Abstract
In the yeast Saccharomyces cerevisiae, sphingolipids are essential for cell growth. Inactivation of sphingolipid biosynthesis, such as by disrupting the serine palmitoyltransferase gene (LCB2), is lethal, but cells can be rescued by supplying an exogenous LCB (long-chain base) like PHS (phytosphingosine) or DHS (dihydrosphingosine). In the present study, supplying SPH (sphingosine), an unnatural LCB for yeast, similarly rescued the Δlcb2 cells, but only when SPH 1-phosphate production was inhibited by deleting the LCB kinase gene LCB4. Exogenously added SPH was adequately converted into phosphoinositol-containing complex sphingolipids. Interestingly, cells carrying SPH-based sphingolipids exhibited a defect in the association of Pma1p with Triton X-100-insoluble membrane fractions, and displayed sensitivities to both Ca2+ and hygromycin B. These results suggest that the SPH-based sphingolipids in these cells have properties that differ from those of the PHS- or DHS-based sphingolipids in regard to lipid microdomain formation, leading to abnormal sensitivities towards certain environmental stresses. The present paper is the first report showing that in sphingolipid-deficient S. cerevisiae, the requirement for LCB can be fulfilled by exogenous SPH, although this supplement results in failure of lipid microdomain formation.
Keywords: dihydrosphingosine, lipid microdomain, long-chain base, phytosphingosine, Saccharomyces cerevisiae, sphingosine
Abbreviations: DHS, dihydrosphingosine; IPC, inositol phosphorylceramide; LCB, long-chain base; M(IP)2C, mannosyldi-inositol phosphorylceramide; MIPC, mannosylinositol phosphorylceramide; OPA, o-phthalaldehyde; PHS, phytosphingosine; SC medium, synthetic complete medium; SPH, sphingosine
INTRODUCTION
Sphingolipids are a major component of the eukaryotic plasma membrane. These compounds have numerous roles, such as regulating signal transduction pathways, directing correct protein sorting, and mediating cell-to-cell interactions and recognition [1,2]. Recent studies have also demonstrated that sphingolipids dynamically cluster with sterols to form lipid microdomains or rafts, which function as platforms for effective signal transduction and correct protein sorting [3].
Structures of sphingolipids vary among species, although the fundamental structure is similar. Basically, a hydrophobic ceramide, i.e. a fatty acid attached through an amide linkage to an LCB (long-chain base), is attached to a head group; it is the head group that varies so greatly in composition [4]. For instance, the mammalian sphingolipid sphingomyelin has phosphocholine as its head group, and mammalian glycosphingolipids, of which there are hundreds, carry carbohydrate head groups [4]. In contrast, the yeast Saccharomyces cerevisiae has only three sphingolipids, IPC (inositol phosphorylceramide), MIPC (mannosylinositol phosphorylceramide) and M(IP)2C (mannosyldi-inositol phosphorylceramide), all containing phosphoinositol [5].
LCBs also differ between mammals and yeast. The major LCB in mammals is SPH (sphingosine), which is desaturated between the C-4 and C-5 positions, although DHS (dihydrosphingosine), which is saturated at that site, is also present. In contrast, SPH does not exist in yeast; instead, PHS (phytosphingosine), which is hydroxylated at the C-4 position, and DHS serve as LCBs. This difference can be attributed to variations in sphingolipid-desaturating and hydroxylating enzymes. Mammals have dihydroceramide Δ4-desaturase (DES1), which generates ceramide from dihydroceramide [6], whereas S. cerevisiae has C-4 hydroxylase (Sur2p), which catalyses the conversion of DHS and dihydroceramide into PHS and phytoceramide respectively [7].
Sphingolipids are essential for viability. Yeast cells carrying a mutation in the LCB2 gene, which encodes a subunit of the first enzyme in sphingolipid biosynthesis, cannot grow unless PHS or DHS is supplied to the medium to compensate for the lack of LCB synthesized de novo [8,9]. Other genes involved in the early stages of sphingolipid synthesis (i.e. those affecting the synthesis of all the sphingolipids) are also essential for cell growth; however, genes involved in the later stages of synthesis [affecting only MIPC and M(IP)2C] can be deleted without interrupting growth [5]. Deletion of the C-4 hydroxylase gene SUR2, which is involved in PHS formation, also has no effect on cell growth [7]. Nevertheless, hydroxylation of LCB and the presence of extended glycans are apparently important during adaptation to harmful environmental conditions, since a loss of either results in altered sensitivity to exogenous Ca2+ or drugs [10–12].
In the present study, we succeeded in constructing yeast cells with sphingolipids that carry SPH as the only LCB, by supplying SPH exogenously to Δlcb2 cells and by deleting the LCB kinase LCB4 gene [13] to prevent generation of toxic SPH 1-phosphate. These cells could grow, but did so much more slowly than those similarly constructed to contain only PHS or DHS, indicating that SPH can compensate for the functions of natural LCBs, but only partly. We found that one of the roles that could not be substituted for by SPH was the ability of natural LCBs to form lipid microdomains. Moreover, those yeast cells with SPH-type sphingolipids exhibited altered sensitivities to exogenous Ca2+ and hygromycin B. These results indicate that SPH-based sphingolipids possess different properties from PHS- or DHS-based sphingolipids.
EXPERIMENTAL
Yeast strains and media
From S. cerevisiae diploid KA31 (MATa/α ura3/ura3 leu2/leu2 his3/his3 trp1/trp1) cells [14], haploid TMY101 (MATa ura3 Δlcb2::LEU2 his3 trp1) cells were constructed as follows. KA31 cells were transfected with a Δlcb2::LEU2 construct that had been generated by replacing the 1.15 kb XbaI–EcoRV region in the LCB2 gene with the LEU2 marker, and selected on a plate of SC (synthetic complete) medium (0.67% yeast nitrogen base and 2%, w/v, glucose) lacking leucine. One of the clones obtained, designated TMY100, exhibited a LCB2/Δlcb2::LEU2 genotype. After TMY100 cells were sporulated, the resulting tetrads were dissected. Each tetrad was then plated on to YPD (1%, w/v, yeast extract, 2%, w/v, peptone and 2% glucose) plates containing 10 μM PHS and 0.0015% Nonidet P40 as a dispersant. One of the haploid cell lines obtained (TMY101), which exhibited a Δlcb2::LEU2 genotype, was chosen. Haploid strains TMY60 (MATa ura3 Δlcb2::LEU2 his3 Δlcb4::TRP1) and TMY85 (MATa ura3 Δlcb2::LEU2 his3 Δlcb4::TRP1 Δsur2::KanMX4) were similarly constructed using Δlcb4::TRP1 [15] and Δsur2::KanMX4 [16] constructs respectively. To provide uniform auxotrophic conditions, the TMY101 cells were further transfected with a TRP1 fragment and selected on SC medium lacking tryptophan, thereby generating TMY77 cells (Δlcb2::LEU2 TRP1).
Lipid extraction
Lipids were extracted from S. cerevisiae by the method of Hanson and Lester [17]. Briefly, the cells were suspended in 100 μl of ethanol/water/diethyl ether/pyridine/15 M ammonia (15:15:5:1:0.018, by vol.) and incubated at 60 °C for 15 min. The residue was centrifuged at 1500 g for 5 min and extracted once more in the same manner. The resulting supernatants were dried and used for further analysis.
LCB analysis
TMY85 (Δlcb2 Δlcb4 Δsur2) yeast cells were cultured overnight in YPD medium containing 5 μM PHS and 0.0015% Nonidet P40. The cultured cells were washed and diluted to 0.1 D600 unit/ml into YPD medium containing 5 μM PHS, DHS or SPH and 0.0015% Nonidet P40. After an 18 h incubation at 30 °C, the cells (2.0 D600 units/ml) were collected and washed with cold distilled water containing 1 mg/ml BSA, and lipids were extracted. The lipids were dissolved in 500 μl of methanol/water (82:18, v/v) containing 1 M HCl, and heated at 80 °C for 18 h; PHS, DHS and SPH standards (1 nmol each) were treated similarly. After the addition of 500 μl of 3 M NH4OH, LCBs were extracted twice with 500 μl of chloroform. The combined chloroform extracts were dried and dissolved in 120 μl of ethanol by heating at 67 °C for 25 min. The dissolved lipid solution was mixed with 15 μl of OPA (o-phthalaldehyde) reagent (1 mg of OPA, 20 μl of ethanol, 2 μl of 2-mercaptoethanol and 1 ml of 3%, w/v, boric acid solution adjusted to pH 10.5) and incubated at room temperature (25 °C) for 30 min. Samples were centrifuged at 10000 g for 5 min, and the resulting supernatants were resolved by HPLC on a pre-packed C18 reversed-phase column (Cosmosil 5C18-AR-II; Nacalai Tesque, Kyoto, Japan) using an isocratic eluent composition of acetonitrile/distilled water (90:10, v/v) and a flow rate of 1 ml/min. The OPA derivatives were monitored at an excitation wavelength of 340 nm and an emission wavelength of 455 nm.
Complex sphingolipid analysis
TMY85 cells were grown overnight at 30 °C in YPD medium containing 0.0015% Nonidet P40 and 5 μM PHS, DHS or SPH. The cells were diluted to 0.5 D600 unit/ml in YPD medium containing 5 μM LCB and incubated at 30 °C for an additional 5 h. After an incubation, cells (1.0 D600 unit/ml) were suspended in fresh YPD medium containing 5 μM LCB and labelled with [3H]myo-inositol (2 μCi; PerkinElmer Life Sciences, Norwalk, CT, U.S.A.) for an additional 5 h at 30 °C. The cells were then washed with cold YPD medium containing 1 mg/ml BSA, and lipids were extracted. Lipids were resolved by TLC on Silica Gel 60 high-performance TLC plates (Merck, Whitestation, NJ, U.S.A.) with chloroform/methanol/4.2 M ammonia (9:7:2, by vol.) as a solvent system. To detect non-radioactive complex sphingolipids, TMY85 cells were grown overnight at 30 °C in YPD medium containing 0.0015% Nonidet P40 and 5 μM PHS, DHS or SPH. The cells were diluted to 0.1 D600 unit/ml in YPD medium containing 5 μM LCB and incubated at 30 °C for an additional 12 h. The cells (3.0 D600 units/ml) were collected and washed with cold distilled water containing 1 mg/ml BSA, and lipids were extracted. Lipids were resolved by TLC with chloroform/methanol/4.2 M ammonia (9:7:2, by vol.) as a solvent system. The TLC plates were treated with an orcinol/H2SO4 reagent to stain carbohydrate (mannose)-containing lipids [12].
Isolation and analysis of Triton X-100-insoluble membrane fractions
Triton X-100-insoluble fractions were isolated by the method of Bagnat et al. [18] with slight modifications. TMY85 cells were grown overnight at 30 °C in YPD medium containing 0.0015% Nonidet P40 and 5 μM PHS, DHS or SPH. The cells were diluted to 0.1 D600 unit/ml in YPD medium containing 5 μM LCB and incubated at 30 °C for an additional 12 h. The cells (5.0 D600 units/ml) were collected, washed with cold distilled water containing 1 mg/ml BSA, and stored at −80 °C. The cell pellets were each lysed in 500 μl of TNE buffer (50 mM Tris/HCl, pH 7.4, 150 mM NaCl and 5 mM EDTA) containing 1 mM dithiothreitol, 1 mM PMSF and a 1× protease inhibitor mixture (Complete™; Roche Diagnostics, Indianapolis, IN, U.S.A.) by vortex-mixing with glass beads for 10 min at 4 °C. Lysates were cleared of unbroken cells and debris by a 5 min centrifugation at 500 g and then incubated with Triton X-100 (1% final) for 30 min on ice. Each lysate (500 μl) was adjusted in volume by adding 1 ml of Optiprep solution (Nycomed Pharma, Oslo, Norway) and was overlaid with 2.4 ml of 30% Optiprep in TXNE (TNE buffer containing 0.1% Triton X-100) and 400 μl of TXNE. The samples were centrifuged at 259000 g for 2 h at 4 °C, and six fractions of equal volume were collected from the top.
For immunoassays, fractions were precipitated with trichloroacetic acid (final concentration of 10%). Precipitates were dissolved in SDS sample buffer (20 mM Tris/HCl, pH 7.5, 1% SDS and 1% 2-mercaptoethanol) and analysed by SDS/PAGE and immunoblotting with an anti-Pma1p antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) and anti-goat IgG–HRP (where HRP stands for horseradish peroxidase) (Santa Cruz Biotechnology, Inc.). Labelling was detected using an ECL® kit (Amersham Biosciences, Piscataway, NJ, U.S.A.).
A 300 μl sample of each density-gradient fraction was analysed for LCB content. Lipids were hydrolysed by adding 1.2 ml of methanol and 136 μl of concentrated HCl and heating at 80 °C for 18 h. Then 100 pmol of DHS was added into each mixture as an internal standard. After the addition of 1.6 ml of 3 M NH4OH, lipids were extracted twice with 1 ml of chloroform. The combined chloroform fractions were dried and dissolved in 120 μl of ethanol by heating at 67 °C for 25 min. The hydrolysed LCBs were derivatized with OPA and quantified by reversed-phase HPLC as described above.
RESULTS
Exogenously added SPH enables cell growth in Δlcb2 Δlcb4 cells
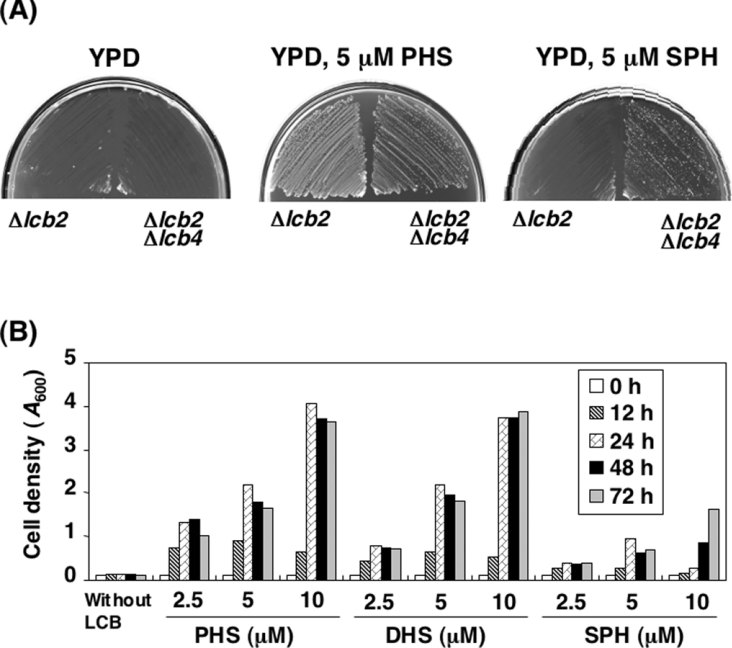
Sphingolipids are essential for cell viability, so S. cerevisiae carrying a mutation in LCB2, which encodes a subunit of serine palmitoyltransferase, grow only when PHS or DHS is supplied to the medium [8,9]; addition of the mammalian LCB SPH cannot similarly compensate [19]. However, previous studies have indicated that SPH added to the culture medium is taken up and phosphorylated to SPH 1-phosphate by LCB kinases [20]. Excessive accumulation of intracellular SPH 1-phosphate is known to cause severe growth defects in yeast [20,21]. Thus, to avoid the toxicity of SPH 1-phosphate in the Δlcb2 cells, we deleted the LCB4 gene, which is responsible for most of the LCB kinase activity in yeast. Neither the Δlcb2 nor the Δlcb2 Δlcb4 cells grew on YPD plates, but both cells grew in the presence of 5 μM PHS (Figure 1A). As reported, the growth defect of the Δlcb2 cells was not rescued by the addition of 5 μM SPH. However, the Δlcb2 Δlcb4 cells were able to grow on the YPD plate containing SPH, although not as efficiently as in the presence of PHS (Figure 1A). To eliminate the possibility that a trace amount of SPH 1-phosphate might be affecting the cell growth, the LCB5 gene, encoding a minor LCB kinase [13], was deleted from the Δlcb2 Δlcb4 cells, but this mutation did not contribute further to the cell growth (results not shown). These results indicate that exogenously supplied SPH can rescue mutants deficient in sphingolipid biosynthesis, but the accumulation of the SPH 1-phosphate generated by LCB4 prevents this rescue.
Figure 1. Deletion of LCB4 overcomes the growth defect of Δlcb2 cells cultured in the presence of SPH.
(A) TMY77 (Δlcb2) and TMY60 (Δlcb2 Δlcb4) cells were streaked on to YPD plates in the absence or presence of 5 μM PHS or SPH and incubated for 3 days at 30 °C. (B) TMY85 (Δlcb2 Δlcb4 Δsur2) cells were cultured in YPD medium containing 5 μM PHS overnight. The cells were washed, diluted (0.1 D600 unit/ml) in YPD medium containing the indicated amounts of PHS, DHS or SPH and incubated at 30 °C. At the indicated times, the cell growth was determined by measuring the attenuance at 600 nm (D600) using a spectrophotometer. Results shown are the average for two independent experiments.
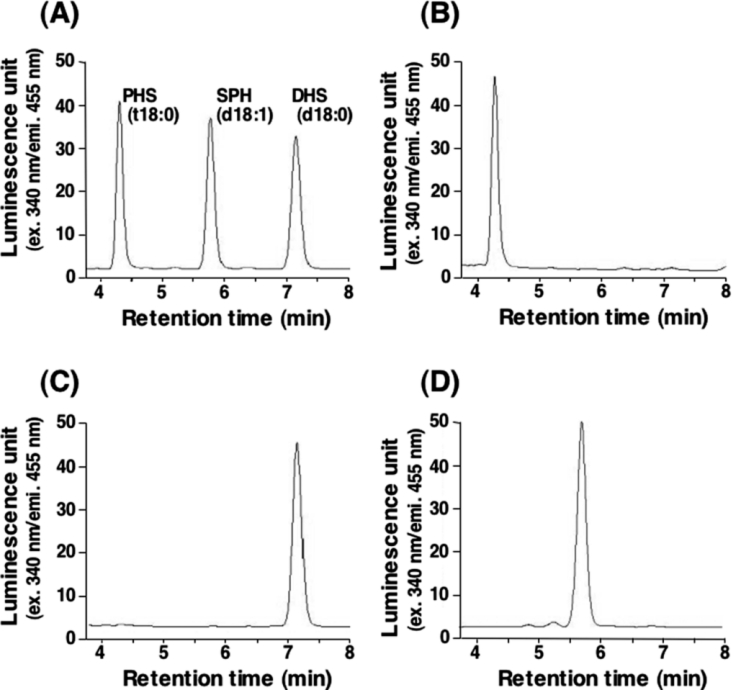
Next we examined the effect of each individual LCB (PHS, DHS and SPH) on the growth of mutants deficient in sphingolipid biosynthesis. To avoid the conversion of exogenously added DHS into PHS by hydroxylation at the C-4 position, we further deleted the SUR2 gene, which encodes sphingolipid C-4 hydroxylase. Addition of the respective LCB to these TMY85 (Δlcb2 Δlcb4 Δsur2) cells produced cells in which the intracellular sphingolipids were reconstituted with PHS, DHS or SPH as their sole LCB. HPLC analysis of lipids extracted from cells cultured for 18 h with each LCB confirmed that the LCB composition corresponded to the exogenously added LCB (Figure 2). When TMY85 cells were cultured in the absence of LCBs, little cell growth was observed (Figure 1B). Addition of PHS or DHS increased the cell growth in a concentration-dependent manner. However, SPH was less effective at promoting cell growth than either PHS or DHS. Furthermore, high concentrations of SPH apparently had a somewhat inhibitory effect on the cell growth, since the growth rate at 24 h was lower in the presence of 10 μM SPH than with 5 μM SPH. However, a longer incubation with 10 μM SPH facilitated the growth of cells (Figure 1B). The ineffective rescue of cell growth by SPH suggests that SPH compensates for the function of natural LCBs (PHS and DHS) only partially.
Figure 2. LCB analysis of LCB-reconstituted cells by HPLC.
TMY85 cells were cultured overnight in YPD medium containing 5 μM PHS. The cells were washed and then diluted (0.1 D600 unit/ml) in YPD medium containing 5 μM PHS (B), 5 μM DHS (C) or 5 μM SPH (D), and incubated for 18 h at 30 °C. Lipids were extracted, hydrolysed by methanol/HCl, derivatized with OPA and analysed by reversed-phase HPLC. Details are provided in the Experimental section. (A) PHS, DHS and SPH standards.
Exogenously added SPH is utilized for complex sphingolipid synthesis
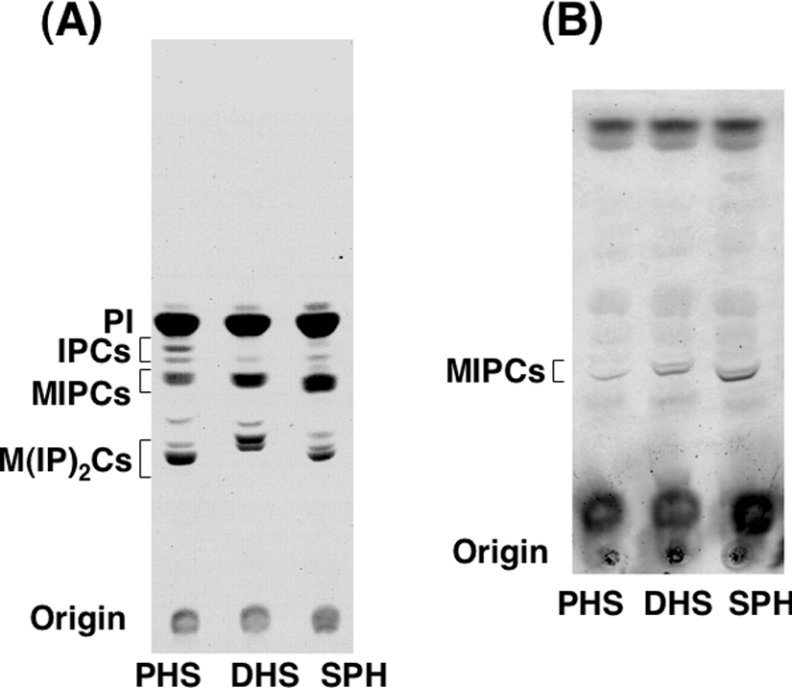
To examine whether the incorporated LCBs were utilized for complex sphingolipid biosynthesis, LCB-reconstituted cells (5 μM, overnight culture) were labelled with [3H]myo-inositol for 5 h, and then lipids were extracted and separated by TLC. 3H-labelled bands corresponding to IPCs, MIPCs and M(IP)2Cs were observed in nearly equal amounts in cells cultured with PHS, DHS or SPH (Figure 3A). In every sample, IPC, MIPC and M(IP)2C bands each contained two bands, which seemed to correspond to sphingolipids having a 2-hydroxy and non-hydroxy fatty acid. In addition, MIPCs were also detected in non-radioactive lipid extracts of every culture by an orcinol/H2SO4 staining (Figure 3B). These results indicate that complex sphingolipids were synthesized normally in cells cultured with not only PHS and DHS, but also SPH.
Figure 3. Synthesis of complex sphingolipids in LCB-reconstituted cells.
(A) [3H]myo-inositol labelling of LCB-reconstituted cells. TMY85 cells cultured with 5 μM PHS, DHS or SPH were labelled with [3H]myo-inositol for 5 h at 30 °C. Incorporated radioactivity was quantified, and equivalent samples (c.p.m.) were used for further analysis. Lipids were extracted and separated by TLC with chloroform/methanol/4.2 M ammonia (9:7:2, by vol.) as the solvent system. (B) Detection of unlabelled MIPCs in LCB-reconstituted cells. TMY85 cells (3.0 D600 units/ml) were cultured with PHS, DHS or SPH as in (A) and collected. Lipids were extracted, separated by TLC and then visualized by orcinol/H2SO4 reagent.
Formation of functional lipid microdomains is impaired in SPH-reconstituted cells
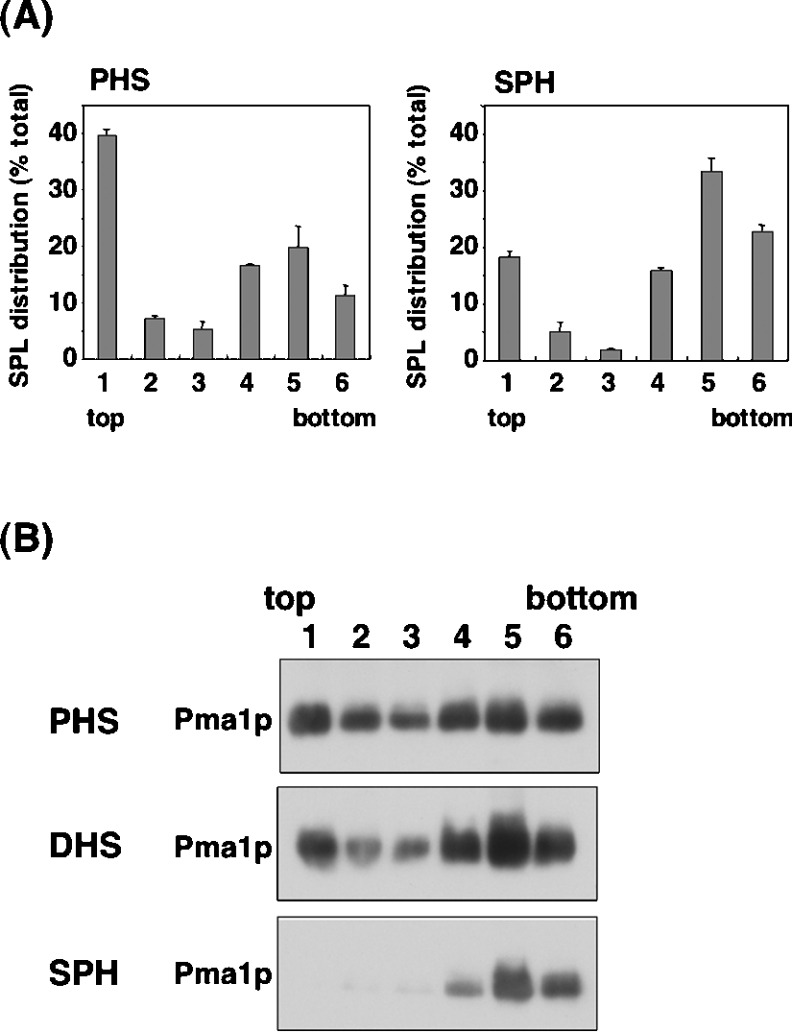
In yeast, sphingolipids and ergosterol form lipid microdomains [18]. Therefore we investigated the ability of reconstituted SPH-based sphingolipids to form normal microdomains. Lysates from TMY85 cells cultured overnight with 5 μM PHS, DHS, or SPH were dissolved in Triton X-100 and then separated over an Optiprep density gradient. The distribution of sphingolipids in each fraction was determined by HPLC after acid hydrolysis and derivatization with OPA. In both PHS- and SPH-reconstituted cells, sphingolipids were detected not only in the high-density Triton X-100-soluble fractions [18], but also in the low-density detergent-insoluble fractions, which contain lipid microdomains (Figure 4A). However, in the PHS-reconstituted cells, 52% of all sphingolipids were detected in the detergent-insoluble fractions (fractions 1–3), whereas in the SPH-reconstituted cells this level had decreased to 25% (Figure 4A). These results suggest that SPH-based sphingolipids are incorporated into the lipid microdomains, but the content is significantly lower than that of PHS-based sphingolipids.
Figure 4. Effect of LCB reconstitution on the formation of lipid microdomains.
TMY85 cells cultured with 5 μM PHS, DHS or SPH were lysed, incubated on ice with 1% Triton X-100 and fractionated by OptiPrep density-gradient centrifugation [49500 rev./min; SW60Ti (Beckman)]. Fractions were collected from the lowest-density (top) to the highest-density (bottom) gradient. (A) Distribution of sphingolipids (SPL) in PHS- or SPH-reconstituted cells. Lipids in each were hydrolysed by methanol/HCl. After derivatization with OPA, the hydrolysed LCBs were quantified by reversed-phase HPLC. Values presented are the amount of LCB in each fraction as a percentage of the total LCB in all fractions, and are the means±S.D. for three independent experiments. SPL, sphingolipids. (B) Association of Pma1p with the lipid microdomains. Fractions were subjected to SDS/PAGE, and Pma1p was detected by immunoblotting with an anti-Pma1p antibody. Details are provided in the Experimental section.
We also examined the distribution of the plasma membrane H+-ATPase, Pma1p, which is predominantly localized in the lipid microdomains in yeast [18]. In the PHS- or DHS-reconstituted cells, Pma1p was detected in both high- and low-density fractions (Figure 4B), suggesting that the protein was partly associated with lipid microdomains. However, in the SPH-reconstituted cells, Pma1p was not apparent in the low-density fractions and was detected only in the high-density fractions (Figure 4B). Taken together, these results suggest that the SPH-reconstituted cells cannot form functional lipid microdomains.
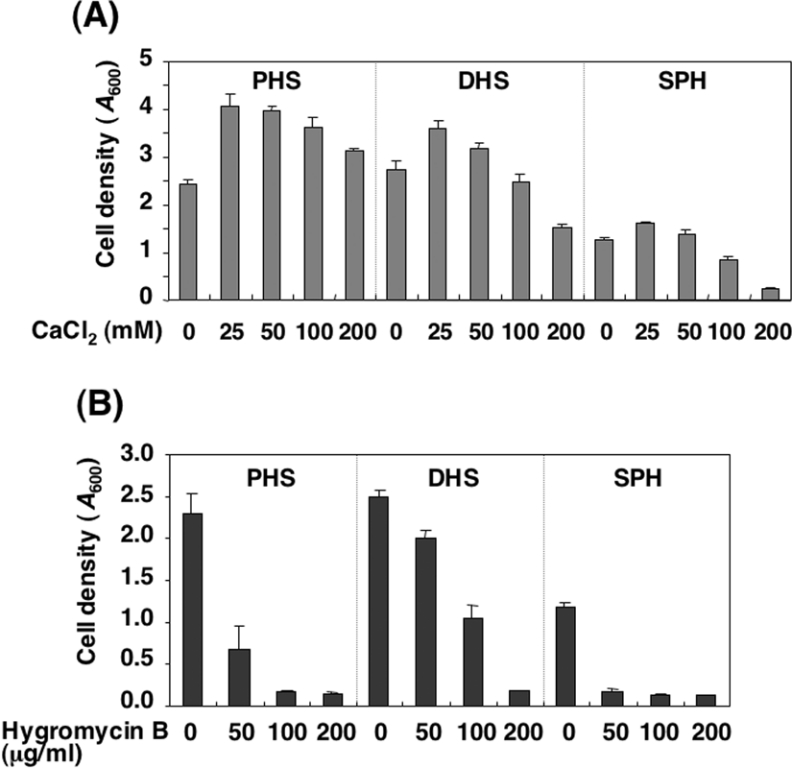
Sensitivity of LCB-reconstituted cells to Ca2+ or hygromycin B
Structural changes in sphingolipids following the deletion of certain biosynthesis enzymes are known to affect Ca2+ and drug sensitivities [10–12]. Therefore, we examined the sensitivity of the LCB-reconstituted cells to Ca2+ and hygromycin B, an aminoglycosidic antibiotic produced by Streptomyces hygroscopicus [22]. Cell growth in the TMY85 cells reconstituted with PHS was increased by the addition of Ca2+ (Figure 5A). Similarly, growth of the cells reconstituted with DHS or SPH was promoted by the addition of up to 50 mM Ca2+, although at 100 mM and above, Ca2+ had a rather inhibitory effect on growth. Notably, 200 mM Ca2+ strongly inhibited growth of the cells reconstituted with SPH (Figure 5A).
Figure 5. Effect of Ca2+ or the antibiotic hygromycin B on the growth of mutants deficient in sphingolipids cultured with various LCBs.
TMY85 cells were cultured overnight in YPD medium containing 5 μM PHS. The cells were washed and diluted to 0.1 D600 unit/ml in YPD medium with 5 μM PHS, DHS or SPH and cultured at 30 °C for an additional 24 h in the absence or presence of increasing concentrations of CaCl2 (A) or hygromycin B (B). After a 24 h incubation, the cell growth was determined by measuring the attenuance at 600 nm (D600) using a spectrophotometer. Results shown are the means±S.D. for three independent experiments.
Treatment with hygromycin B inhibited the growth of every LCB-reconstituted cell line, although the sensitivities of the cells differed. Growth of the SPH-reconstituted cells was strongly inhibited by hygromycin B, while the PHS-reconstituted cells were relatively resistant. Strong resistance was also observed for DHS-reconstituted cells (Figure 5B). These results indicate that sensitivities to Ca2+ and hygromycin B are greatly affected by LCB composition in cells.
DISCUSSION
A previous report indicated that supplying SPH exogenously could not compensate for the LCB auxotrophy exhibited by S. cerevisiae mutants deficient in sphingolipid biosynthesis [19]. In the present study, we have clearly demonstrated that deletion of the gene encoding the LCB kinase LCB4 enables growth of Δlcb2 cells when supplied with SPH. In S. cerevisiae, phosphorylated LCBs are involved in heat stress resistance, diauxic shift and Ca2+ mobilization [23–25], while their excessive intracellular accumulation leads to growth inhibition [20,21]. Thus it is plausible that SPH 1-phosphate production by Lcb4p interferes with the rescue of Δlcb2 cells by exogenously added SPH. SPH incorporated by these Δlcb2 Δlcb4 cells was found to be adequately utilized for complex sphingolipid synthesis (Figure 3). The present paper is the first report showing that in S. cerevisiae, sphingolipids containing SPH, a major LCB in mammals but one lacking in yeast, can substitute for the endogenous sphingolipids formed from PHS or DHS. Although the structures of sphingolipid LCBs differ among organisms, the present study suggests that sphingolipids, regardless of structure, can be substituted for by those of another organism, at least for cell growth. On the other hand, the S. cerevisiae mutant SLC bypasses the need to synthesize LCBs for growth and instead synthesizes a novel phosphoinositol-containing glycerolipid that mimics the structure of complex sphingolipids [26]. From these observations, one might postulate that for cell growth, the structural requirement of the hydrophobic portion of the sphingolipid has some flexibility.
Studies using the lcb1-100 temperature-sensitive yeast mutant, which is deficient in sphingolipid biosynthesis when cultured at an elevated temperature, demonstrated that sphingolipids are essential for lipid microdomain formation [18]. In the study presented here, SPH-based sphingolipids were recovered in the low-density fractions containing the microdomain fractions, although their distribution was reduced compared with that of PHS-based sphingolipids (Figure 4A). Considering that the microdomain localization of Pma1p was completely abolished (Figure 4B), SPH-based sphingolipids may be unable to fulfil their functions in lipid microdomains. It is unclear, though, how the C-4, C-5-double bond of LCBs affects the microdomain formation in S. cerevisiae. The existence of lipid microdomains has been confirmed in a variety of organisms, including mammals, plants, fungi and insects, although the structures of the sphingolipids and sterols composing the domains differ [27]. Mammals and S. cerevisiae contain different sterols (cholesterol and ergosterol respectively) and different sphingolipids (SPH/DHS-based sphingomyelin or glycosphingolipids and PHS/DHS-based phosphoinositol-containing sphingolipids respectively). In studies of membrane models, the ability of sterols to pack tightly with sphingolipids was found to be key in their promotion of lipid domain formation, and sterols with different structures had different effects [27]. Thus it is possible that the C-4, C-5-desaturation of sphingolipids is critical to their affinity towards ergosterol and eventually affects the lipid microdomain formation. This notion implies that the combination of sphingolipids and sterols in each organism is vital for unique lipid microdomain formation.
Previous studies have shown that certain sphingolipids play a key role in Ca2+ and drug sensitivities [10–12]. Additionally, transcription of IPT1, which codes for an enzyme involved in M(IP)2C synthesis, is controlled by key transcription factors (Pdr1p and Pdr3p) that regulate multidrug-resistance genes, supporting a role for sphingolipid composition in drug resistance [28]. In the present study, we also found that yeast cells carrying PHS-, DHS- or SPH-based sphingolipids exhibit different sensitivities to Ca2+ and the antibiotic hygromycin B (Figure 5). Generally, it is still unclear how sphingolipids affect these phenomena. However, it is likely that a change in sphingolipid composition would affect the properties of the plasma membrane itself and the activity of the membrane proteins interacting with extracellular factors. In this context, abnormal lipid microdomain formation in cells carrying SPH-based sphingolipids may affect the cells' sensitivities to Ca2+ and hygromycin B (Figures 4 and 5).
In summary, we found that in S. cerevisiae mutants deficient in sphingolipid biosynthesis, the requirement for a LCB can be fulfilled by exogenous SPH instead of PHS or DHS, although SPH results in incomplete or abnormal lipid microdomain formation. Further studies using LCB reconstitution of the mutant would help to elucidate the structural features of LCBs that are required for proper functioning, such as lipid microdomain formation.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (B) (12140201) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Degroote S., Wolthoorn J., van Meer G. The cell biology of glycosphingolipids. Semin. Cell Dev. Biol. 2004;15:375–387. doi: 10.1016/j.semcdb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Futerman A. H., Hannun Y. A. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons K., Ikonen E. Functional rafts in cell membranes. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 4.Merrill A. H., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 5.Dickson R. C., Lester R. L. Yeast sphingolipids. Biochim. Biophys. Acta. 1999;1426:347–357. doi: 10.1016/s0304-4165(98)00135-4. [DOI] [PubMed] [Google Scholar]
- 6.Ternes P., Franke S., Zahringer U., Sperling P., Heinz E. Identification and characterization of a sphingolipid Δ4-desaturase family. J. Biol. Chem. 2002;277:25512–25518. doi: 10.1074/jbc.M202947200. [DOI] [PubMed] [Google Scholar]
- 7.Haak D., Gable K., Beeler T., Dunn T. Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J. Biol. Chem. 1997;272:29704–29710. doi: 10.1074/jbc.272.47.29704. [DOI] [PubMed] [Google Scholar]
- 8.Buede R., Rinker-Schaffer C., Pinto W. J., Lester R. L., Dickson R. C. Cloning and characterization of LCB1, a Saccharomyces gene required for biosynthesis of the long-chain base component of sphingolipids. J. Bacteriol. 1991;173:4325–4332. doi: 10.1128/jb.173.14.4325-4332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagiec M. M., Baltisberger J. A., Wells G. B., Lester R. L., Dickson R. C. The LCB2 gene of Saccharomyces and the related LCB1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7899–7902. doi: 10.1073/pnas.91.17.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grilley M. M., Stock S. D., Dickson R. C., Lester R. L., Takemoto J. Y. Syringomycin action gene SYR2 is essential for sphingolipid 4-hydroxylation in Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:11062–11068. doi: 10.1074/jbc.273.18.11062. [DOI] [PubMed] [Google Scholar]
- 11.Beeler T., Gable K., Zhao C., Dunn T. A novel protein, CSG2p, is required for Ca2+ regulation in Saccharomyces cerevisiae. J. Biol. Chem. 1994;269:7279–7284. [PubMed] [Google Scholar]
- 12.Dickson R. C., Nagiec E. E., Wells G. B., Nagiec M. M., Lester R. L. Synthesis of mannose-(inositol-P)2-ceramide, the major sphingolipid in Saccharomyces cerevisiae, requires the IPT1 (YDR072c) gene. J. Biol. Chem. 1997;272:29620–29625. doi: 10.1074/jbc.272.47.29620. [DOI] [PubMed] [Google Scholar]
- 13.Nagiec M. M., Skrzypek M., Nagiec E. E., Lester R. L., Dickson R. C. The LCB4 (YOR171c) and LCB5 (YLR260w) genes of Saccharomyces encode sphingoid long chain base kinases. J. Biol. Chem. 1998;273:19437–19442. doi: 10.1074/jbc.273.31.19437. [DOI] [PubMed] [Google Scholar]
- 14.Irie K., Takase M., Lee K. S., Levin D. E., Araki H., Matsumoto K., Oshima Y. KK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol. Cell. Biol. 1993;13:3076–3083. doi: 10.1128/mcb.13.5.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwaki S., Kihara A., Sano T., Igarashi Y. Phosphorylation by Pho85 cyclin-dependent kinase acts as a signal for the down-regulation of the yeast sphingoid long-chain base kinase Lcb4 during the stationary phase. J. Biol. Chem. 2005;280:6520–6527. doi: 10.1074/jbc.M410908200. [DOI] [PubMed] [Google Scholar]
- 16.Uemura S., Kihara A., Inokuchi J., Igarashi Y. Csg1p and newly identified Csh1p function in mannosylinositol phosphorylceramide synthesis by interacting with Csg2p. J. Biol. Chem. 2003;278:45049–45055. doi: 10.1074/jbc.M305498200. [DOI] [PubMed] [Google Scholar]
- 17.Hanson B. A., Lester R. L. The extraction of inositol-containing phospholipids and phosphatidylcholine from Saccharomyces cerevisiae and Neurospora crassa. J. Lipid Res. 1980;21:309–315. [PubMed] [Google Scholar]
- 18.Bagnat M., Keranen S., Shevchenko A., Shevchenko A., Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G. B., Lester R. L. The isolation and characterization of a mutant strain of Saccharomyces cerevisiae that requires a long chain base for growth and for synthesis of phosphosphingolipids. J. Biol. Chem. 1983;258:10200–10203. [PubMed] [Google Scholar]
- 20.Saba J. D., Nara F., Bielawska A., Garrett S., Hannun Y. A. The BST1 gene of Saccharomyces cerevisiae is the sphingosine-1-phosphate lyase. J. Biol. Chem. 1997;272:26087–26090. doi: 10.1074/jbc.272.42.26087. [DOI] [PubMed] [Google Scholar]
- 21.Kim S., Fyrst H., Saba J. D. Accumulation of phosphorylated sphingoid long chain bases results in cell growth inhibition in Saccharomyces cerevisiae. Genetics. 2000;156:1519–1529. doi: 10.1093/genetics/156.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez A., Jimenez A., Vazquez D., Davies J. E., Schindler D. Studies on the mode of action of hygromycin B, an inhibitor of translocation in eukaryotes. Biochim. Biophys. Acta. 1978;521:459–469. doi: 10.1016/0005-2787(78)90287-3. [DOI] [PubMed] [Google Scholar]
- 23.Skrzypek M. S., Nagiec M. M., Lester R. L., Dickson R. C. Analysis of phosphorylated sphingolipid long-chain bases reveals potential roles in heat stress and growth control in Saccharomyces. J. Bacteriol. 1999;181:1134–1140. doi: 10.1128/jb.181.4.1134-1140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottlieb D., Heideman W., Saba J. D. The DPL1 gene is involved in mediating the response to nutrient deprivation in Saccharomyces cerevisiae. Mol. Cell Biol. Res. Commun. 1999;1:66–71. doi: 10.1006/mcbr.1999.0109. [DOI] [PubMed] [Google Scholar]
- 25.Birchwood C. J., Saba J. D., Dickson R. C., Cunningham K. W. Calcium influx and signaling in yeast stimulated by intracellular sphingosine 1-phosphate accumulation. J. Biol. Chem. 2001;276:11712–11718. doi: 10.1074/jbc.M010221200. [DOI] [PubMed] [Google Scholar]
- 26.Lester R. L., Wells G. B., Oxford G., Dickson R. C. Mutant strains of Saccharomyces cerevisiae lacking sphingolipids synthesize novel inositol glycerophospholipids that mimic sphingolipid structures. J. Biol. Chem. 1993;268:845–856. [PubMed] [Google Scholar]
- 27.Xu X., Bittman R., Duportail G., Heissler D., Vilcheze C., London E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J. Biol. Chem. 2001;276:33540–33546. doi: 10.1074/jbc.M104776200. [DOI] [PubMed] [Google Scholar]
- 28.Hallstrom T. C., Lambert L., Schorling S., Balzi E., Goffeau A., Moye-Rowley W. S. Coordinate control of sphingolipid biosynthesis and multidrug resistance in Saccharomyces cerevisiae. J. Biol. Chem. 2001;276:23674–23680. doi: 10.1074/jbc.M101568200. [DOI] [PubMed] [Google Scholar]