Abstract
Mammalian metabolism of ingested cysteine is conducted principally within the liver. The liver tightly regulates its intracellular cysteine pool to keep levels high enough to meet the many catabolic and anabolic pathways for which cysteine is needed, but low enough to prevent toxicity. One of the enzymes the liver uses to regulate cysteine levels is CDO (cysteine dioxygenase). Catalysing the irreversible oxidation of cysteine, CDO protein is up-regulated in the liver in response to the dietary intake of cysteine. In the present study, we have evaluated the contribution of the ubiquitin–26 S proteasome pathway to the diet-induced changes in CDO half-life. In the living rat, inhibition of the proteasome with PS1 (proteasome inhibitor 1) dramatically stabilized CDO in the liver under dietary conditions that normally favour its degradation. Ubiquitinated CDO intermediates were also seen to accumulate in the liver. Metabolic analyses showed that PS1 had a significant effect on sulphoxidation flux secondary to the stabilization of CDO but no significant effect on the intracellular cysteine pool. Finally, by a combination of in vitro hepatocyte culture and in vivo whole animal studies, we were able to attribute the changes in CDO stability specifically to cysteine rather than the metabolite 2-mercaptoethylamine (cysteamine). The present study represents the first demonstration of regulated ubiquitination and degradation of a protein in a living mammal, inhibition of which had dramatic effects on cysteine catabolism.
Keywords: cysteine, dioxygenase, liver, proteasome, ubiquitin, ubiquitin-26 S proteasome system
Abbreviations: CDO, cysteine dioxygenase; CSA, cysteine sulphinic acid; CSAD, CSA decarboxylase; CYS, cysteine; HP diet, high-protein diet; LP diet, low-protein diet; MEA, 2-mercaptoethylamine or cysteamine; OPA, o-phthalaldehyde; PS1, proteasome inhibitor 1; SBD-F, ammonium 4-fluoro-7-sulphobenzofurazan; THF, tetrahydrofuran
INTRODUCTION
The intracellular free amino acid pool of cysteine is tightly regulated in the mammalian liver. In rats, for instance, intracellular cysteine is maintained between 20 and 100 nmol/g even when dietary protein or sulphur amino acid intake is varied from sub-requirement to above-requirement levels for this species. The narrow range of permissible cysteine concentrations fulfils two homoeostatic requirements. Liver tissue must keep cysteine levels sufficiently high to meet the needs of protein synthesis and the production of other essential molecules such as glutathione, CoA, taurine and inorganic sulphur. At the same time, however, cysteine concentrations must also be kept below the threshold of cytotoxicity. The potent toxicity of excess cysteine has been demonstrated in several animal models [1–3], whereas, in humans, chronically high levels of cysteine have been closely associated with rheumatoid arthritis [4–6], Parkinson's disease [5], Alzheimer's disease [5], increased risk of cardiovascular disease [7] and adverse pregnancy outcomes [8].
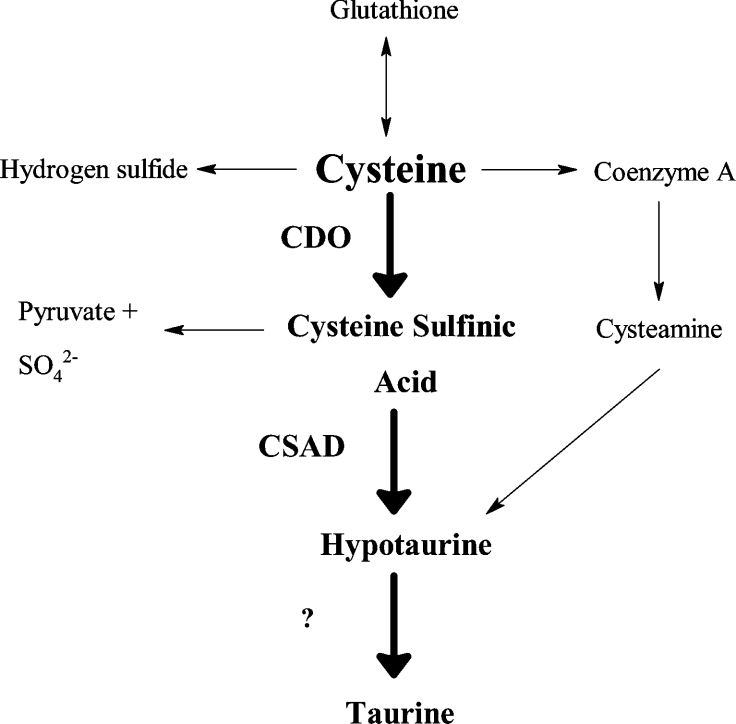
An important enzyme that contributes to the regulation of steady-state intracellular cysteine levels is CDO (cysteine dioxygenase; EC 1.13.11.20). Expressed at high levels in the liver, with lower levels in the kidney, brain and lung, this Fe2+ metalloenzyme catalyses the oxidation of the thiol group of cysteine, yielding CSA (cysteine sulphinic acid). The oxidative catabolism of cysteine to CSA by CDO represents an irreversible loss of cysteine from the free amino acid pool; CSA is shuttled into the pathways of hypotaurine/taurine synthesis, inorganic sulphur production, and use of the carbon backbone as pyruvate for gluconeogenesis or oxidative decarboxylation and cellular respiration; a metabolic flowchart in Figure 1 highlights CDO within the context of cysteine's catabolic pathways. In vivo results suggest that the liver, the organ with the highest amount of CDO protein expression and activity, may use CDO as a means of catabolizing cysteine obtained through the diet and, in the process, conveniently generates CSA, the biosynthetic precursor of the essential metabolites sulphate, hypotaurine and taurine [9].
Figure 1. A metabolic flowchart illustrating the position of CDO within the many pathways of cysteine catabolism.
CDO catalyses the first step in the CSAD pathway, cysteine→CSA→hypotaurine→taurine (highlighted in boldface), and shunts cysteine towards the production of CSA, pyruvate, sulphate, hypotaurine and taurine. For the purpose of clarity, multistep pathways apart from the CSAD route have been condensed to a single arrow. ‘?’, unidentified enzyme.
Steady-state levels of hepatic CDO protein are exquisitely regulated by the availability of dietary sulphur amino acids. Hepatic CDO activity is barely detectable in rats fed with LP (low-protein; i.e. sulphur amino acid poor) diets but increases as much as 35-fold in rats fed with diets enriched with methionine, cystine or total protein [10,11]. The regulation of CDO appears to be specifically associated with changes in intracellular cysteine concentration; other non-sulphur amino acids have no effect on CDO levels and blocking the synthesis of cysteine from methionine with the trans-sulphuration inhibitor propargylglycine inhibits the effect of methionine, but not of cysteine, on CDO levels [12].
Cysteine's ability to regulate CDO levels is rather unique in that it is an exclusively post-translational phenomenon [13]. Recent evidence collected from rat primary hepatocyte cultures has shown that cysteine availability significantly affects the half-life of CDO protein [14]. In that study, under conditions where the supply of cysteine in the medium was limiting, cultured hepatocytes rapidly ubiquitinated and degraded CDO by the 26 S proteasome system. On the other hand, when cysteine availability was high, the ubiquitination of CDO was markedly attenuated and the half-life of the protein was significantly prolonged. The metabolic signal for ubiquitination in vitro appeared to be specific to the reduced form of cysteine, because oxidized metabolites of cysteine such as CSA were not able to prevent CDO degradation. Interestingly, the reduced cysteine metabolite MEA (2-mercaptoethylamine or cysteamine) was found to be as effective as cysteine in attenuating CDO degradation in vitro. This finding raised the possibility that, contrary to what has been logically presumed, the proximal metabolite for the signalling of CDO stabilization in vivo is actually MEA rather than cysteine.
Because previous work describing the cysteine-dependent regulation of CDO by the ubiquitin–26 S proteasome system has been limited to cell culture models [14], we decided to explore whether this same system is responsible for the dietary regulation of CDO protein observed in vivo. We also sought to identify whether MEA is the actual in vivo regulator of CDO turnover. To address the first objective, pharmacological inhibition of the 26 S proteasome enabled us to prevent the degradation of CDO and even witness accumulation of ubiquitinated CDO intermediates under dietary conditions that otherwise would have led to its rapid proteolysis. Metabolic investigations revealed a significant enhancement of sulphoxidation flux upon inhibition of CDO degradation. To test the second objective, we fed rats with LP diets enriched in MEA and monitored tissue MEA accumulation and CDO protein levels. Surprisingly, we found that even under conditions of significant MEA accumulation, this metabolite failed to stabilize CDO in vivo. These results directly point to cysteine as the master regulator of CDO degradation in vivo.
EXPERIMENTAL
Animal feeding studies
Male Sprague–Dawley rats (170–210 g) were purchased from Harlan Sprague Dawley (Indianapolis, IN, U.S.A.). Rats were housed in polycarbonate cages containing paper bedding in a room maintained at 20 °C and 60–70% humidity with light from 18:00 to 06:00 h. These animals had ad libitum access to water but had access to food only during the dark cycle 06:00 to 18:00 h, wherein food was provided in ceramic cups. To ensure high expression of hepatic CDO protein, all rats were initially fed with a HP (high-protein) diet for 1 week prior to the treatment day. This diet contained 40% casein by weight. On the treatment day, rats were randomly assigned to the following five experimental treatments: maintenance on HP diet; switch to an LP (10% casein by weight) diet; switch to an LP diet supplemented with 8.12 g/kg dietary cysteine (LP+CYS); switch to an LP diet supplemented with 7.2 g/kg dietary MEA (LP+MEA); or switch to an LP diet [LP+PS1 (proteasome inhibitor 1)] plus an intraperitoneal injection of the proteasome inhibitor, PS1 [N-carboxybenzyl-Ile-Glu(O-t-butyl)-Ala-Leu-aldehyde; 17 mg/kg in DMSO]. PS1 has been previously used to inhibit proteasome activity in vivo [15] and, in our hands, has proven to be an effective inhibitor of proteasome-mediated degradation of CDO in vitro [14]. Both the LP and HP diets were prepared by Dyets (Bethlehem, PA, U.S.A.) and were a modification of the AIN-93A diet [16]. The LP diet mixture was initially prepared with 25 g/kg sucrose excluded from it. For the LP and LP+PS1 diets, all of this sucrose was added back in. For the LP+CYS diet, 16.88 g/kg sucrose was added along with 8.12 g/kg cysteine to fully reconstitute the diet. For the LP+MEA diet, 14.4 g/kg sucrose was added along with 10.6 g/kg MEA HCl (7.2 g of MEA) to create the complete diet. Following reconstitution, all diets were prepared as gel cubes by the addition of a hot 3% (w/v) agar solution, followed by casting in containers at 4 °C and cutting into easily managed cubes.
At the end of the fasting, light cycle on treatment day (time=0 h), three rats were killed to establish baseline values, and the remaining rats were switched to their assigned dietary treatments (six rats/treatment). Animals were subsequently killed 6 and 10 h after the diet switch (three rats from each group per time point). Animals in the LP+PS1 group received an intraperitoneal injection of PS1 at 2.5 h after the diet switch. At the appropriate time point, rats were weighed and anaesthetized using sodium pentobarbital (30 mg/ml in 15%, v/v, ethanol) at a dose of 90 mg/kg. Whole livers were removed, rinsed with ice-cold saline, and immediately frozen in liquid nitrogen. The animal protocol used in the present study was approved by the Cornell University Institutional Animal Care and Use Committee.
Western-blot analysis
Western-blot analysis was conducted as described previously but with some minor modifications [11]. Briefly, livers were homogenized in 4 vol. of lysis buffer containing 50 mM Tris/HCl, 150 mM NaCl, 1 mM EDTA, 0.5% (v/v) Nonidet P40, 10 mM orthovanadate, 1× protease inhibitor cocktail (Sigma, St. Louis, MO, U.S.A.), 10 mM N-ethylmaleimide and 20 μM MG-132 (Boston Biochem, Boston, MA, U.S.A.), pH 7.4. Homogenates were centrifuged at 16500 g for 20 min. Equivalent amounts of total supernatant protein from each experimental group, as determined by bicinchoninic acid assay (Pierce, Rockford, IL, U.S.A.), were separated by one-dimensional SDS/PAGE (either 12 or 15%, w/v, acrylamide) and then electroblotted overnight on to 0.45 μm Immobilin-P PVDF membranes (Millipore, Medford, MA, U.S.A.). Immunoreactive protein was detected by chemiluminescence using rabbit anti-rat CDO polyclonal antibody [18], rabbit anti-rat actin (Sigma) and horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Supersignal Pico; Pierce) with exposure to a Kodak X-OMAT film. Developed films were scanned using a desktop scanner. With the obtained electronic images, two-dimensional quantitative densitometric analysis was performed on protein bands using AlphaEase software (Alpha Innotech, San Leandro, CA, U.S.A.). Linearity of the developed signal was verified by examining dilutions of liver supernatant from the 0 h HP group that were run on each gel. The apparent molecular masses of native CDO (which ran as a double band on SDS/PAGE with a molecular mass of ∼23 kDa) and ubiquitinated CDO [which also ran as double bands with molecular masses of ∼23+(n×8) kDa, where n is the number of attached ubiquitin moieties] were consistent with previously published values [17,18]. For the quantification of native CDO, both bands were used for densitometry.
Hepatocyte culture
Hepatocytes were isolated from rats that had been fed with the HP diet for 3 days and were cultured on 100 mm collagen-coated dishes in modified Williams' medium E as described previously [12,14]. The basal sulphur amino acid-free medium used for culturing contained no cysteine but was supplemented with 100 μM methionine. After harvesting hepatocytes, cells were acclimatized for 24 h in basal medium supplemented with 1 mM cysteine to minimize CDO degradation prior to evaluating the effects of various cysteine analogues in maintaining CDO stability. At the beginning of the cysteine analogue time-course study (0 h), cells were washed with sterile PBS and then cultured in 15 ml of basal medium supplemented with one of the following: 0 mM cysteine, 1 mM cysteine, 1 mM MEA, 1 mM 2-mercaptoethanol, 1 mM 3-mercaptopropionic acid or 1 mM penicillamine (2-amino-3-mercapto-3-methylbutanoic acid). Cells were harvested at 0, 4, 12 and 24 h in the same lysis buffer as described for the Western-blot procedures. For culture periods longer than 12 h, the medium was replaced at 12 h. Experiments were replicated at least three times using hepatocytes isolated from different rats.
Analysis of tissue cysteine, MEA and hypotaurine content by HPLC
Determination of tissue cysteine levels was performed by modification of a previously described method [19]. Frozen livers were homogenized in 4 vol. of the same lysis buffer as used for the Western-blot analysis except that N-ethylmaleimide was not included. Following centrifugation at 16500 g for 25 min, 75 μl of the sample supernatant was diluted with 25 μl of PBS and then reduced with 10 μl of 100 g/l tris(2-carboxyethyl)phosphine for 30 min at room temperature (22 °C). Reduced samples were deproteinized by the addition of 90 μl of 100 g/l trichloroacetic acid containing 1 mM EDTA. Samples were centrifuged for 10 min at 13000 g. For thiol derivatization, 50 μl of sample supernatant was added to an autosampler vial containing 10 μl of 1.55 mM NaOH, 125 μl of 125 mM borate buffer containing 4 mM EDTA (pH 9.5) and 50 μl of 1 g/l SBD-F (ammonium 4-fluoro-7-sulphobenzofurazan; Dojindo Laboratories, Kumamato, Japan) dissolved in 125 mM borate buffer with 4 mM EDTA (pH 9.5). Thiol derivatization was done for 60 min at 60 °C. Chromatographic separation was done on a 4.6 mm×150 mm Sunfire analytical column with C18 5 μm spherical packing material (Waters, Milford, MA, U.S.A.) protected by a C18 guard cartridge (5 μm spherical particles; Alltech Associates, Deerfield, IL, U.S.A.). A total of 80 μl of sample was injected on to the column by an automated sample injector (WISP 717 Plus; Waters). Derivatized thiols were separated by isocratic elution using buffer A, which consisted of 0.1 M acetic acid (pH 5.5) with 3% (v/v) HPLC-grade methanol at a flow rate of 0.7 ml/min at room temperature. After elution of all thiols (10.5 min), the column was washed with 50% buffer B, consisting of 0.1 M acetic acid (pH 5.5) with 50% HPLC-grade methanol. The column was then equilibrated for 6 min with 100% buffer A prior to the next injection. SBD-F-derivatized thiols were detected by a fluorescence detector (LC240; PerkinElmer, Boston, MA, U.S.A.) at an excitation wavelength of 360 nm and an emission wavelength of 515 nm. Analogue signals from the fluorescence detector were managed by a personal computer containing Empower software (version 5.0; Waters). Chromatographic peaks were integrated using this software and quantified against cysteine standards (5–50 μM) that were reduced, derivatized and run in parallel with the samples.
MEA and hypotaurine contents were assessed from the acid extracts of liver homogenates that were prepared by homogenizing frozen liver samples in 4 vol. of 5% (w/v) sulphosalicylic acid. Homogenates were then centrifuged at 10000 g for 10 min. Acid supernatant (1.5 ml) was removed and added to 50 μl of 0.2 mM m-Cresol Purple with mixing. While mixing the sample, 0.48 ml of 2 M KOH/2.4 M KHCO3 was added followed by 50 μl of 50 mM dithiothreitol. The mixture was incubated at 37 °C for 30 min. After the incubation, 50 μl of 200 mM iodoacetate was added with mixing, and the mixture was placed in the dark for 10 min to alkylate free thiols. Chromatography of derivatized samples was conducted on a 4.6 mm×150 mm column packed with Nova-Pak C18 4 μm spherical packing material (Waters) equipped with a C18 guard cartridge (5 μm spherical particles; Alltech Associates). Under conditions of no-flow, 75 μl of OPA (o-phthalaldehyde)–2-mercaptoethanol derivatizing reagent and then a 50 μl volume of the sample acid supernatant or standard solution were injected into the precolumn tubing by an automatic sample injector (WISP Model 712; Waters). The derivatizing reagent was prepared fresh daily by mixing 3.5 mg of OPA with 50 μl of 95% ethanol, 5 ml of 100 mM borate buffer (pH 10.4) and 10 μl of 2-mercaptoethanol. A 3 min delay was programmed prior to initiating flow of mobile phase to allow reaction of amines with OPA. Amino acids were separated by gradient elution using two buffers. Buffer A was 100 mM potassium phosphate buffer plus 3% (v/v) THF (tetrahydrofuran) (pH 7.0) and buffer B was 100 mM potassium phosphate buffer plus 3% THF and 40% (v/v) acetonitrile (pH 7.0). Buffers were filtered through 0.45 μm filters before use. Flow rate was 1.0 ml/min and column temperatures were maintained at room temperature. Mobile phase was started isocratically for the first 1 min at 3% B, increased to 30% B over 6 min, then increased to 55% buffer B over 13 min, and then increased to 100% B over 2 min and held at 100% B for 3 min. At 25 min into the run, the mobile phase was decreased to 3% B over 10 min and the column was allowed to equilibrate for another 11 min before the next sample injection. Detection of OPA-derivatized amino acids was performed using a Spectra/glo Filter fluorimeter (Gilson Medical Electronics, Middleton, WI, U.S.A.) equipped with a 5 μl flow cell and filters for excitation and emission peaks at 360 and 455 nm respectively. The fluorimeter was connected to a personal computer equipped with Peak Simple Chromatography Data System version 3.21 (LabAlliance, State College, PA, U.S.A.) for the integration of chromatographic peaks.
Statistical analysis
All quantitative data are expressed as means±S.D. Statistical analyses were conducted by ANOVA and Tukey's post-test procedure using Prism 3 (GraphPad Software, San Diego, CA, U.S.A.). Differences were considered significant at P≤0.05. Unless otherwise stated, fold changes are relative to 0 h values.
RESULTS
Proteasome inhibition prevents hepatic CDO degradation
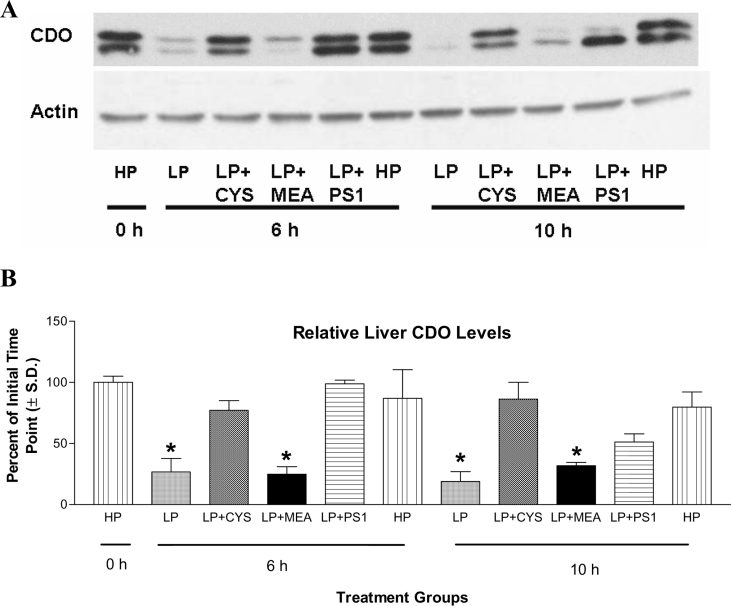
Rats switched from the HP diet to the LP diet exhibited a significant decrease in CDO protein over the time course of the study; by the 10 h time point, CDO levels had decreased by more than 80% (Figure 2). CDO levels were stabilized, however, by maintaining animals on the HP diet, supplementing the LP diet with cysteine, or providing an injection of PS1. Although the effect of PS1 was comparable with that of rats maintained on the HP diet or given the LP diet at 6 h, its ability to attenuate CDO degradation was diminished by approx. 50% at 10 h. Nevertheless, animals receiving a PS1 injection retained significantly more CDO protein than animals on the LP diet alone.
Figure 2. Effects of dietary manipulation and proteasome inhibition on levels of liver CDO protein in rats.
For SDS/PAGE analysis, 60 μg of soluble liver protein was loaded per lane on to a 12% (w/v) polyacrylamide gel. (A) A representative Western blot, probed first for CDO and then for actin as a loading control, is shown for one such analysis. (B) Average relative hepatic CDO protein levels. To determine the relative changes in protein levels for all samples, the absorbance (A) of each CDO band was measured by densitometry. Absorbances were then normalized by HP 0 h control CDO values (run on each gel) and expressed as a percentage of these controls in the bar graph (B). LP+CYS, LP diet +8.12 g of cysteine/kg of diet; LP+MEA, LP diet +7.2 g of MEA/kg of diet; LP+PS1, LP diet+intraperitoneal injection of PS1 (17 mg/kg). *P<0.01 versus initial (0 h HP) value. N=three rats per group.
The expression of actin, a protein with an extremely long half-life [20], was included as a loading control in the analysis of CDO levels. Steady-state levels of actin in the liver were not affected by any of the treatment groups within the time course of the study (Figure 2A).
Effect of dietary treatments on hepatic CDO ubiquitination
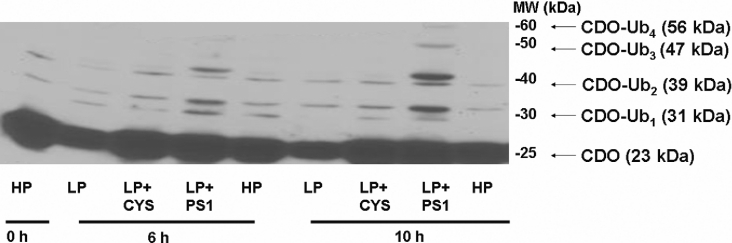
Primary hepatocyte culture studies have shown that a reduction in cysteine availability causes an increase in the amount of polyubiquitinated CDO [14]. These ubiquitinated species, which constitute a relatively small fraction of the total CDO protein pool (<10%), were seen as a higher-molecular-mass ladder differing from the molecular mass of CDO by integer multiples of approx. 8 kDa (the molecular mass of monomeric ubiquitin is ∼8 kDa). As shown in Figure 3, mono- and di-ubiquitinated forms of CDO were present in the livers of rats regardless of the treatment group, though the total quantity of the ubiquitinated species was treatment-sensitive. There was a marked accumulation of these species in the LP+PS1-treated animals, particularly at the 10 h time point, as would be expected from conditions wherein CDO ubiquitination is enhanced and proteasome activity is inhibited. Higher-molecular-mass forms of ubiquitinated CDO (tri- and tetra-ubiquitinated CDO) were also apparent at the 10 h time point, further demonstrating the accumulation of modified CDO following proteasome inhibition.
Figure 3. Effects of dietary manipulation and proteasome inhibition on levels of ubiquitinated CDO.
A Western blot showing the effects of dietary manipulation and proteasome inhibition on steady-state levels of ubiquitinated liver CDO. Liver homogenate (125 μg) was loaded into each lane. A molecular mass ladder (MW) is shown. Ubiquitinated species are designated CDO-Ub, with the total number of attached ubiquitin moieties indicated by a subscript numeral and their corresponding molecular masses.
Effects of CDO stabilization on cysteine and hypotaurine levels in vivo
Given the effectiveness of proteasome inhibition in stabilizing CDO levels in vivo, we asked whether there would be any accompanying perturbation in the concentrations of CSA pathway metabolites. In particular, we anticipated that PS1 would decrease the intracellular cysteine pool and increase the intracellular hypotaurine pool. These predictions were based on several previously established observations concerning CSA pathway flux in vivo. First, CDO catalyses the flux regulating step of the cysteine→CSA→hypotaurine pathway [21–23]. The concentration of CSA, which is either rapidly decarboxylated by CSAD (CSA decarboxylase) to yield hypotaurine or deaminated by aspartate aminotransferase to yield pyruvate, does not change dramatically as intracellular levels are kept very low (frequently beneath the limits of detection) [24]. The intracellular pool of hypotaurine, however, has been shown to be closely tied to acute changes in cysteine sulphoxidation flux [25]. Indeed, hypotaurine rapidly accumulates in tissues as the capacity of hypotaurine oxidation to taurine appears to be slow and is readily saturable at high rates of hypotaurine generation from cysteine [26–28]. Although total reduced cysteine levels declined rapidly and significantly within the livers of animals switched to the LP diet, there was no additional decrease in hepatic cysteine levels in PS1-treated rats (Table 1). Thus it would appear that the stabilization of CDO by PS1 had no significant effect on the total cysteine pool in this organ.
Table 1. Effects of experimental diets and proteasome inhibition on total cysteine levels (nmol/mg of protein) in liver.
Values with the same superscript letters are significantly different by ANOVA and Tukey's post-test procedure (P≤0.05). N=three rats per treatment group.
| Cysteine level (nmol/mg of protein) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h (fasting) | 6 h (fed) | 10 h (fed) | |||||||
| Time… Diet… | HP | LP | LP+CYS | LP+PS1 | HP | LP | LP+CYS | LP+PSI | HP |
| 0.90±0.21ab | 0.37±0.18bc | 1.0±0.35a | 0.45±0.20bc | 0.82±0.14a | 0.25±0.11c | 0.87±0.33a | 0.24±0.04c | 0.58±0.12a | |
Concordant with the important role of CDO in the control of CSA pathway flux and hypotaurine synthesis, however, there was a clear association between liver CDO protein levels and liver hypotaurine content in the present study. The decrement in CDO protein within the LP group was accompanied by a sharp decline in hypotaurine content (Table 2). In contrast, the LP+CYS, LP+PS1 and HP groups, which had elevated levels of CDO protein relative to the LP groups, also displayed significantly elevated levels of hypotaurine.
Table 2. Effects of experimental diets and proteasome inhibition on hypotaurine levels in liver.
Values with the same superscript letters are significantly different by ANOVA and Tukey's post-test procedure (P≤0.05). N=three rats per treatment group.
| Hypotaurine level (pmol/mg of protein) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h (fasting) | 6 h (fed) | 10 h (fed) | |||||||
| Time… Diet… | HP | LP | LP+CYS | LP+PS1 | HP | LP | LP+CYS | LP+PS1 | HP |
| 158±25a | 37±3b | 701±247c | 685±123c | 405±121c | 37±10b | 999±516c | 249±191ac | 337±75ac | |
MEA stabilizes CDO in vitro but is not the in vivo signal for CDO stabilization
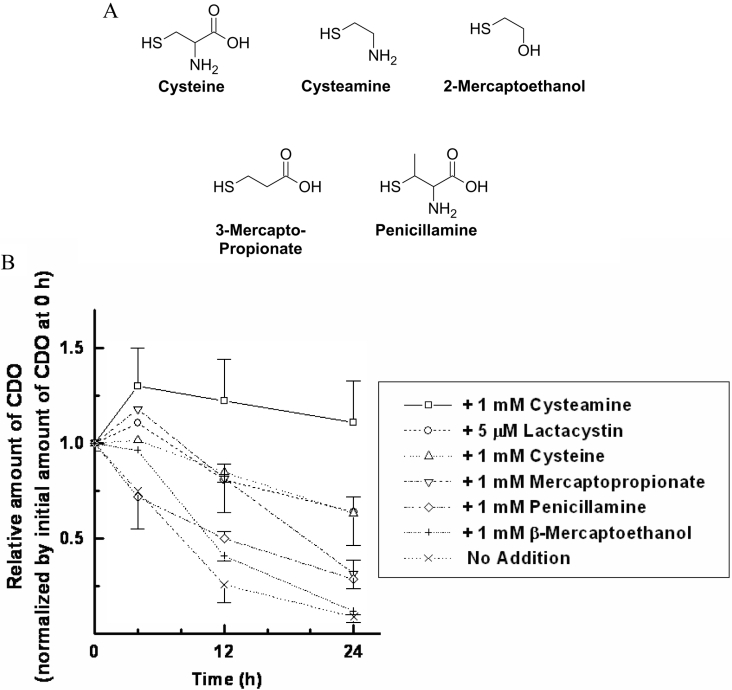
Recently, it has been shown that MEA is as effective as cysteine in attenuating CDO degradation in vitro [14]. Using primary hepatocyte cultures, we conducted a more complete analysis of the ability of cysteine structural analogues to stabilize CDO in order to reveal what components of the cysteine molecule were necessary and sufficient for achieving CDO stabilization (Figure 4A). Although these compounds are structurally related to cysteine, they possess no significant inhibitory or stimulatory effect on CDO catalysis [17]. The ability of these compounds to attenuate CDO degradation under cysteine-limiting conditions is shown in Figure 4(B). Although an amine group, two-carbon aliphatic backbone and reduced thiol group appeared to be necessary for preventing CDO degradation, none were sufficient on their own to mediate this effect. This would therefore appear to rule out a general redox effect on CDO turnover. Similarly, 1 mM ascorbic acid and 1 mM hydrogen sulphide – a reducing agent and cysteine metabolite – failed to prevent CDO degradation (results not shown). These results, coupled with previous studies in hepatocytes that have shown that oxidized metabolites of cysteine [12,14] do not affect CDO turnover, effectively ruled out all other cysteine metabolites except MEA as potential mediators of CDO degradation.
Figure 4. A comparative evaluation of cysteine-like compounds to prevent CDO degradation in primary hepatocyte cultures.
(A) Structures of the cysteine analogues tested. (B) The effect of each of these compounds on CDO protein, determined by SDS/PAGE and Western blotting. Lactacystin, a specific proteasome inhibitor, was used at a final concentration of 5 μM in the medium for comparison purposes. All other compounds were tested at a concentration of 1 mM. Results shown in (B) are presented as means±S.E.M.
Because MEA is an indirect cysteine metabolite produced by the constitutive turnover of CoA in mammals, we decided to explore if the dietary stabilization of CDO by cysteine in vivo is proximally mediated by its subsequent metabolism to MEA. Switching rats from the HP to the LP diet significantly reduced MEA levels in the liver (Table 3). There was a partial restoration of tissue MEA by the LP+CYS diet, but the levels were still less than 30% of those in tissues from rats fed with the HP diet. Dietary supplementation with a quantity of MEA that was equimolar to the amount of cysteine in the LP+CYS group significantly increased tissue MEA beyond that seen in the HP groups. In spite of this accumulation of MEA, however, the LP+MEA diet failed to induce significant CDO stabilization in the liver (Figure 2).
Table 3. Effects of experimental diets on total reduced cysteamine levels in liver.
Values with the same superscript letters are significantly different by ANOVA and Tukey's post-test procedure (P≤0.05). N=three rats per treatment group.
| Cysteamine level (pmol/mg of protein) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h (fasting) | 6 h (fed) | 10 h (fed) | |||||||
| Time… Diet… | HP | LP | LP+CYS | LP+MEA | HP | LP | LP+CYS | LP+MEA | HP |
| 64±17a | 6.1±1.6b | 13±15b | 114±14c | 44±6.6ab | 14±19b | 13±16b | 131±17c | 45±13ab | |
DISCUSSION
Although the ubiquitin–26 S proteasome pathway is thought to be responsible for the half-lives of most if not all intracellular mammalian proteins, direct evidence for targeted ubiquitination and degradation has been gathered for only a handful of proteins – the majority being short-lived transcription factors and signal transduction molecules [29,30]. Studies involving these proteins have relied heavily upon evidence derived from in vitro cell-free or intact cell culture models, with no demonstration of physiological regulation of the queried protein by the ubiquitin–26 S proteasome system in a living mammal. In the present paper, however, we have shown that CDO – a protein involved in intermediary amino acid metabolism – is robustly regulated by the ubiquitin–26 S proteasome system in vivo and that this regulation has a significant impact on flux through the CSA pathway. We have also demonstrated that it is cysteine, rather than its reduced metabolite MEA, that is the proximal signal for the dietary regulation of CDO ubiquitination and degradation.
Cysteine-mediated ubiquitination and degradation in the liver
Rats on the LP diet rapidly down-regulated CDO protein in response to low cysteine availability. This down-regulation was prevented by pharmacological inhibition of the proteasome. Moreover, ubiquitinated species of CDO were elevated in the livers of rats that received a specific proteasome inhibitor. These results are consistent with what we have previously seen in cultured primary rat hepatocytes and transfected HepG2 cells, which rapidly ubiquitinate and degrade CDO under conditions of low cysteine availability [14]. The degradation of liver CDO was also prevented by increasing the intracellular concentration of cysteine and was also accompanied by a decrease in the relative steady-state levels of ubiquitinated CDO after normalization by native CDO. Again, this parallels nicely with the results of our previous cell culture studies, wherein hepatocyte CDO ubiquitination and degradation were markedly attenuated upon incubation in a high-cysteine medium [14].
The striking change in hepatic CDO protein stability seen in the present study occurred over a very narrow range of intracellular cysteine concentrations: approx. 0.3–0.6 nmol/mg of protein. The regulation of CDO stability by its substrate in vivo is therefore an extremely sensitive and robust system. Whether the cysteine-mediated regulation of steady-state CDO ubiquitination is due to a decrease in the rate of ubiquitin attachment or an increase in the rate of de-ubiquitination is not clear; no such conclusion can be deduced on the basis of total steady-state ubiquitinated CDO. Work is currently under way to resolve this issue as well as to identify the putative E2/E3 ubiquitin ligases that initiate CDO ubiquitination. We do know, however, that the signal for ubiquitination and degradation does not appear to be due to structural changes in CDO arising from substrate binding. This conclusion is based on two pieces of evidence. First, MEA can effectively prevent CDO degradation in spite of the fact that it is neither a substrate for the enzyme nor an effective inhibitor of activity. Secondly, we have recently elucidated the crystal structure of CDO with cysteine complexed in its active site. Compared with the apo-enzyme, there are no significant changes in the solvent-exposed surfaces of CDO as a consequence of cysteine binding (C. R. Simmons, Q. Hao and M. H. Stipanuk, unpublished work).
Impact of CDO on CSA pathway flux and steady-state cysteine levels
Because CDO catalyses the first step in the CSA pathway, it is thought to be strategically positioned for the regulation of this pathway's flux. The results presented in this paper lend support to this notion of flux control, as there was a consistent positive association between CDO protein and hypotaurine levels. Moreover, significant hepatic hypotaurine accumulation could be accomplished even when intracellular cysteine levels were low by artificially increasing CDO levels through proteasome inhibition.
CDO is thought to be an important regulator of the hepatic intracellular free cysteine pool partly due to the dramatic rise in CDO protein/activity that follows cysteine intake and partly due to the lack of a metabolic pathway in mammals for the regeneration of cysteine from CSA. In light of these well-established observations, it was surprising that the hepatic intracellular cysteine concentrations of animals on a cysteine-limited diet were not significantly lowered when the degradation of CDO was inhibited by PS1. Metabolic data clearly demonstrated under these conditions that cysteine was being actively siphoned from the free amino acid pool for hypotaurine synthesis. This interesting floor effect on cysteine levels suggests that other mechanisms exist in the liver that prevent intracellular cysteine levels from decreasing below a critical level in vivo. A reduction in glutathione synthesis does not appear to be one such mechanism, as total GSH levels were not significantly changed in the organs of any of the treatment groups over the study time course (results not shown). Alternative possibilities include an increase in trans-sulphuration flux from methionine to cysteine or a reduction of cysteine incorporation in protein. Both of these adaptive phenomena occur in yeast under conditions of limited sulphur/cysteine availability [31,32], but additional work is needed to substantiate these responses in mammals.
MEA does not mediate CDO regulation in vivo
Cell culture work has consistently shown the cysteine metabolite MEA to be as effective as cysteine in preventing hepatic CDO degradation [14]. Our whole-animal studies, however, indicate that intracellular MEA is not the in vivo metabolic signal for CDO degradation. More specifically, animals on the LP diet and animals on the LP+CYS diet showed no significant change in intracellular MEA levels and yet had remarkable differences in CDO protein. Similarly, elevating MEA levels by dietary supplementation had no effect on CDO stability. Discrepancies between the in vitro and in vivo results for MEA may be attributable to the absolute magnitude of intracellular MEA accumulation in each system. In our experiments with primary hepatocyte cultures, we have found that when exposed to either 1 mM MEA or 1 mM cysteine, primary hepatocytes accumulate both amino acids to roughly comparable levels: approx. 7 nmol/mg of protein (results not shown). In contrast, even when animals were fed with MEA, MEA levels remained well below the lowest cysteine levels observed in rat tissue (i.e. those in the tissues of rats fed with the LP diet). Hence it is unlikely that MEA ever accumulates to levels sufficiently high to produce CDO stabilization in vivo. Collectively, these results point to cysteine, rather than MEA, as the signalling molecule that dictates CDO half-life in vivo.
Out of the panoply of sulphur amino acid metabolites synthesized and catabolized by eukaryotes, many have evolved to use intracellular cysteine levels as a homoeostatic sensor of sulphur flux. This amino acid has been shown to co-ordinate the expression of many enzymes involved in plant [33] and fungal [34,35] sulphur metabolism. In the present study, we show that mammals also use intracellular cysteine levels to co-ordinate aspects of their sulphur metabolism. Moreover, cysteine levels specifically alter the half-life of CDO through the proteasome pathway and thus directly manipulate the rate of sulphoxidation flux in vivo. This is the first demonstration of a post-translational level of regulation mediated by cysteine. In the broader evolutionary context, the conserved usage of cysteine as a regulator of sulphur flux raises the interesting question of how organisms sense intracellular cysteine levels. At least within the context of the present study, the phenomenon does not appear to involve a simple redox-dependent sensor. The identification of this sensor shall provide a more integrated view of sulphur amino acid metabolism regulation across many kingdoms of organisms.
Acknowledgments
This research was supported by a grant from the National Institutes of Health, grant PHS DK056649, awarded to M. H. S. J. E. D. was supported by a graduate student fellowship from the National Science Foundation. We thank Dr Jeong-In Lee and Mr Chad Simmons (Division of Nutritional Sciences, Cornell University) for their technical assistance as well as their valuable scientific discussions during the course of this study.
References
- 1.Andine P., Orwar O., Jacobson I., Sandberg M., Hagberg H. Extracellular acidic sulfur-containing amino acids and gamma-glutamyl peptides in global ischemia: postischemic recovery of neuronal activity is paralleled by a tetrodotoxin-sensitive increase in cysteine sulfinate in the CA1 of the rat hippocampus. J. Neurochem. 1991;57:230–236. doi: 10.1111/j.1471-4159.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann A. Alterations in hippocampal extracellular amino acids and purine catabolites during limbic seizures induced by folate injections into the rabbit amygdala. Neuroscience. 1987;22:573–578. doi: 10.1016/0306-4522(87)90354-x. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann A., Hagberg H., Orwar O., Sandberg M. Cysteine sulphinate and cysteate: mediators of cysteine toxicity in the neonatal rat brain? Eur. J. Neurosci. 1993;5:1398–1412. doi: 10.1111/j.1460-9568.1993.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 4.Bradley H., Gough A., Sokhi R. S., Hassell A., Waring R., Emery P. Sulfate metabolism is abnormal in patients with rheumatoid arthritis. Confirmation by in vivo biochemical findings. J. Rheumatol. 1994;21:1192–1196. [PubMed] [Google Scholar]
- 5.Heafield M. T., Fearn S., Steventon G. B., Waring R. H., Williams A. C., Sturman S. G. Plasma cysteine and sulphate levels in patients with motor neurone, Parkinson's and Alzheimer's disease. Neurosci. Lett. 1990;110:216–220. doi: 10.1016/0304-3940(90)90814-p. [DOI] [PubMed] [Google Scholar]
- 6.Gordon C., Bradley H., Waring R. H., Emery P. Abnormal sulphur oxidation in systemic lupus erythematosus. Lancet. 1992;339:616–617. doi: 10.1016/0140-6736(92)90144-r. [DOI] [PubMed] [Google Scholar]
- 7.El-Khairy L., Vollset S. E., Refsum H., Ueland P. M. Plasma total cysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2003;77:467–472. doi: 10.1093/ajcn/77.2.467. [DOI] [PubMed] [Google Scholar]
- 8.Karlsen R. L., Pedersen O. O. A morphological study of the acute toxicity of L-cysteine on the retina of young rats. Exp. Eye Res. 1982;34:467–472. doi: 10.1016/0014-4835(82)90009-4. [DOI] [PubMed] [Google Scholar]
- 9.Garcia R. A., Stipanuk M. H. The splanchnic organs, liver and kidney have unique roles in the metabolism of sulfur amino acids and their metabolites in rats. J. Nutr. 1992;122:1693–1701. doi: 10.1093/jn/122.8.1693. [DOI] [PubMed] [Google Scholar]
- 10.Bella D. L., Hahn C., Stipanuk M. H. Effects of nonsulfur and sulfur amino acids on the regulation of hepatic enzymes of cysteine metabolism. Am. J. Physiol. 1999;277:E144–E153. doi: 10.1152/ajpendo.1999.277.1.E144. [DOI] [PubMed] [Google Scholar]
- 11.Bella D. L., Hirschberger L. L., Hosokawa Y., Stipanuk M. H. Mechanisms involved in the regulation of key enzymes of cysteine metabolism in rat liver in vivo. Am. J. Physiol. 1999;276:E326–E335. doi: 10.1152/ajpendo.1999.276.2.E326. [DOI] [PubMed] [Google Scholar]
- 12.Kwon Y. H., Stipanuk M. H. Cysteine regulates expression of cysteine dioxygenase and gamma-glutamylcysteine synthetase in cultured rat hepatocytes. Am. J. Physiol. Endocrinol. Metab. 2001;280:E804–E815. doi: 10.1152/ajpendo.2001.280.5.E804. [DOI] [PubMed] [Google Scholar]
- 13.Bella D. L., Kwon Y. H., Hirschberger L. L., Stipanuk M. H. Post-transcriptional regulation of cysteine dioxygenase in rat liver. Adv. Exp. Med. Biol. 2000;483:71–85. doi: 10.1007/0-306-46838-7_7. [DOI] [PubMed] [Google Scholar]
- 14.Stipanuk M. H., Hirschberger L. L., Londono M. P., Cresenzi C. L., Yu A. F. The ubiquitin-proteasome system is responsible for cysteine-responsive regulation of cysteine dioxygenase concentration in liver. Am. J. Physiol. Endocrinol. Metab. 2004;286:E439–E448. doi: 10.1152/ajpendo.00336.2003. [DOI] [PubMed] [Google Scholar]
- 15.Fischer D., Gang G., Pritts T., Hasselgren P. O. Sepsis-induced muscle proteolysis is prevented by a proteasome inhibitor in vivo. Biochem. Biophys. Res. Commun. 2000;270:215–221. doi: 10.1006/bbrc.2000.2398. [DOI] [PubMed] [Google Scholar]
- 16.Stipanuk M. H., Londono M., Lee J. I., Hu M., Yu A. F. Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J. Nutr. 2002;132:3369–3378. doi: 10.1093/jn/132.11.3369. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi K., Hosokawa Y., Kohashi N., Kori Y., Sakakibara S., Ueda I. Rat liver cysteine dioxygenase (cysteine oxidase). Further purification, characterization, and analysis of the activation and inactivation. J. Biochem. (Tokyo) 1978;83:479–491. doi: 10.1093/oxfordjournals.jbchem.a131935. [DOI] [PubMed] [Google Scholar]
- 18.Stipanuk M. H., Londono M., Hirschberger L. L., Hickey C., Thiel D. J., Wang L. Evidence for expression of a single distinct form of mammalian cysteine dioxygenase. Amino Acids. 2004;26:99–106. doi: 10.1007/s00726-003-0001-4. [DOI] [PubMed] [Google Scholar]
- 19.Pfeiffer C. M., Huff D. L., Gunter E. W. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin. Chem. 1999;45:290–292. [PubMed] [Google Scholar]
- 20.Chu F. F., Doyle D. Turnover of plasma membrane proteins in rat hepatoma cells and primary cultures of rat hepatocytes. J. Biol. Chem. 1985;260:3097–3107. [PubMed] [Google Scholar]
- 21.Drake M. R., De La Rosa J., Stipanuk M. H. Metabolism of cysteine in rat hepatocytes. Evidence for cysteinesulphinate-independent pathways. Biochem. J. 1987;244:279–286. doi: 10.1042/bj2440279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de la Rosa J., Drake M. R., Stipanuk M. H. Metabolism of cysteine and cysteinesulfinate in rat and cat hepatocytes. J. Nutr. 1987;117:549–558. doi: 10.1093/jn/117.3.549. [DOI] [PubMed] [Google Scholar]
- 23.de la Rosa J., Stipanuk M. H. Evidence for a rate-limiting role of cysteinesulfinate decarboxylase activity in taurine biosynthesis in vivo. Comp. Biochem. Physiol. B. 1985;81:565–571. doi: 10.1016/0305-0491(85)90367-0. [DOI] [PubMed] [Google Scholar]
- 24.Bagley P. J., Hirschberger L. L., Stipanuk M. H. Evaluation and modification of an assay procedure for cysteine dioxygenase activity: high-performance liquid chromatography method for measurement of cysteine sulfinate and demonstration of physiological relevance of cysteine dioxygenase activity in cysteine catabolism. Anal. Biochem. 1995;227:40–48. doi: 10.1006/abio.1995.1250. [DOI] [PubMed] [Google Scholar]
- 25.Kasai T., Ogo Y., Otobe Y., Kiriyama S. Accumulation of hypotaurine in tissues and urine of rats fed an excess methionine diet. J. Nutr. Sci. Vitaminol. (Tokyo) 1992;38:93–101. doi: 10.3177/jnsv.38.93. [DOI] [PubMed] [Google Scholar]
- 26.Oja S. S., Kontro P. Oxidation of hypotaurine in vitro by mouse liver and brain tissues. Biochim. Biophys. Acta. 1981;677:350–357. doi: 10.1016/0304-4165(81)90246-4. [DOI] [PubMed] [Google Scholar]
- 27.Stipanuk M. H., Rotter M. A. Metabolism of cysteine, cysteinesulfinate and cysteinesulfonate in rats fed adequate and excess levels of sulfur-containing amino acids. J. Nutr. 1984;114:1426–1437. doi: 10.1093/jn/114.8.1426. [DOI] [PubMed] [Google Scholar]
- 28.Coloso R. M., Hirschberger L. L., Dominy J. E., Lee J., Stipanuk M. H. Cysteamine dioxygenase: evidence for the physiological conversion of cysteamine to hypotaurine in rat and mouse tissues. Adv. Exp. Med. Biol. 2006 doi: 10.1007/978-0-387-33504-9_3. in the press. [DOI] [PubMed] [Google Scholar]
- 29.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat. Rev. Mol. Cell. Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 30.Ciechanover A., Ben-Saadon R. N-terminal ubiquitination: more protein substrates join in. Trends Cell. Biol. 2004;14:103–106. doi: 10.1016/j.tcb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Lafaye A., Junot C., Pereira Y., Lagniel G., Tabet J., Ezan E., Labarre J. Combined proteome and metabolite-profiling analyses reveal surprising insights into yeast sulfur metabolism. J. Biol. Chem. 2005;280:24723–24730. doi: 10.1074/jbc.M502285200. [DOI] [PubMed] [Google Scholar]
- 32.Fuachon M., Lagniel G., Aude J. C., Lombardia L., Soularue P., Petat C., Marguerie G., Sentenac A., Werner M., Labarre J. Sulfur sparing in the yeast proteome in response to sulfur demand. Mol. Cell. 2002;9:713–723. doi: 10.1016/s1097-2765(02)00500-2. [DOI] [PubMed] [Google Scholar]
- 33.Nikiforova V., Freitag J., Kempa S., Adamik M., Hesse H., Hoefgen R. Transcriptome analysis of sulfur depletion in Arabidopsis thaliana: interlacing biosynthetic pathways provides response specificity. Plant J. 2003;33:633–650. doi: 10.1046/j.1365-313x.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 34.Hansen J., Johannesen P. F. Cysteine is essential for transcriptional regulation of the sulfur assimilation genes in Saccharomyces cerevisiae. Mol. Gen. Genet. 2000;263:535–542. doi: 10.1007/s004380051199. [DOI] [PubMed] [Google Scholar]
- 35.Ono B.-I., Naito K., Shirahige Y.-I., Yamamoto M. Regulation of cystathionine γ-lyase in Saccharomyces cerevisiae. Yeast. 1991;7:843–848. doi: 10.1002/yea.320070809. [DOI] [PubMed] [Google Scholar]