −Se |
| GPX | 32.0±4.9 | 6.3±1.4* |
| TxrR | 100.1±2.2 | 47.3±7.1* |
| ROS | 6.7±1.4 | 13.9±3.4* |
| 15-HETE | 519±27.2 | 254±15.7* |
| 15-HPETE | 239±19.4 | 60±17.6* |
Elevation of HO-1 in ECs during Se deficiency
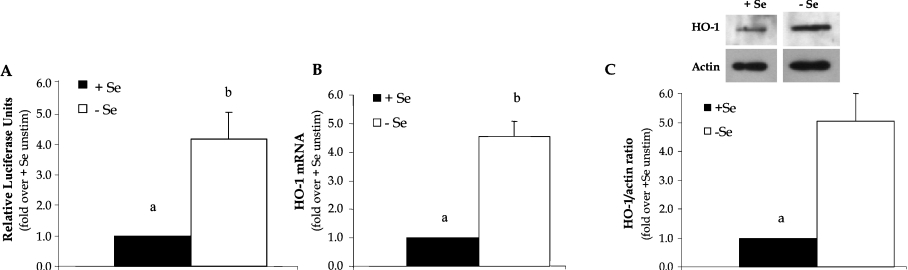
To confirm that Se deficiency increased the expression of the protective antioxidant HO-1 in BAECs, a luciferase reporter construct that contained a 4.0 kb portion of the human HO-1 promoter was used in these studies. Luciferase activity in +Se and −Se BAECs was measured 48 h after transfection, and the results showed that there was a 4-fold increase in luciferase activity in −Se BAECs compared with +Se BAECs (Figure 1A). Next, HO-1 mRNA levels were measured in order to determine whether increased activity of the promoter resulted in increased HO-1 transcription. In −Se BAECs, the levels of HO-1 mRNA were significantly increased >4-fold as determined using QC RT-PCR (Figure 1B). This was confirmed further at the protein level using Western blot analysis, where HO-1 was elevated in whole-cell lysates from −Se BAECs compared with +Se BAECs (Figure 1C).
Figure 1. Se deficiency increases expression of HO-1 mRNA and protein.
ECs were cultured in +Se or −Se medium for two to three passages. (A) Cells were transiently transfected with HO-1 4.0 kb Luc plasmid and 48 h later were analysed for luciferase activity. Luciferase activity is expressed as fold increase of firefly/Renilla over +Se unstimulated BAECs (unstim). Results are means±S.E.M. for three separate experiments. (B) RNA was isolated and QC RT–PCR was performed. PCR products were separated on a 2% agarose gel, and densitometry was performed in order to quantify the amount of HO-1 mRNA. Results are expressed as fold increase of HO-1 mRNA over +Se unstimulated BAECs (unstim) and are means±S.E.M. for five separate experiments. (C) Whole-cell lysates were harvested, equal amounts of protein were resolved by SDS/12% PAGE, and a Western blot was performed. Results are expressed as fold increase of HO-1 protein/actin ratio over +Se unstimulated BAECs (unstim) and are means±S.E.M. for three separate experiments. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined using a paired Student's t test.
Differential levels of HO-1 following stimulation with 15-HPETE
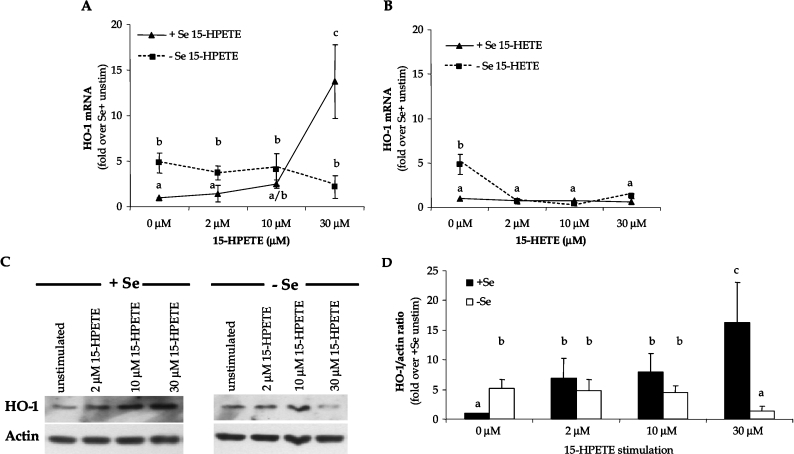
Since FAHPs (fatty acid hydroperoxides) can induce HO-1 expression, we sought to determine whether pro-oxidant challenge with the FAHP from the LOX pathway, 15-HPETE, alters HO-1 expression. The selection of 15-HPETE as the pro-oxidant agonist was based on earlier studies documenting the role of this FAHP in contributing to vascular dysfunction during Se deficiency [30]. The reduced form of 15-HPETE, 15-HETE, also was used to confirm that the hydroperoxy metabolite is responsible for activation of HO-1 during increased AA metabolism through the 15-LOX pathway. Following stimulation of +Se and −Se BAECs with increasing doses of each metabolite (2–30 μM), levels of HO-1 mRNA and protein were measured. Results showed that, while −Se cells demonstrated elevated levels of HO-1 under basal conditions, further exposure to higher doses of 15-HPETE resulted in a downward trend in HO-1 expression (Figure 2A). Alternatively, exposure of high doses of 15-HPETE to +Se BAECs significantly increased the levels of HO-1 mRNA above basal levels and demonstrated a dose–response with increasing concentrations of 15-HPETE (Figure 2A). This elevation in HO-1 mRNA levels was due to an activation of de novo transcription, because the induction of HO-1 was prevented in the presence of the RNA polymerase inhibitor, actinomycin D (results not shown). In contrast, stimulation with 15-HETE resulted in no significant change in HO-1 mRNA in +Se BAECs and decreased levels in −Se BAECs, confirming that the hydroperoxy group in 15-HPETE was responsible for HO-1 activation (Figure 2B). In order to confirm that the trend observed at the mRNA level resulted in similar protein levels, cell lysates were collected following stimulation with 15-HPETE and levels of HO-1 protein expression were measured through Western blot analysis (Figures 2C and 2D). At the protein level, there was a significant decrease in HO-1 expression observed in −Se BAECs upon exogenous 15-HPETE stimulation. Strikingly, HO-1 levels were increased in +Se BAECs following the same stimulation, similar to the results observed at the mRNA level.
Figure 2. Differential levels of HO-1 expression in +Se and −Se BAECs following 15-HPETE stimulation.
Following stimulation of ECs with dose titration of (A) 15-HPETE or (B) 15-HETE for 2 h, total RNA was isolated, and QC RT–PCR for HO-1 mRNA was then performed. PCR products were separated on a 2% agarose gel, and densitometry of HO-1 mRNA was measured. Results are expressed as fold increase of HO-1 mRNA over +Se unstimulated BAECs (unstim) and are means±S.E.M. for three separate experiments. (C) Following stimulation of ECs with dose titration of 15-HPETE for 2 h, whole-cell lysates were harvested, and equal amounts of protein were resolved by SDS/12% PAGE for Western blot analysis. (D) Densitometry results from three separate experiments in (C) were averaged and means±S.E.M. for three separate experiments are shown. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined by ANOVA.
Induction of apoptosis following 15-HPETE stimulation
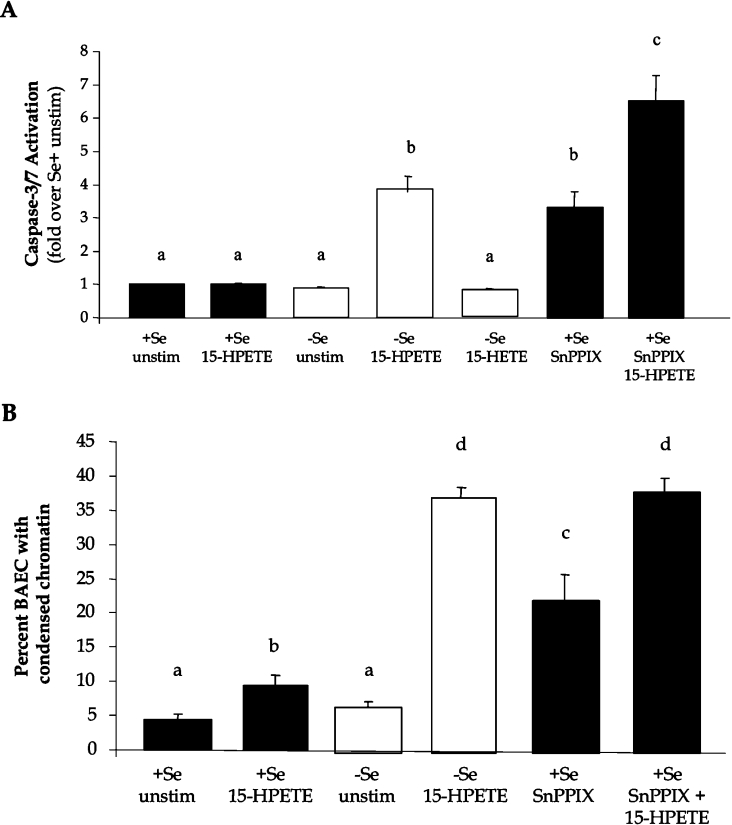
The next objective was to determine whether 15-HPETE-induced changes in HO-1 expression with respect to Se status correlated with changes in the expression of apoptosis markers. Therefore +Se and −Se BAECs were cultured with 30 μM 15-HPETE or 15-HETE, and activation of caspase 3/7 was measured as a biochemical indicator of apoptosis. Following 6 h of stimulation with 15-HPETE, there was a 4-fold increase in caspase 3/7 activity in −Se BAECs, whereas there was no change in the levels of apoptosis in +Se BAECs (Figure 3A). Alternatively, upon stimulation with 15-HETE, caspase 3/7 activity remain unchanged in both +Se and −Se BAECs. In order to determine whether the lower levels of caspase 3/7 activation in +Se BAECs was due in part to the up-regulation of HO-1 following the same stimulation, HO-1 activity was inhibited using the chemical inhibitor SnPPIX, and apoptosis was measured following 15-HPETE stimulation. Following a 15 h pre-treatment with SnPPIX and a 2 h stimulation with 15-HPETE, there was an increase in the magnitude of caspase 3/7 activation in +Se BAECs that exceeded the levels observed in −Se BAECs (Figure 3A). In order to corroborate the activation of apoptotic signalling pathways with morphological changes, chromatin condensation was measured using Hoechst 33342 staining under the same conditions. Following stimulation with 15-HPETE, there was a significant increase in condensed chromatin of −Se BAECs compared with +Se BAECs (Figure 3B). Following inhibition of HO-1 with SnPPIX and stimulation with 15-HPETE, there was a significant increase in cell death in +Se BAECs, which approached similar levels observed in −Se BAECs following the same stimulation (Figure 3B).
Figure 3. HO-1 activity regulates apoptosis during Se deficiency.
+Se and −Se BAECs were pre-treated with the indicated doses of the HO-1 inhibitor, SnPPIX, for 15 h, followed by treatment with 30 μM 15-HETE or 15-HPETE for 6 h. (A) Caspase 3/7 activation was measured, and results are expressed as fold increase in caspase-3/7 activation over +Se unstimulated BAECs (unstim) as means±S.E.M. for four separate experiments. (B) For Hoechst 33342 staining, approx. 200 ECs per well, in triplicate per treatment, were manually scored for apoptosis based on the presence or absence of nucleic acid condensation and results are reported as the percentage of BAECs with condensed chromatin. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined by ANOVA.
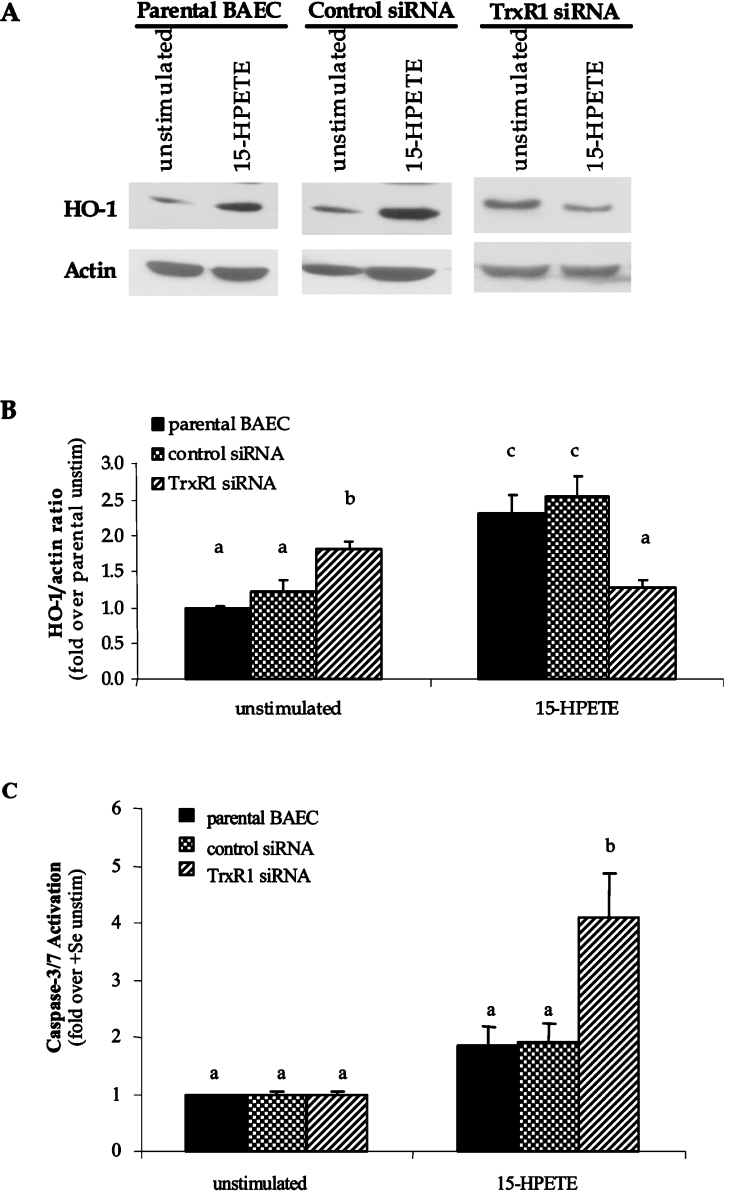
Inhibition of TrxR1 reverses HO-1 induction in +Se BAECs
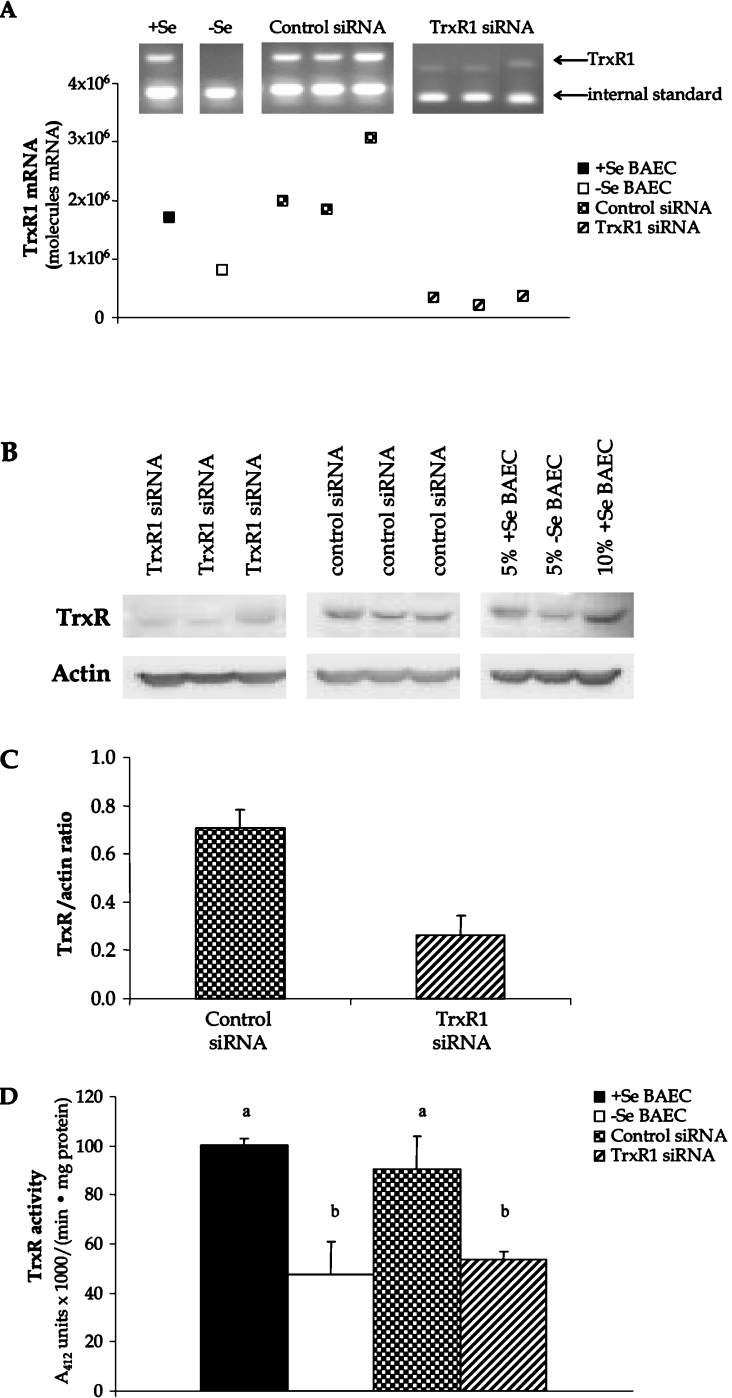
To verify whether Se-mediated changes in HO-1 expression were due to altered activity of the TrxR/Trx system, TrxR was inhibited and HO-1 expression was measured. Preliminary experiments were conducted in BAECs using the chemical inhibitor, DNCB, to parallel other studies in the literature that routinely use this chemical in TrxR inhibition studies [31,32]. Interestingly, a 3 h pre-treatment of +Se BAECs with 10 nM DNCB reversed the induction of HO-1 following 15-HPETE stimulation, suggesting a positive role for TrxR1 in HO-1 gene expression (+15-HPETE/−DNCB, 15.8±4.1-fold increase in HO-1 mRNA; +15-HPETE/+DNCB, 3.9±3.1-fold increase in HO-1 mRNA, P<0.05). Due to the non-specific nature of DNCB treatment and the controversial results obtained in other studies, an alternative method for TrxR inhibition employing siRNA was developed to verify these results. A siRNA construct was developed against the N-terminal region of bovine TrxR1 that was confirmed to be present in all known splice variants of TrxR1 identified in other species [26,27]. Following stable transfection of BAECs with TrxR1 siRNA and control siRNA plasmids, BAECs were cultured to confluence under antibiotic selection in +Se medium. There was no difference in viability or proliferation of TrxR1 siRNA-transfected cells compared with control siRNA cells as measured by Trypan Blue exclusion and caspase 3/7 activity assays (results not shown). Confirmation of a decrease in TrxR1 levels was determined through the measurement of TrxR1 mRNA and protein expression using QC RT–PCR (Figure 4A) and Western blot analysis (Figures 4B and 4C), as well as the measurement of TrxR activity using an insulin reduction method (Figure 4D). These results confirmed a significant decrease in TrxR1 mRNA (87% decrease), protein (63% decrease) and activity levels (41% decrease), which is commonly observed in siRNA studies as opposed to a complete elimination of protein expression associated with knockout systems [32]. Furthermore, the results of control siRNA BAECs were comparable with the levels observed in +Se parental BAECs, demonstrating that the transfection and antibiotic selection process did not interfere with the expression or activity of this selenoprotein in BAECs. BAECs stably expressing TrxR1 siRNA showed a basal increase in HO-1 mRNA and protein levels compared with cells that were transfected with a control siRNA sequence (Figures 5A and 5B). Following 15-HPETE stimulation, TrxR1 siRNA BAECs demonstrated a decrease in HO-1 expression, similar to the effect observed in −Se BAECs stimulated under the same conditions. In control siRNA BAECs cultured in the presence of Se, 15-HPETE stimulation caused an increase in HO-1 expression to levels similar to those observed in parental +Se BAECs. When analysing indicators of apoptosis in siRNA cells following stimulation with 15-HPETE, TrxR1 siRNA BAECs exhibited increased levels of caspase 3/7 activity compared with those in either control siRNA BAECs or the parental BAECs (Figure 5C).
Figure 4. TrxR1 siRNA decreases TrxR mRNA, protein and activity.
BAECs were transfected with siRNA for TrxR1 or control sequence and were grown to confluence under antibiotic selection for stable expression of siRNA construct. (A) Total RNA was isolated, and QC RT–PCR for TrxR1 mRNA was then performed. PCR products were separated on a 2% agarose gel, and densitometry of TrxR1 mRNA and internal standard was measured. (B) Whole-cell lysates were harvested, equal amounts of protein were resolved by SDS/10% PAGE, and a Western blot was performed. A representative blot is shown. (C) Results in (B) are expressed as fold increase TrxR protein over actin and are means±S.E.M. for three separate experiments. (D) Whole-cell lysates were harvested and tested for TrxR1 activity using the insulin-based method. Results are expressed in A412 units×1000/min per mg of protein, and are means±S.E.M. for three separate clones. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined by ANOVA.
Figure 5. TrxR1 siRNA inhibits HO-1 induction in BAECs following 15-HPETE stimulation.
BAECs were transfected with siRNA for TrxR1 or control sequence and were grown to confluence under antibiotic selection for expression of siRNA construct. (A) Whole-cell lysates from control and TrxR1 siRNA-transfected cells were harvested, and equal amounts of protein were resolved by SDS/12% PAGE for measurement of HO-1 protein levels. (B) Densitometry results from three separate experiments in (A) were averaged, and results are means±S.E.M. for three separate experiments are shown. (C) Caspase 3/7 activation was measured in TrxR siRNA and control siRNA BAECs following 2 h of 15-HPETE stimulation, and results are expressed as fold increase in caspase-3/7 activation over unstimulated BAECs (unstim). Results are means±S.E.M. for four separate experiments. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined by ANOVA.
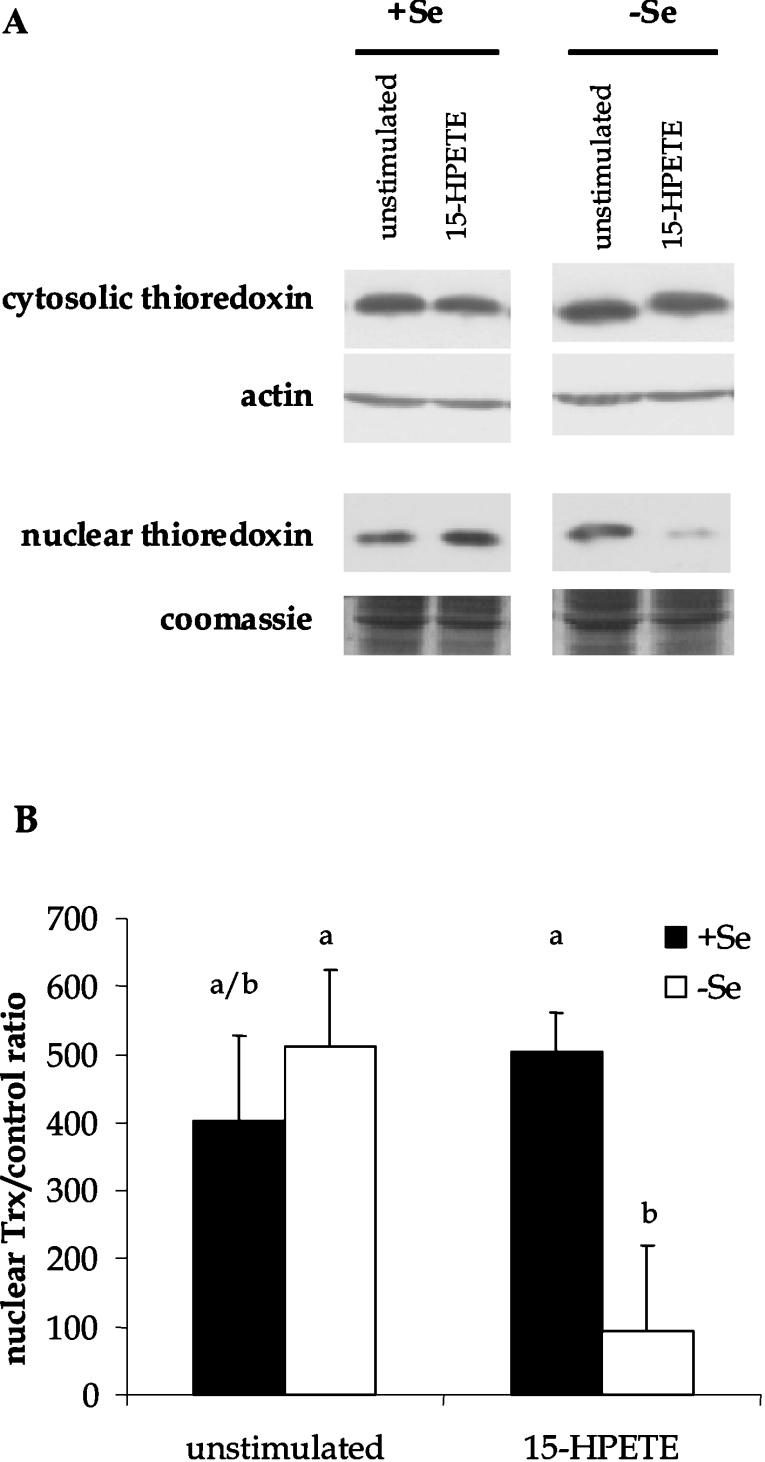
Alterations in Trx protein localization and activity following 15-HPETE stimulation
The next objective was to determine whether differences in the redox environment following 15-HPETE stimulation correlated with the differences in HO-1 expression. Prominent redox proteins in ECs were measured in order to monitor changes in redox environment. Measurement of GSH and GSSG levels in whole-cell lysates demonstrated no significant changes before or after 15-HPETE stimulation regardless of Se status (Table 2). However, there were changes in the levels of Trx protein in certain cellular compartments. Following 15-HPETE stimulation, cytosolic fractions and nuclear extracts were collected from +Se and −Se BAECs, and Trx protein levels were measured by Western blot techniques. There was no measurable difference in protein levels in cytosolic fractions between +Se and −Se BAECs either before or after stimulation (Figure 6). However, there was a significant decrease in nuclear Trx levels in −Se BAECs following stimulation with 15-HPETE. Since there were significant changes in Trx protein localization, we next sought to determine whether there were accompanying changes in Trx activity levels. Upon stimulation with 15-HPETE, nuclear Trx activity was diminished significantly in −Se BAECs (1897±822 ng of thiols formed per mg of protein) when compared with +Se BAECs (3823±911 ng of thiols formed per mg of protein), indicating depletion of both Trx protein and activity levels in the nuclear compartment upon pro-oxidant challenge.
Table 2. Redox potential following 15-HPETE stimulation.
BAECs cultured in F-12K medium supplemented with 5% (v/v) FBS in the presence and absence of Se (10 ng/ml) and stimulated with 30 μM 15-HPETE for 2 h in serum-free medium. GSH and GSSG levels were measured using the GSH/GSSG-412 kit. Results are means±S.E.M. for three individual experiments.
| GSH levels | GSSG levels | |
|---|---|---|
| +Se unstimulated | 37.5±1.9 | 1.5±0.8 |
| +Se 15-HPETE | 40.0±9.7 | 1.3±0.7 |
| −Se unstimulated | 36.8±4.6 | 1.7±0.8 |
| −Se 15-HPETE | 36.8±3.7 | 2.0±1.0 |
Figure 6. Trx localization following 15-HPETE stimulation.
+Se and −Se BAECs were stimulated with 30 μM 15-HPETE or 15-HPETE for 2 h. (A) Cytosolic and nuclear extracts were separated, and equal amounts of protein were resolved by SDS/15% PAGE. (B) Densitometry results from three separate experiments performed with nuclear extracts in (A) were averaged, and results are means±S.E.M. for three separate experiments. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined by ANOVA.
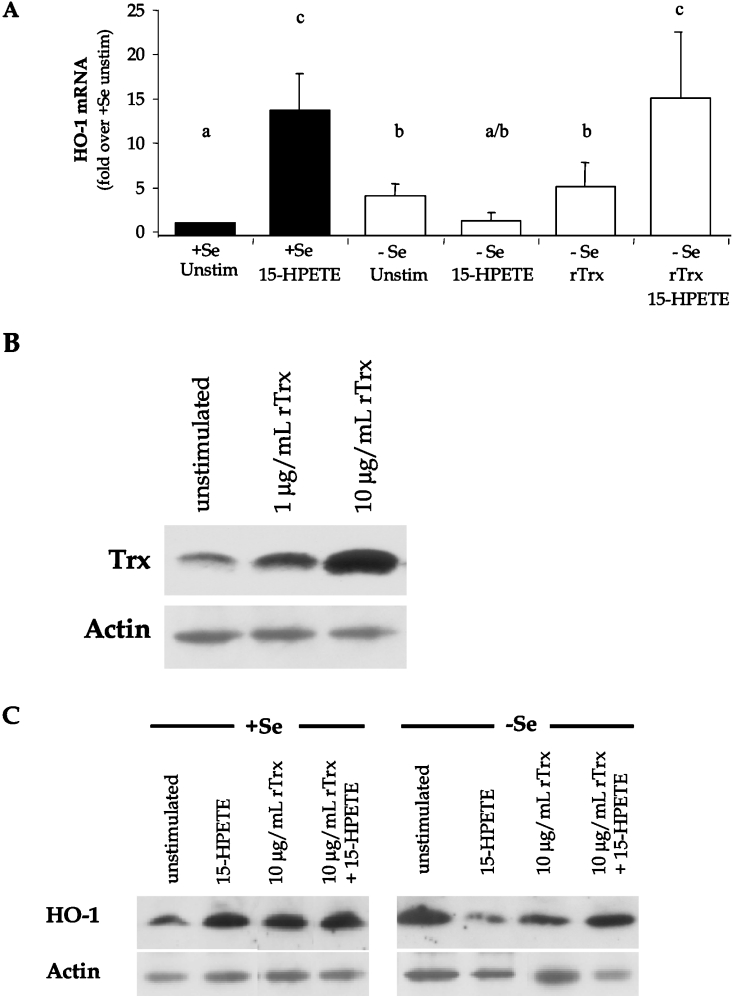
Trx restores HO-1 expression during oxidant stress
In order to determine whether changes in intracellular redox environment following 15-HPETE stimulation were involved in differential activation of HO-1 expression, rTrx in the reduced form was added to the medium before 15-HPETE stimulation. This method of treating cells with rTrx has been demonstrated previously to alter activation of HO-1 transcription [33]. Initially, it was necessary to verify an increase in intracellular Trx levels following rTrx pre-treatment by measuring total Trx in whole-cell lysates by Western blot analysis (Figure 7B). In order to eliminate the possibility that exogenous rTrx was merely associating with the plasma membrane, membrane fractions were removed from the whole-cell lysate preparations through centrifugation methods. Results confirmed that rTrx treatment increased intracellular Trx expression as demonstrated by elevated Trx protein levels. Next, HO-1 mRNA was measured in rTrx-treated BAECs in order to address whether exogenous rTrx treatment of BAECs altered HO-1 expression. Following stimulation of −Se BAECs with 15-HPETE, there was a characteristic decrease in HO-1 expression. Repletion of these cells with rTrx before 15-HPETE stimulation was able to restore HO-1 mRNA and protein levels that approached the levels of HO-1 expression observed in +Se BAECs (Figures 7A and 7C). Interestingly, there was an increase in HO-1 levels following rTrx treatment of unstimulated −Se BAECs, suggesting that rTrx positively influences basal levels of HO-1 expression in EC.
Figure 7. Recombinant Trx induces levels of HO-1 in −Se BAECs following 15-HPETE stimulation.
(A) +Se and −Se BAECs were pre-treated with the indicated doses of rTrx for 3 h, followed by treatment with 30 μM 15-HPETE for 2 h. RNA was isolated, and QC RT–PCR was performed. PCR products were separated on a 2% agarose gel, and densitometry was performed in order to quantify the amount of HO-1 mRNA. Results are expressed as fold increase HO-1 mRNA over +Se unstimulated (unstim), and results are means±S.E.M. for three separate experiments. Values denoted by different lower-case letters are statistically different from each other (P<0.05) as determined by ANOVA. (B) BAECs were stimulated with 1 or 10 μg/ml rTrx for 3 h, whole-cell lysates were harvested, and equal amounts of protein were resolved by SDS/15% PAGE. A Western blot was performed to confirm increased intracellular Trx protein following rTrx treatment. (C) +Se and −Se BAECs were pre-treated with 10 μg/ml rTrx for 3 h followed by treatment with 30 μM 15-HPETE for 2 h. Whole-cell lysates were harvested, equal amounts of protein were resolved by SDS/12.5% PAGE and a Western blot was performed for HO-1 protein levels. A representative blot of three is shown.
DISCUSSION
During atherosclerosis, there is an increase in oxidant stress in the vascular endothelium following exposure to high levels of ROS in the vessel wall [34]. A deficiency in the micronutrient Se causes a decrease in the activity of selenoprotein antioxidants, including GPX-1 and TrxR1, requiring ECs to combat ROS with alternative sources of antioxidants in order to prevent cell damage [1]. Recent studies demonstrate that HO-1 is up-regulated during Se deficiency and suggest that this antioxidant may compensate for the loss of Se-dependent antioxidants [19]. However, there remains a positive correlation between cardiovascular disease and Se deficiency, suggesting that this compensatory increase in HO-1 is not sufficient to protect against vascular dysfunction [2]. In order to investigate this relationship between Se deficiency and HO-1 expression at the site of vascular injury, we studied the regulation of HO-1 activation in aortic ECs. In the present study, there was an increase in HO-1 levels during Se deficiency in aortic ECs, thus correlating our research with previous findings observed in vivo where there was an increase in HO-1 expression in the Kupffer cell fraction of livers obtained from Se-deficient rats [19]. However, research also demonstrates that Se-deficient BAECs exhibit an increase in adhesion molecule expression, pro-inflammatory cytokine production and elevated levels of apoptosis which contradicts the cytoprotective properties of HO-1 [30,35]. Therefore we propose that the magnitude of HO-1 activation in a pro-oxidant environment may be critical towards the protection against the damaging effects observed during oxidant stress.
In order to investigate further the relative levels of HO-1 activation during oxidant stress, −Se BAECs were exposed to pro-oxidant challenge, and HO-1 expression was measured. Previous studies demonstrated an accumulation of reactive FAHPs at the site of atherosclerotic lesion formation, including the arachidonate 15-LOX-1 metabolites, 15-HETE and 15-HPETE [36,37]. These studies performed in situ measured up to micromolar doses of these fatty acids in atherosclerotic lesions confirming that high doses of 15-LOX-1 metabolites come into contact with ECs during atherosclerosis [38,39]. Therefore the pro-oxidant, 15-HPETE, was used as an agonist to mimic the microenvironment associated with atherosclerotic lesion formation. Following this stimulation, there were differential levels of HO-1 activation depending on the Se status of the cells and the level of HO-1 expression correlated with the level of caspase 3/7 activation. These data suggest that while −Se BAECs had higher basal levels of HO-1 expression, these cells did not up-regulate this antioxidant following 15-HPETE exposure, whereas +Se BAECs significantly increased HO-1 expression during the same stimulation. We propose that the ability of +Se BAECs to up-regulate HO-1 during increased exposure to oxidative damage may partially protect these cells from apoptosis. Alternatively, the lack of HO-1 induction following pro-oxidant stimulation during Se deficiency may trigger pro-apoptotic events that would provide an explanation for the detrimental outcome associated with decreased Se nutritional status and cardiovascular diseases. In conclusion, we provide evidence for the requirement of Se to combat oxidant stress through the up-regulation of alternative non-Se-dependent antioxidants, including HO-1.
Previous research by Ejima et al. [20] demonstrated that transfection of Trx into macrophages promoted an increase in HO-1 protein during pro-inflammatory conditions. A separate study also identified a relationship between HO-1 activation and TrxR activity, although this study was performed in an Se-deficient model and suggested a negative role for TrxR in the regulation of HO-1 expression in the liver [9]. Because of these conflicting reports, we sought to clarify the correlation between TrxR activity and HO-1 expression in ECs following stimulation with the pro-oxidant, 15-HPETE. In order to directly compare our results with previous findings, a commonly utilized TrxR chemical inhibitor, DNCB, was evaluated for its effect on HO-1 expression. Results showed that DNCB treatment of +Se BAECs prevented induction of HO-1 expression following pro-oxidant stimulation, suggesting a direct relationship between TrxR activity and up-regulation of HO-1. Due to the non-specific nature of DNCB, including its ability to alter other cellular antioxidant systems such as glutathione and GPX, we chose to develop a more sensitive and direct method for the inhibition of TrxR1 employing siRNA technology. siRNA was directed against a region located 30 bp downstream of the ATG start codon of TrxR1 and was shown to significantly reduce TrxR1 mRNA, protein and activity compared with a nonspecific scramble control siRNA construct. There have been numerous reports of TrxR1 variants in other species that are due to alternative splicing in the first exon, upstream of the ATG start codon [26,27]. While the methods that we employed for detecting TrxR could not differentiate between splice variants, we were able to confirm that the region targeted by the siRNA construct is present in all reported variants and therefore would be sensitive to siRNA inhibition. Therefore any residual TrxR activity following siRNA inhibition is most likely because of the partial inhibition that is commonly associated with siRNA techniques or residual activity from the mitochondrial isoform, TrxR2, as opposed to the expression of splice variants that are not sensitive to siRNA inhibition [32]. When investigating the effect of the TrxR1 siRNA on the level of HO-1 activation in BAECs, we were able to confirm the results observed in the DNCB experiments, where there was a lack of induction of HO-1 in TrxR1 siRNA BAECs compared with the control siRNA BAECs following 15-HPETE stimulation. This is the first time that direct inhibition of TrxR1 using siRNA has been reported and conclusively demonstrates a relationship between TrxR1 and HO-1 expression in endothelial cells. The ability of TrxR1 to positively regulate HO-1 induction upon prooxidant challenge underlies the importance for selenoprotein antioxidant activity in the regulation of other cytoprotective genes. Furthermore, the siRNA data confirm that any inhibition of TrxR1 activity, including depleted Se nutritional status, can significantly impair HO-1 activation and render ECs more susceptible to pro-apoptotic stimuli during oxidant stress.
The regulation of HO-1 activation by TrxR1 may involve the ability of the TrxR system to control the intracellular redox balance. Previous studies show that depletion of GSH levels either through pro-oxidant stimulation or treatment with glutathione-depleting agents correlates with an increase in HO-1 levels [12,40]. Other research implicates a role for Trxred in the regulation of HO-1 gene activation [20,33]. Therefore it was important to address the effect of 15-HPETE stimulation on two redox couples that are prominent regulators of EC redox balance, GSH/GSSG and Trxred/Trxox (oxidized Trx). While there were no significant changes in GSH/GSSG ratios, there were significant alterations in Trx protein and activity levels in both cytosolic and nuclear compartments of −Se BAECs stimulated with 15-HPETE, which may alter redox sensitive signalling pathways. Furthermore, repletion of BAECs with rTrx restored HO-1 expression in −Se BAECs, suggesting that the decreased Trx activity plays a role in the lack of HO-1 activation. For instance, a decrease in cytosolic Trx activity may result in the oxidation of redox-sensitive kinases and phosphatases that are implicated in the signal transduction pathways upstream of HO-1 gene transcription, including PKC (protein kinase C) and protein tyrosine phosphatases [41,42]. Relative to our findings, lower levels of Trx activity in the nucleus may support an oxidized nuclear environment, which may result in the oxidation of critical cysteine residues in transcription factors which inhibit DNA binding to promoter elements [43]. There are two redox-sensitive transcription factors, Nrf2 (NF-E2-related factor-2) and AP-1 (activator protein-1), that are implicated in control of HO-1 gene transcription and therefore may be susceptible to decreased Trx activity in the nucleus [44–46]. Therefore fluctuations in Trx activity following 15-HPETE stimulation provide a potential explanation for the alterations in HO-1 induction patterns between +Se and −Se BAECs. Depletion of Trx activity in our system is most likely to be caused by the generation of ROS from stimulation with the pro-oxidant, 15-HPETE. Studies describe 15-HPETE as a pro-oxidant that can alter the intracellular redox status through the ability to stimulate LOX and COX enzyme activity, increase lipid peroxidation and therefore deplete antioxidant defences through the generation of ROS [47,48]. Furthermore, a recent study reports that 15-HPETE can inhibit the activity of TrxR1 directly, which causes further depletion of Trxred during Se deficiency [48]. Therefore, exposure of Se-deficient ECs to pro-oxidant metabolites such as those present during atherosclerosis can antagonize already depleted Se-dependent antioxidants. The ability of the ECs to maintain TrxR1 activity and provide an ample supply of Trxred upon pro-oxidant exposure may dictate the ability to up-regulate alternative antioxidants and further prevent oxidant stress.
The present study provides a potential explanation for the paradox associated with an increase in HO-1 expression during Se deficiency and the lack of protection against the development of atherosclerosis. Even though there are elevated levels of HO-1 during Se deficiency, a subsequent pro-oxidant challenge does not result in further activation of the antioxidant to protective levels. The present study demonstrates that this lack of induction of HO-1 is due to decreased TrxR1 activity and the resulting decrease in the levels of Trxred. It will be of considerable interest to identify further the regulation of the TrxR system during Se deficiency as it may provide insight into the ability of selenoproteins to regulate other redox-sensitive gene responses and contribute further to our understanding of the mechanisms that are involved in the progression to atherosclerosis.
Acknowledgments
We acknowledge the contribution of Matthew J. Sylte and Sixtine-Pacifique Valdelievre in the technical assistance of this manuscript, and Dr Xi-Lin Chen for providing the HO-1–luciferase construct. This work was supported, in part, by an endowment from the Matilda R. Wilson Fund (Detroit, MI, U.S.A.).
References
- 1.Oster O., Prellwitz W. Selenium and cardiovascular disease. Biol. Trace Elem. Res. 1990;24:91–103. doi: 10.1007/BF02917198. [DOI] [PubMed] [Google Scholar]
- 2.Alissa E. M., Bahijri S. M., Ferns G. A. The controversy surrounding selenium and cardiovascular disease: a review of the evidence. Med. Sci. Monit. 2003;9:RA9–RA18. [PubMed] [Google Scholar]
- 3.Kuhn H., Borchert A. Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Radical Biol. Med. 2002;33:154–172. doi: 10.1016/s0891-5849(02)00855-9. [DOI] [PubMed] [Google Scholar]
- 4.Huang H. S., Chen C. J., Suzuki H., Yamamoto S., Chang W. C. Inhibitory effect of phospholipid hydroperoxide glutathione peroxidase on the activity of lipoxygenases and cyclooxygenases. Prostaglandins Other Lipid Mediators. 1999;58:65–75. doi: 10.1016/s0090-6980(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 5.Schnurr K., Belkner J., Ursini F., Schewe T., Kuhn H. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxygenase with complex substrates and preserves the specificity of the oxygenation products. J. Biol. Chem. 1996;271:4653–4658. doi: 10.1074/jbc.271.9.4653. [DOI] [PubMed] [Google Scholar]
- 6.Bjornstedt M., Hamberg M., Kumar S., Xue J., Holmgren A. Human thioredoxin reductase directly reduces lipid hydroperoxides by NADPH and selenocystine strongly stimulates the reaction via catalytically generated selenols. J. Biol. Chem. 1995;270:11761–11764. doi: 10.1074/jbc.270.20.11761. [DOI] [PubMed] [Google Scholar]
- 7.Ochi H., Morita I., Murota S. Mechanism for endothelial cell injury induced by 15-hydroperoxyeicosatetraenoic acid, an arachidonate lipoxygenase product. Biochim. Biophys. Acta. 1992;1136:247–252. doi: 10.1016/0167-4889(92)90113-p. [DOI] [PubMed] [Google Scholar]
- 8.Ochi H., Morita I., Murota S. Roles of glutathione and glutathione peroxidase in the protection against endothelial cell injury induced by 15-hydroperoxyeicosatetraenoic acid. Arch. Biochem. Biophys. 1992;294:407–411. doi: 10.1016/0003-9861(92)90704-z. [DOI] [PubMed] [Google Scholar]
- 9.Mostert V., Hill K. E., Burk R. F. Loss of activity of the selenoenzyme thioredoxin reductase causes induction of hepatic heme oxygenase-1. FEBS Lett. 2003;541:85–88. doi: 10.1016/s0014-5793(03)00309-0. [DOI] [PubMed] [Google Scholar]
- 10.Kietzmann T., Samoylenko A., Immenschuh S. Transcriptional regulation of heme oxygenase-1 gene expression by MAP kinases of the JNK and p38 pathways in primary cultures of rat hepatocytes. J. Biol. Chem. 2003;278:17927–17936. doi: 10.1074/jbc.M203929200. [DOI] [PubMed] [Google Scholar]
- 11.Terry C. M., Clikeman J. A., Hoidal J. R., Callahan K. S. Effect of tumor necrosis factor-α and interleukin-1α on heme oxygenase-1 expression in human endothelial cells. Am. J. Physiol. 1998;274:H883–H891. doi: 10.1152/ajpheart.1998.274.3.H883. [DOI] [PubMed] [Google Scholar]
- 12.Andre M., Felley-Bosco E. Heme oxygenase-1 induction by endogenous nitric oxide: influence of intracellular glutathione. FEBS Lett. 2003;546:223–227. doi: 10.1016/s0014-5793(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 13.Jeney V., Balla J., Yachie A., Varga Z., Vercellotti G. M., Eaton J. W., Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 14.Perrella M. A., Yet S. F. Role of heme oxygenase-1 in cardiovascular function. Curr. Pharm. Des. 2003;9:2479–2487. doi: 10.2174/1381612033453776. [DOI] [PubMed] [Google Scholar]
- 15.Tsui T. Y., Wu X., Lau C. K., Ho D. W., Xu T., Siu Y. T., Fan S. T. Prevention of chronic deterioration of heart allograft by recombinant adeno-associated virus-mediated heme oxygenase-1 gene transfer. Circulation. 2003;107:2623–2629. doi: 10.1161/01.CIR.0000066911.03770.8D. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa K., Sugawara D., Goto J., Watanabe Y., Kawamura K., Shiomi M., Itabe H., Maruyama Y. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation. 2001;104:1831–1836. doi: 10.1161/hc3901.095897. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa K., Sugawara D., Wang X., Suzuki K., Itabe H., Maruyama Y., Lusis A. J. Heme oxygenase-1 inhibits atherosclerotic lesion formation in LDL-receptor knockout mice. Circ. Res. 2001;88:506–512. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- 18.Juan S. H., Lee T. S., Tseng K. W., Liou J. Y., Shyue S. K., Wu K. K., Chau L. Y. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;104:1519–1525. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- 19.Mostert V., Hill K. E., Ferris C. D., Burk R. F. Selective induction of liver parenchymal cell heme oxygenase-1 in selenium-deficient rats. Biol. Chem. 2003;384:681–687. doi: 10.1515/BC.2003.076. [DOI] [PubMed] [Google Scholar]
- 20.Ejima K., Layne M. D., Carvajal I. M., Nanri H., Ith B., Yet S. F., Perrella M. A. Modulation of the thioredoxin system during inflammatory responses and its effect on heme oxygenase-1 expression. Antioxid. Redox Signaling. 2002;4:569–575. doi: 10.1089/15230860260220067. [DOI] [PubMed] [Google Scholar]
- 21.Sordillo L. M., SooHoo H., Aherne K. M., Reddy C. C., Hogan J. S. A method to reduce glutathione peroxidase levels in primary endothelial cell cultures. Methods Cell Sci. 1998;19:243–253. [Google Scholar]
- 22.Holmgren A., Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- 23.Holmgren A. Thioredoxin. Annu. Rev. Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 24.Vanden Heuvel J. P., Clark G. C., Kohn M. C., Tritscher A. M., Greenlee W. F., Lucier G. W., Bell D. A. Dioxin-responsive genes: examination of dose–response relationships using quantitative reverse transcriptase–polymerase chain reaction. Cancer Res. 1994;54:62–68. [PubMed] [Google Scholar]
- 25.Kong Y., Johnson S. E., Taparowsky E. J., Konieczny S. F. Ras p21Val inhibits myogenesis without altering the DNA binding or transcriptional activities of the myogenic basic helix–loop–helix factors. Mol. Cell. Biol. 1995;15:5205–5213. doi: 10.1128/mcb.15.10.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadley K. B., Sunde R. A. Selenium regulation of thioredoxin reductase activity and mRNA levels in rat liver. J. Nutr. Biochem. 2001;12:693–702. doi: 10.1016/s0955-2863(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 27.Furman C., Rundlof A. K., Larigauderie G., Jaye M., Bricca G., Copin C., Kandoussi A. M., Fruchart J. C., Arner E. S., Rouis M. Thioredoxin reductase 1 is upregulated in atherosclerotic plaques: specific induction of the promoter in human macrophages by oxidized low-density lipoproteins. Free Radical Biol. Med. 2004;37:71–85. doi: 10.1016/j.freeradbiomed.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y. Z., Reddy C. C., Sordillo L. M. Altered eicosanoid biosynthesis in selenium-deficient endothelial cells. Free Radical Biol. Med. 2000;28:381–389. doi: 10.1016/s0891-5849(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 29.Cao Y. Z., Weaver J. A., Reddy C. C., Sordillo L. M. Selenium deficiency alters the formation of eicosanoids and signal transduction in rat lymphocytes. Prostaglandins Other Lipid Mediators. 2002;70:131–143. doi: 10.1016/s0090-6980(02)00018-7. [DOI] [PubMed] [Google Scholar]
- 30.Weaver J. A., Maddox J. F., Cao Y. Z., Mullarky I. K., Sordillo L. M. Increased 15-HPETE production decreases prostacyclin synthase activity during oxidant stress in aortic endothelial cells. Free Radical Biol. Med. 2001;30:299–308. doi: 10.1016/s0891-5849(00)00466-4. [DOI] [PubMed] [Google Scholar]
- 31.Arner E. S., Bjornstedt M., Holmgren A. 1-Chloro-2,4-dinitrobenzene is an irreversible inhibitor of human thioredoxin reductase: loss of thioredoxin disulfide reductase activity is accompanied by a large increase in NADPH oxidase activity. J. Biol. Chem. 1995;270:3479–3482. doi: 10.1074/jbc.270.8.3479. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa A., Kubota Y., Murayama T., Nomura Y. Cell death by 1-chloro-2,4-dinitrobenzene, an inhibitor of thioredoxin reductase and its dual regulation by nitric oxide in rats. Neurosci. Lett. 1999;277:99–102. doi: 10.1016/s0304-3940(99)00850-2. [DOI] [PubMed] [Google Scholar]
- 33.Wiesel P., Foster L. C., Pellacani A., Layne M. D., Hsieh C. M., Huggins G. S., Strauss P., Yet S. F., Perrella M. A. Thioredoxin facilitates the induction of heme oxygenase-1 in response to inflammatory mediators. J. Biol. Chem. 2000;275:24840–24846. doi: 10.1074/jbc.M000835200. [DOI] [PubMed] [Google Scholar]
- 34.Wassmann S., Wassmann K., Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension. 2004;44:381–386. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- 35.Maddox J. F., Aherne K. M., Reddy C. C., Sordillo L. M. Increased neutrophil adherence and adhesion molecule mRNA expression in endothelial cells during selenium deficiency. J. Leukocyte Biol. 1999;65:658–664. doi: 10.1002/jlb.65.5.658. [DOI] [PubMed] [Google Scholar]
- 36.Waddington E. I., Croft K. D., Sienuarine K., Latham B., Puddey I. B. Fatty acid oxidation products in human atherosclerotic plaque: an analysis of clinical and histopathological correlates. Atherosclerosis. 2003;167:111–120. doi: 10.1016/s0021-9150(02)00391-x. [DOI] [PubMed] [Google Scholar]
- 37.Hiltunen T., Luoma J., Nikkari T., Yla-Herttuala S. Induction of 15-lipoxygenase mRNA and protein in early atherosclerotic lesions. Circulation. 1995;92:3297–3303. doi: 10.1161/01.cir.92.11.3297. [DOI] [PubMed] [Google Scholar]
- 38.Kuhn H., Belkner J., Zaiss S., Fahrenklemper T., Wohlfeil S. Involvement of 15-lipoxygenase in early stages of atherogenesis. J. Exp. Med. 1994;179:1903–1911. doi: 10.1084/jem.179.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter K. L., Taylor S. E., van der Veen C., Williamson B. K., Ballantine J. A., Mitchinson M. J. Lipids and oxidised lipids in human atherosclerotic lesions at different stages of development. Biochim. Biophys. Acta. 1995;1256:141–150. doi: 10.1016/0005-2760(94)00247-v. [DOI] [PubMed] [Google Scholar]
- 40.Ewing J. F., Maines M. D. Glutathione depletion induces heme oxygenase-1 (HSP32) mRNA and protein in rat brain. J. Neurochem. 1993;60:1512–1519. doi: 10.1111/j.1471-4159.1993.tb03315.x. [DOI] [PubMed] [Google Scholar]
- 41.Bloom D. A., Jaiswal A. K. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 2003;278:44675–44682. doi: 10.1074/jbc.M307633200. [DOI] [PubMed] [Google Scholar]
- 42.van der Wijk T., Blanchetot C., den Hertog J. Regulation of receptor protein-tyrosine phosphatase dimerization. Methods. 2005;35:73–79. doi: 10.1016/j.ymeth.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Watson W. H., Jones D. P. Oxidation of nuclear thioredoxin during oxidative stress. FEBS Lett. 2003;543:144–147. doi: 10.1016/s0014-5793(03)00430-7. [DOI] [PubMed] [Google Scholar]
- 44.Hirota K., Matsui M., Iwata S., Nishiyama A., Mori K., Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alam J., Killeen E., Gong P., Naquin R., Hu B., Stewart D., Ingelfinger J. R., Nath K. A. Heme activates the heme oxygenase-1 gene in renal epithelial cells by stabilizing Nrf2. Am. J. Physiol. Renal Physiol. 2003;284:F743–F752. doi: 10.1152/ajprenal.00376.2002. [DOI] [PubMed] [Google Scholar]
- 46.Itoh K., Tong K. I., Yamamoto M. Molecular mechanism activating Nrf2–Keap1 pathway in regulation of adaptive response to electrophiles. Free Radical Biol. Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 47.Schewe T. 15-Lipoxygenase-1: a prooxidant enzyme. Biol. Chem. 2002;383:365–374. doi: 10.1515/BC.2002.041. [DOI] [PubMed] [Google Scholar]
- 48.Yu M. K., Moos P. J., Cassidy P., Wade M., Fitzpatrick F. A. Conditional expression of 15-lipoxygenase-1 inhibits the selenoenzyme thioredoxin reductase: modulation of selenoproteins by lipoxygenase enzymes. J. Biol. Chem. 2004;279:28028–28035. doi: 10.1074/jbc.M313939200. [DOI] [PubMed] [Google Scholar]