Abstract
ERK8 (extracellular-signal-regulated protein kinase 8) expressed in Escherichia coli or insect cells was catalytically active and phosphorylated at both residues of the Thr-Glu-Tyr motif. Dephosphorylation of the threonine residue by PP2A (protein serine/threonine phosphatase 2A) decreased ERK8 activity by over 95% in vitro, whereas complete dephosphorylation of the tyrosine residue by PTP1B (protein tyrosine phosphatase 1B) decreased activity by only 15–20%. Wild-type ERK8 expressed in HEK-293 cells was over 100-fold less active than the enzyme expressed in bacteria or insect cells, but activity could be increased by exposure to hydrogen peroxide, by incubation with the protein serine/threonine phosphatase inhibitor okadaic acid, or more weakly by osmotic shock. In unstimulated cells, ERK8 was monophosphorylated at Tyr-177, and exposure to hydrogen peroxide induced the appearance of ERK8 that was dually phosphorylated at both Thr-175 and Tyr-177. IGF-1 (insulin-like growth factor 1), EGF (epidermal growth factor), PMA or anisomycin had little effect on activity. In HEK-293 cells, phosphorylation of the Thr-Glu-Tyr motif of ERK8 was prevented by Ro 318220, a potent inhibitor of ERK8 in vitro. The catalytically inactive mutants ERK8[D154A] and ERK8[K42A] were not phosphorylated in HEK-293 cells or E. coli, whether or not the cells had been incubated with protein phosphatase inhibitors or exposed to hydrogen peroxide. Our results suggest that the activity of ERK8 in transfected HEK-293 cells depends on the relative rates of ERK8 autophosphorylation and dephosphorylation by one or more members of the PPP family of protein serine/threonine phosphatases. The major residue in myelin basic protein phosphorylated by ERK8 (Ser-126) was distinct from that phosphorylated by ERK2 (Thr-97), demonstrating that, although ERK8 is a proline-directed protein kinase, its specificity is distinct from ERK1/ERK2.
Keywords: extracellular-signal-regulated kinase 8 (ERK8), mass spectrometry, mitogen-activated protein kinase (MAPK), oxidative stress, protein phosphatase
Abbreviations: DYRK, dual tyrosine-regulated phosphorylated kinase; EGF, epidermal growth factor; ERK, extracellular-signal-regulated protein kinase; GSK3, glycogen synthase kinase 3; GST, glutathione S-transferase; HA, haemagglutinin; IGF-1, insulin-like growth factor 1; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MBP, myelin basic protein; MKK, MAPK kinase; MKP, MAPK phosphatase; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; PP2A, protein serine/threonine phosphatase 2A; PTP1B, protein tyrosine phosphatase 1B; PTPase, protein tyrosine phosphatase
INTRODUCTION
Many members of the MAPK (mitogen-activated protein kinase) subfamily are components of protein kinase cascades that are activated in response to extracellular signals. Thus ERKs (extracellular-signal-regulated protein kinases) 1, 2 and 5 (ERK1, ERK2 and ERK5) are strongly activated by growth factors and other mitogenic signals, but can also be activated, to some extent, by pro-inflammatory cytokines, pathogenic infection and cellular stresses. In contrast, the p38 MAPKs (p38α, p38β, p38γ and p38δ) and the JNKs (c-Jun N-terminal kinases; JNK1, JNK2 and JNK3) are activated most strongly by pro-inflammatory cytokines, infection and stress. These ten MAPKs are all characterized by the presence of a Thr-Xaa-Tyr motif located in the ‘activation loop’ in subdomain VIII of the catalytic unit. The threonine and tyrosine residues both become phosphorylated when these enzymes are activated by extracellular signals, phosphorylation being catalysed by members of the MKK (MAPK kinase) subfamily. Thus MKK1 and MKK2 phosphorylate ERK1/ERK2, MKK5 phosphorylates ERK5, MKK3 and MKK6 phosphorylate the p38 MAPKs, and MKK4 and MKK7 phosphorylate the JNKs (reviewed in [1]). Components of MAPK cascades have become important targets for therapeutic intervention, and drugs that inhibit MKK1 and p38α MAPK are undergoing human clinical trials for the treatment of colon cancer and rheumatoid arthritis respectively (reviewed in [2]).
The two most recently described MAPK family members, ERK7 and ERK8, resemble ERK1, ERK2 and ERK5 in possessing a Thr-Glu-Tyr motif in the activation loop. ERK7 was originally cloned from a rat cDNA library [3], and a gene encoding a protein that is 99% identical with ERK7 is present in the mouse genome. ERK7 was reported not to be activated by growth factors or cellular stresses, to possess appreciable constitutive activity even in serum-starved cells, and to be capable of phosphorylating and activating itself [4]. The expression of ERK7 appears to be regulated by ubiquitylation and subsequent destruction by the proteasome [5], and it has been reported that ERK7 transfected into human cells can enhance the ubiquitylation and subsequent destruction of the oestrogen receptor α [6]. Although the physiological roles of ERK7 are unknown, it has been reported to interact with CLIC3, a protein showing significant homology with chloride channels [7], and to phosphorylate c-Myc and c-Fos in vitro [3].
ERK8 is encoded by a gene located on human chromosome 8 at q24.3 [8]. Its amino acid sequence resembles ERK7 most closely, and the human genome does not appear to encode any other MAPK that is more similar to ERK7. However, whether ERK8 is simply the human homologue of ERK7 in rodents is unclear because the sequence identity between the human and rodent proteins (69%) is far lower than that observed between other human and rodent MAPKs, which is typically greater than 95%. The activity of ERK8 was reported to be enhanced by co-transfection of COS cells with the protein tyrosine kinase Src and, in contrast with ERK7, ERK8 was reported not to phosphorylate c-Myc and c-Fos [8]. The loss of immunoreactivity towards an anti-ERK7 antibody in human breast cancer cell lines correlates with breast cancer progression [6], raising the possibility that ERK8 may be a tumour suppressor. The physiological functions of ERK8 are unknown.
In the present paper we show that, surprisingly, the activity of ERK8 is largely determined by the phosphorylation of the threonine residue of the Thr-Glu-Tyr motif. Our results suggest that the activity of ERK8 in transfected mammalian cells is a balance between the rate of ERK8 autophosphorylation and dephosphorylation catalysed by one or more members of the PPP family of protein serine/threonine phosphatases. ERK8 activity in HEK-293 cells is increased by exposure to hydrogen peroxide and, to a lesser extent, by osmotic shock. Finally, we demonstrate that ERK8 is a proline-directed protein kinase, with a specificity distinct from that of ERK2.
MATERIALS AND METHODS
Materials
[γ-32P]ATP was obtained from Amersham Biosciences (Little Chalfont, Bucks, U.K.), Ro 318220 was from Calbiochem (Nottingham, U.K.), microcystin-LR was from Dr Linda Lawton (School of Life Sciences, Robert Gordon University, Aberdeen, U.K.), okadaic acid was from Qbiogene-Alexis (Nottingham, U.K.) and N-acetyl-L-cysteine was from Sigma Chemical Co. (Poole, Dorset, U.K.). The sources of other reagents have been described previously [9,10].
DNA Constructs
DNA encoding human ERK8 (AY065978) was amplified from IMAGE EST 5242481 with Expand HiFi polymerase (Roche Molecular Sciences, Lewes, East Sussex, U.K.). The product was cloned into pCR2.1 (Invitrogen, Paisley, U.K.) and the sequence was verified. The derived fragment contained a 50 bp insert, which was removed by standard PCR methods. This fragment was cloned into pEBG2T and pGEX6P-1 (Pharmacia Biotech) to produce pEBG2T ERK8 and pGEX6P-1 ERK8 for expression as a GST (glutathione S-transferase) fusion protein in mammalian cells and Escherichia coli respectively. PCR was used to add an HA (haemagglutinin) tag to the 5′ end of the ERK8 open reading frame, and this fragment was ligated into pCMV5. The T175A, Y177F, D154A and K42A mutations were introduced using the QuikChange® Site-Directed Mutagenesis Kit (Stratagene, Amsterdam, The Netherlands). Constructs for expression in mammalian cells were transformed into E. coli strain DH5α, and DNA was prepared using the Plasmid Mega Kit (Qiagen, Crawley, West Sussex, U.K.) according to the manufacturer's guidelines. The DNA encoding ERK8 was also cloned into the pFASTBAC1 vector, and this vector used to generate His6-tagged ERK8 in insect Sf21 cells.
Protein preparations
pGEX6P-1 ERK8, or the same vector expressing ERK8[T175A], ERK8[Y177F], ERK8[D154A] and ERK8[K42A] mutants, was transformed into E. coli strain BL21 pLys S, and expression was induced with 50 μM IPTG (isopropyl β-thiogalactoside) for 16 h at 26 °C. For expression in mammalian cells, pEBG2T ERK8 was transfected into HEK-293 cells as described below. At 36 h post-transfection, the cells were lysed and the GST fusion proteins were purified by affinity chromatography on glutathione–Sepharose. The baculovirus expressing His6–ERK8 was used to infect insect Sf21 cells, and the expressed protein was purified by affinity chromatography on nickel–nitrilotriacetate–agarose (Qiagen). All ERK8 preparations were dialysed into 50 mM Tris/HCl, pH 7.5, 0.1 mM EGTA, 50% (v/v) glycerol and 0.1% (v/v) 2-mercaptoethanol and stored at −20 °C.
GST–ERK2 was expressed in E. coli, purified and activated as described previously [11]. The PP2A1 (protein serine/threonine phosphatase 2A1) holoenzyme was purified from rabbit skeletal muscle by Dr James Hastie (Division of Signal Transduction Therapy, Dundee, U.K.) or purchased from Upstate Inc. (Dundee, U.K.). PTP1B (protein tyrosine phosphatase 1B) was generously given by Professor David Barford (Institute of Cancer Research, London, U.K.). Bovine MBP (myelin basic protein) was purchased from Invitrogen.
Antibodies
An antibody that recognizes phosphorylated and unphosphorylated ERK8 equally well was generated by injecting sheep with His6-tagged ERK8 and affinity-purified on an ERK8–Sepharose column. It was used for immunoblotting at 0.28 μg/ml. Polyclonal antibody that recognizes the phosphorylated Thr-Glu-Tyr motifs of ERK1 and ERK2 (catalogue number 9101; Lot 16) was purchased from Cell Signaling Technologies (Hitchin, Herts., U.K.). The anti-phosphotyrosine antibody clone 4G10 was from Upstate (Milton Keynes, U.K.), and anti-HA antibody clone 12CA5 was from Roche (Lewes, U.K.). Rabbit anti-sheep IgG, goat anti-rabbit IgG and rabbit anti-mouse IgG peroxidase-conjugated antibodies were from Perbio Science (Tattenhall, Cheshire, U.K.).
Cell culture, cell transfection and cell lysis
HEK-293 cells were cultured at 37 °C in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% foetal bovine serum and 1% (v/v) antibiotic/antimycotic solution. The cells were transfected using either calcium phosphate [12] or poly-ethyleneimine [13] with EBG2T ERK8 to express GST–ERK8 or pCMV5 ERK8 to express HA–ERK8. At 36 h post-transfection, the medium was aspirated, cells were rinsed with ice-cold PBS and then lysed in 50 mM Tris/HCl, pH 7.5, containing 1 mM EDTA, 1 mM EGTA, 1 mM sodium orthovanadate, 10 mM sodium β-glycerophosphate, 5 mM sodium pyrophosphate, 50 mM sodium fluoride, 0.27 M sucrose, 1% (v/v) Triton X-100, 0.1% (v/v) 2-mercaptoethanol and Complete™ proteinase inhibitor cocktail (Roche; one tablet per 50 ml). Lysates were centrifuged at 13000 g for 10 min at 4 °C, and the supernatants (termed ‘cell extract’) were removed, frozen in liquid nitrogen and stored at −20 °C until use.
Assay of ERK8
ERK8 preparations or ERK8 immunoprecipitated from cell extracts were assayed at 30 °C in 50 μl reaction mixtures containing 50 mM Tris/HCl, pH 7.5, 0.1 mM EGTA, 10 mM magnesium acetate, 0.1% (v/v) 2-mercaptoethanol, 0.1 mM sodium orthovanadate, 0.33 mg/ml MBP and 0.1 mM [γ-32P]ATP (106 c.p.m./nmol). After 10 min, the reaction was stopped and incorporation of phosphate into MBP was measured by spotting 40 μl aliquots on to phosphocellulose P81 paper, followed by washing with 75 mM orthophosphoric acid to remove [γ-32P]ATP, drying and Cerenkov counting. One unit of ERK8 activity was that amount which catalysed the phosphorylation of 1 nmol of MBP in the standard assay.
Reversible activation/inactivation of ERK8
ERK8 preparations from E. coli or Sf21 cells (typically 10 μg/ml) were incubated at 30 °C with or without PP2A1 (10 units/ml) and/or PTP1B (50 μg/ml) in 50 mM Tris/HCl, pH 7.5, containing 0.1 mM EGTA and 0.1% (v/v) 2-mercaptoethanol. Phosphatase-treated ERK8 was reactivated by incubation at 30 °C in the same buffer containing 10 mM magnesium acetate, 0.1 mM unlabelled ATP and the phosphatase inhibitors sodium orthovanadate (0.1 mM) or microcystin-LR (0.1 μM).
Mass spectrometry
ERK8 samples were separated by SDS/PAGE, stained with colloidal Coomassie Blue and digested with trypsin [14]. The tryptic digests were dissolved in 0.2 ml of 1% formic acid/4 mM EDTA in water, and 0.02 ml aliquots were analysed by LC–MS with precursor 79 scanning on a 4000 Q-TRAP system [14,15]. Extracted ion chromatograms from precursor ion scans were generated using Analyst 1.4.1 software (MDS-Sciex, Canada). The sequences of the peptides and sites of phosphorylation were confirmed both by database searching using Mascot v2.1(Matrix-Science, U.K.) run on a local server, and by manual interpretation of the MS/MS spectra.
RESULTS
Characterization of recombinant ERK8
ERK8 expressed in E. coli as a GST fusion protein or in insect Sf21 cells as a His6-tagged protein had similar specific activities towards MBP of approx. 120 units/mg. Since the genome of E. coli does not encode any MAPKs or conventional MKKs, it is unlikely that an activator of ERK8 is present in these bacteria. Therefore the finding that ERK8 was constitutively active suggested that activity was generated by ERK8 itself via an autophosphorylation reaction.
It is well established that the activation of ERK1 and ERK2 requires their phosphorylation at both a threonine and a tyrosine residue located in a Thr-Glu-Tyr motif in the ‘activation loop’ of the catalytic domain. Since ERK8 also contains a Thr-Glu-Tyr motif at the equivalent position, we investigated whether its activation also resulted from the phosphorylation of these residues. ERK8 expressed in E. coli or insect Sf21 cells was recognized by a polyclonal phosphospecific antibody from Cell Signaling Technologies that recognizes the phosphorylated Thr-Glu-Tyr motif of ERK1 and ERK2. ERK8 was also recognized by an anti-phosphotyrosine antibody. In contrast, the catalytically inactive mutant ERK8[D154A] was not recognized by either antibody (Figure 1A), again indicating that ERK8 activity was generated by autophosphorylation.
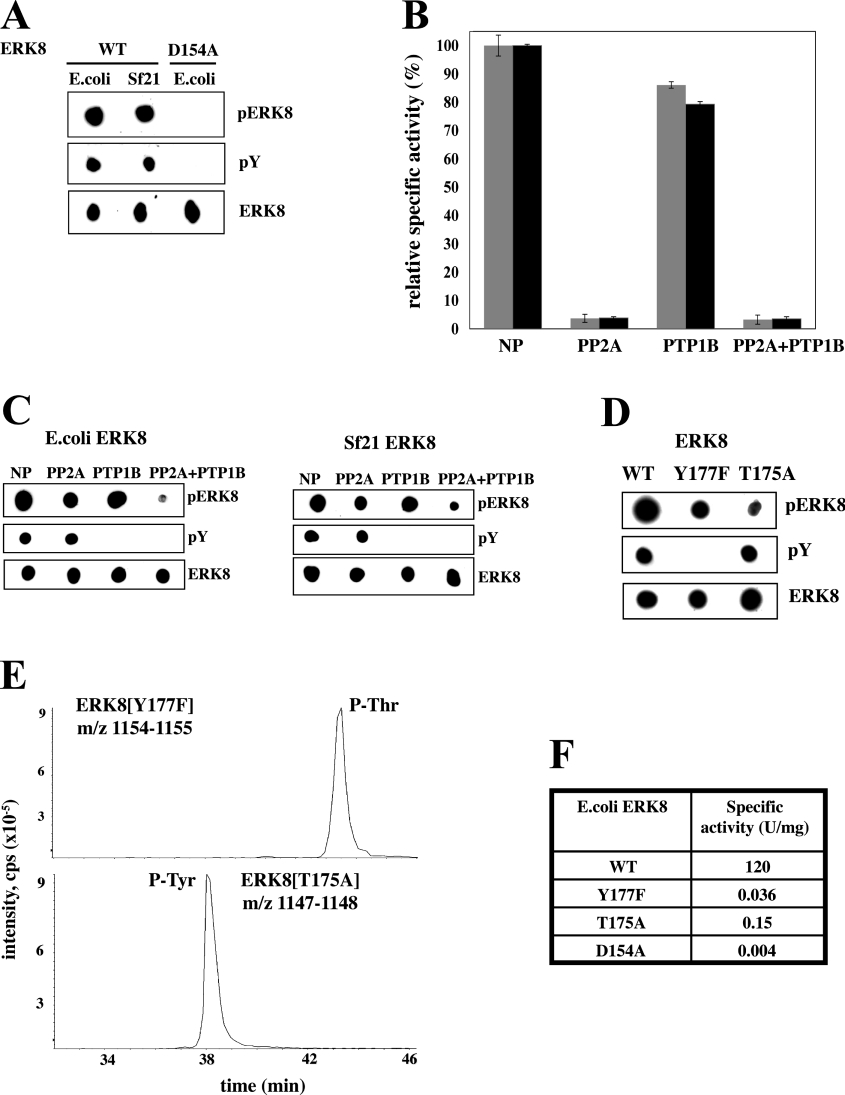
Figure 1. State of phosphorylation of the activation loop of ERK8 and effect of phosphatases.
Wild-type (WT) ERK8, and the catalytically inactive D154A mutant were expressed as GST fusions in E. coli and His6-tagged WT ERK8 was expressed in Sf21 cells. (A) The purified proteins (100 ng) were spotted on to nitrocellulose membranes and immunoblotted using the ECL® detection system (Amersham Pharmacia Biotech, Little Chalfont, U.K.). The antibodies used were a phosphospecific antibody that recognizes the phosphorylated Thr-Glu-Tyr motif of ERK1 and ERK2, but also recognizes the same region in ERK8 (pERK8), an anti-phosphotyrosine antibody (pY), and an antibody that recognizes phosphorylated and unphosphorylated ERK8 equally well (ERK8). (B) Wild-type GST–ERK8 (grey bars) or His6–ERK8 (black bars) were incubated with PP2A, PTP1B, both phosphatases or in the absence of any phosphatase (NP; no phosphatase) as described in the Materials and methods section. Aliquots of each reaction were then assayed for ERK8 activity using MBP as a substrate. (C) Further aliquots of the incubations from (B) were spotted on to nitrocellulose membranes and immunoblotted with the antibodies used in (A). (D) The experiment was carried out as in (A), except that the ERK8[T175A] and the ERK8[Y177F] mutants were used, as well as the wild-type enzyme. (E) MS analysis of tryptic digests of ERK8[Y177F] and ERK8[T175A] showing the extracted ion chromatograms for the doubly charged ([M−2H]2−) tryptic phosphopeptide ions. Upper panel: the peptide comprising Ser-161–Arg-182 of ERK8[Y177F] (m/z=1154–1155); lower panel: the peptide comprising Ser-161–Arg-182 of ERK8[T175A] (m/z=1147–1148). (F) The specific activities of wild-type ERK8, ERK8[Y177F], ERK8[T175A] and ERK8[D154A] were determined using MBP as substrate. Protein concentrations were determined by the method of Bradford.
ERK1 and ERK2 are inactivated by treatment with either a PTPase (protein tyrosine phosphatase) or a protein serine/threonine phosphatase, demonstrating that phosphorylation of both residues in the Thr-Glu-Tyr motif is required to generate significant activity. As expected, the activity of ERK8 expressed in E. coli or insect Sf21 cells was decreased by 95% after treatment with the serine/threonine-specific phosphatase PP2A, but, surprisingly, treatment with the tyrosine-specific phosphatase PTP1B only decreased activity by 15–20% (Figure 1B). This was not explained by an inability of PTP1B to dephosphorylate ERK8, because PTP1B-treated ERK8 was no longer recognized by the anti-phosphotyrosine antibody (Figure 1C). These results indicate that phosphorylation of the threonine residue is the major determinant of ERK8 activity, the phosphorylation of the tyrosine apparently only having a minor effect.
Interestingly, ERK8 inactivated by PP2A or PTP1B, in which the phosphothreonine or phosphotyrosine residue had been dephosphorylated respectively, were still recognized by the antibody against the phosphorylated Thr-Glu-Tyr motif (Figure 1C), indicating that this particular polyclonal antibody recognizes ERK8 when it is phosphorylated at either residue. This was confirmed by the finding that the ERK8[T175A] and ERK8[Y177F] mutants in which the threonine or tyrosine of the Thr-Glu-Tyr motif were changed to a non-phosphorylatable residue were still recognized by the same phosphospecific antibody (Figure 1D). MS analysis confirmed that the T175A mutant was still heavily phosphorylated at Tyr-177, whereas the Y177F mutant was heavily phosphorylated at Thr-175 (Figure 1E). However, these monophosphorylated mutants both had less than 0.1% of the activity of the wild-type enzyme towards MBP (Figure 1F).
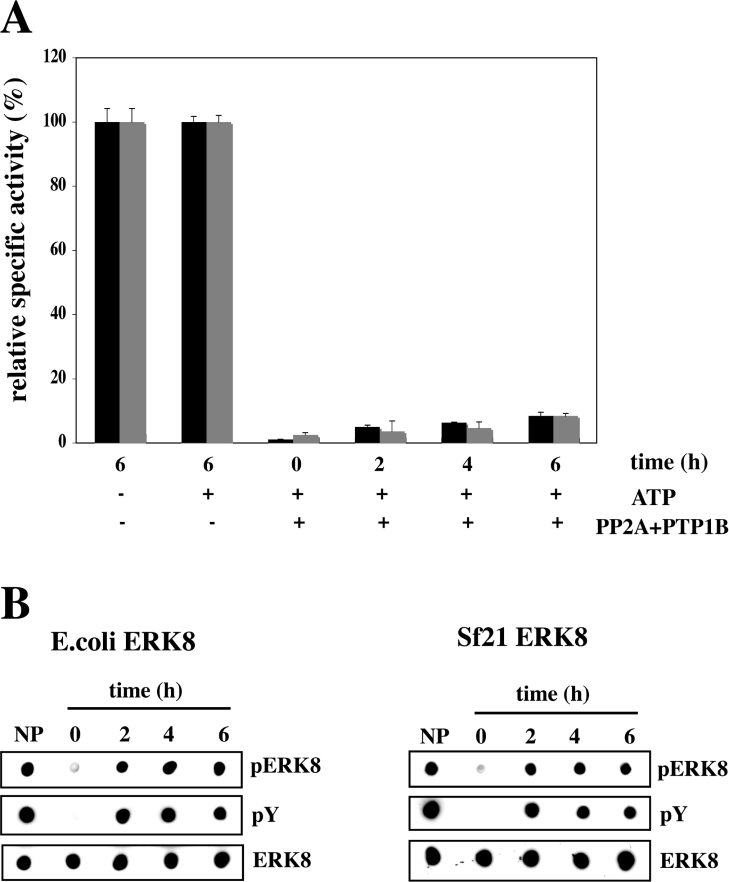
To investigate whether ERK8 can autoactivate in vitro, we incubated the bacterially expressed and the insect cell-expressed enzymes with PP2A plus PTP1B (Figure 2A), then inhibited the PP2A and PTP1B with microcystin-LR and sodium orthovanadate respectively, followed by incubation with MgATP. Under these conditions, ERK8 from either E. coli or insect Sf21 cells was only capable of slight reactivation (Figure 2A). However, reactivation was accompanied by substantial rephosphorylation of the Thr-Glu-Tyr motif, as judged by immunoblotting with the anti-phospho-ERK1/ERK2 and anti-phosphotyrosine antibodies (Figure 2B). The activity of ERK8, which had not been phosphatase-treated, was unaffected by incubation with MgATP (Figure 2A).
Figure 2. Slight reactivation of phosphatase-treated ERK8 after incubation with MgATP.
(A) Wild-type GST–ERK8 (grey bars) or His6–ERK8 (black bars) were incubated in the absence or presence of PP2A plus PTP1B as in Figure 1(B), and the phosphatases were then inhibited by the addition of the PP2A inhibitor microcystin–LR (0.1 μM) and the PTP1B inhibitor sodium orthovanadate (0.1 mM). The untreated or inactivated ERK8 were then incubated with MgATP, and aliquots of the reactions were removed at the times indicated and assayed for activity using MBP as a substrate. (B) Further aliquots from (A) were spotted on to nitrocellulose membranes and immunoblotted as in Figure 1. NP, no phosphatase; ERK8 not pre-treated with PP2A plus PTP1B and incubated for 6 h without MgATP.
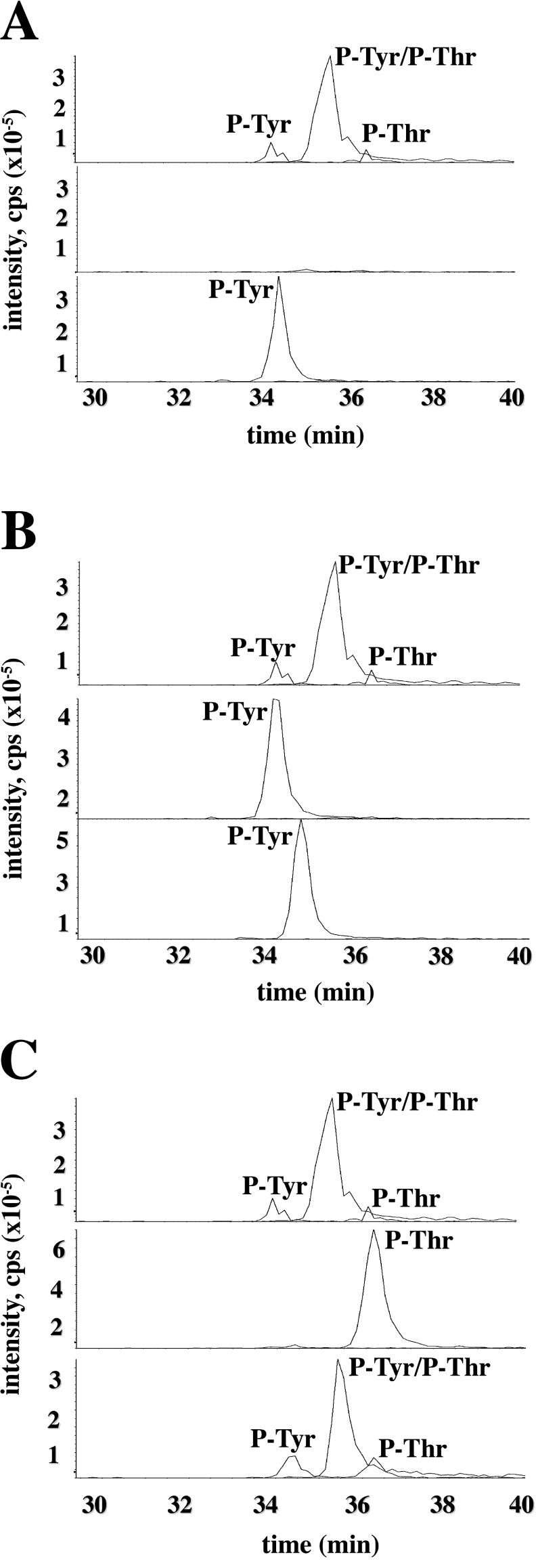
We next examined the dephosphorylation and rephosphorylation of bacterially expressed ERK8 in greater detail using MS. Tryptic digestion followed by precursor ion scanning analysis showed that bacterially expressed ERK8 was isolated predominantly as the diphosphorylated form phosphorylated at both residues of the Thr-Glu-Tyr motif, with small amounts of each of the monophosphorylated species (Figure 3A, top panel). Following incubation with PP2A plus PTP1B, ERK8 was completely dephosphorylated (Figure 3A, middle panel) and subsequent incubation with MgATP led to rephosphorylation of the tyrosine residue (Figure 3A, bottom panel). Interestingly, however, rephosphorylation of the threonine residue was not detected. Consistent with this finding, treatment with PP2A alone to generate the tyrosine monophosphorylated species (Figure 3B, middle panel), followed by incubation with MgATP, also did not lead to detectable rephosphorylation of the threonine residue (Figure 3B, bottom panel). On the other hand, incubation with PTP1B alone to generate ERK8 that was monophosphorylated on the threonine residue (Figure 3C, middle panel), followed by incubation with MgATP, led to reformation of the dually phosphorylated species (Figure 3C, bottom panel). These results are considered further in the Discussion. It should also be noted that it was critical to include EDTA in the samples to avoid losing the diphosphorylated peptides during chromatography on the LC–MS column.
Figure 3. MS analysis of bacterially expressed ERK8.
Tryptic digests of GST–ERK8 from untreated (A–C, top panels), ERK8 incubated with PP2A and PTP1B (A, middle panel), PP2A alone (B, middle panel) or PTP1B alone (C, middle panel) and from phosphatase-treated ERK8 that had been rephosphorylated by incubation for 6 h with 10 mM magnesium acetate and 0.1 mM ATP (A–C, bottom panels) were analysed by LC–MS with precursor 79 scanning. Shown is the relevant section of the extracted ion chromatograms for the doubly charged peptide ions ([M−2H]2−) representing the singly phosphorylated (m/z=1162–1163) and doubly phosphorylated (m/z=1202–1203) peptide comprising residues Ser-161–Arg-182.
Effects of different agonists and inhibitors on the phosphorylation of ERK8 in HEK-293 cells
In order to study the regulation of ERK8 activity in mammalian cells, we initially expressed it as a GST fusion protein in HEK-293 cells. The cells were lysed in the presence of protein phosphatase inhibitors and, following affinity purification on glutathione–Sepharose in the continued presence of phosphatase inhibitors, the purified ERK8 was found to have an extremely low activity, approx. 100-fold lower than that of the protein expressed in E. coli or Sf21 cells. This suggested that the autoactivation of ERK8 was effectively counteracted by protein phosphatase activities present in mammalian cells.
We next transfected HEK-293 cells with wild-type HA-tagged ERK8 or two catalytically inactive mutants, HA–ERK8[D154A] (Figure 4A) and HA–ERK8[K42A] (results not shown). The activation loop of wild-type HA–ERK8 was phosphorylated as expected, but neither the HA–ERK8[D154A] (Figure 4A) nor HA–ERK8[K42A] mutants were phosphorylated at the Thr-Glu-Tyr motif before or after stimulation with any agonist (results not shown). This suggested that the phosphorylation of the activation loop of ERK8 was an autophosphorylation event in HEK-293 cells, as was the case for the protein expressed in bacteria or insect cells.
Figure 4. Effect of hydrogen peroxide and Ro 318220 on the phosphorylation of ERK8.
(A) HEK-293 cells were transfected with vectors expressing either wild-type (WT) HA–ERK8 or HA–ERK8[D154A] as described in the Materials and methods section. At 36 h post-transfection, the cells were exposed for 10 or 30 min to 1 mM hydrogen peroxide, and lysed. The lysates (60 μg) were denatured in SDS, subjected to SDS/PAGE and, after transfer to PVDF membranes, immunoblotted with the phosphospecific antibody that recognizes the phosphorylated Thr-Glu-Tyr motifs of ERK1, ERK2 and ERK8, and an antibody that recognizes phosphorylated and unphosphorylated ERK8 equally well. (B) HA–ERK8 in 30 mg of cell lysate protein was immunoprecipitated from HEK-293 cells exposed to hydrogen peroxide (lower panel) or left unstimulated (upper panel) was digested with trypsin and analysed by LC–MS. The extracted ion chromatograms for the doubly charged peptide ions ([M−2H]2−) representing the singly phosphorylated (m/z=1162–1163) and doubly phosphorylated (m/z=1202–1203) forms of the peptide comprising Ser-161–Arg-182 are shown. (C) HEK-293 cells transfected with HA–ERK8 as in (A) were incubated for 1 h with the indicated concentrations of Ro 318220, then exposed for 10 or 30 min to 1 mM hydrogen peroxide and lysed. Cell lysate (45 μg) was then analysed as in (A). (D) Wild-type GST–ERK8 and GST–ERK2 (both from E. coli) were assayed in vitro using MBP as substrate at the indicated concentrations of Ro 318220.
The HEK-293 cells transfected with wild type HA–ERK8 were then stimulated with IGF-1 (insulin-like growth factor-1), EGF (epidermal growth factor) or PMA, or exposed to an osmotic shock (0.5 M sorbitol), the protein synthesis inhibitor anisomycin (10 μg/ml) or 1 mM hydrogen peroxide. Under the conditions used in Figure 4(A), the only agonist that reproducibly stimulated the phosphorylation of wild-type HA–ERK8 was hydrogen peroxide. The effect of hydrogen peroxide was maximal after 10 min and was sustained for 30 min (Figure 4A). Analysis of tryptic digests of the immunoprecipitated ERK8 showed the presence of the monophosphorylated species phosphorylated only at the tyrosine residue of the Thr-Glu-Tyr motif in unstimulated cells (Figure 4B, upper panel). Exposure of the cells to hydrogen peroxide led to the appearance of both the diphosphorylated species as well as a monophosphorylated species phosphorylated only at Thr-175 (Figure 4B, lower panel).
The endogenous ERK1 and ERK2 in the same extracts also became phosphorylated at their Thr-Glu-Tyr motifs in response to hydrogen peroxide, but more slowly than ERK8 (Figure 4A). Interestingly, the basal level of phosphorylation of ERK1/ERK2 in serum-grown HEK-293 cells was consistently suppressed by expression of the vector encoding wild-type ERK8, but not by a vector encoding the catalytically inactive ERK8[D154A] mutant (Figure 4A).
The basal or hydrogen peroxide-induced phosphorylation of ERK8 was unaffected by incubating the cells with PD 184352 (2 μM), SB 203580 (10 μM) or wortmannin (100 nM), alone or in combination, which are relatively specific inhibitors of MKK1/MKK2 (the upstream activators of ERK1/ERK2), p38α/p38β MAPKs and PI3K (phosphoinositide 3-kinase) respectively. In contrast, these inhibitors blocked the hydrogen peroxide-induced phosphorylation of ERK1 and ERK2, the phosphorylation of MAPKAP-K2 (MAPK-activated protein kinase 2) at Thr-334 (a target of p38α MAPK) and the phosphorylation of protein kinase B at Ser-473 (a downstream target of the PI3K pathway) respectively, in the same extracts (results not shown).
The phosphorylation of ERK8 has been reported to increase to a small extent in COS cells when co-transfected with a vector expressing the protein tyrosine kinase Src [8]. However, we were unable to suppress the phosphorylation of ERK8 in HEK-293 cells using the Src family inhibitor PP2 at 10 μM, a concentration that completely blocks the phosphorylation of physiological substrates of Src family members in cells (results not shown).
Interestingly, phosphorylation of the Thr-Glu-Tyr motif of ERK8 in unstimulated cells or in cells exposed to hydrogen peroxide was completely suppressed by the indoyl bismaleimide Ro 318220 (Figure 4C), originally developed as an inhibitor of PKC (protein kinase C). This initially suggested that ERK8 might be phosphorylated by a distinct Ro 318220-sensitive kinase, since the activities of ERK2 and other MAPKs, such as JNK1 and p38 MAPK isoforms, are unaffected by this compound [11]. However, surprisingly, we found that ERK8 is inhibited by Ro 318220 exceptionally potently in vitro, with an IC50 of 5–10 nM (Figure 4D), similar to the concentration that inhibits PKC. Therefore the simplest interpretation of this result is that Ro 318220 is exerting its effect by inhibiting the autophosphorylation of ERK8, and not by inhibiting an ‘upstream’ kinase. The finding that ERK8 is not phosphorylated at the Thr-Glu-Tyr motif in HEK-293 cells expressing catalytically inactive ERK8 (Figure 4A) is consistent with this view.
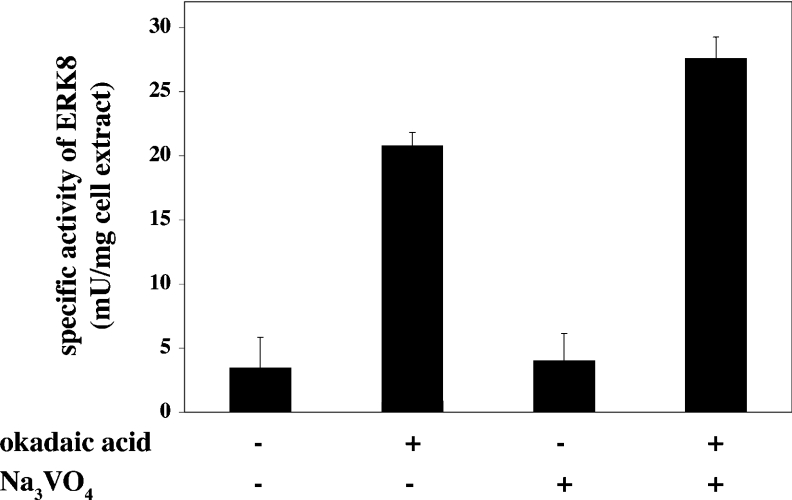
The results presented in Figure 4 suggest that the activity of ERK8 in HEK-293 cells is a balance between the autophosphorylating activity of ERK8 and one or more ERK8 phosphatases. In order to test this hypothesis we therefore exposed cells to okadaic acid, a cell-permeant specific inhibitor of most members of the PPP family of protein serine/threonine phosphatases, and/or sodium orthovanadate, an inhibitor of both PTPases and the structurally related ‘dual-specificity’ MKPs (MAPK phosphatases). Exposure of cells to okadaic acid induced a 5-fold increase in the activity of ERK8, whereas orthovanadate only had a minor effect on activity. A slightly higher activity was observed in cells exposed to both okadaic acid and orthovanadate (Figure 5).
Figure 5. Effect of phosphatase inhibitors on the activity of ERK8 in HEK-293 cells.
The cells were transfected with a vector encoding wild-type HA–ERK8 as in Figure 4(A). At 36 h post-transfection, the cells were incubated for 1 h with or without 1 μM okadaic acid, 1 mM sodium orthovanadate (Na3VO4), or both phosphatase inhibitors, and lysed. ERK8 was immunoprecipitated from cell extracts using an anti-HA antibody and assayed for activity using MBP as a substrate.
Effect of antioxidant on the activity of ERK8 in HEK-293 cells
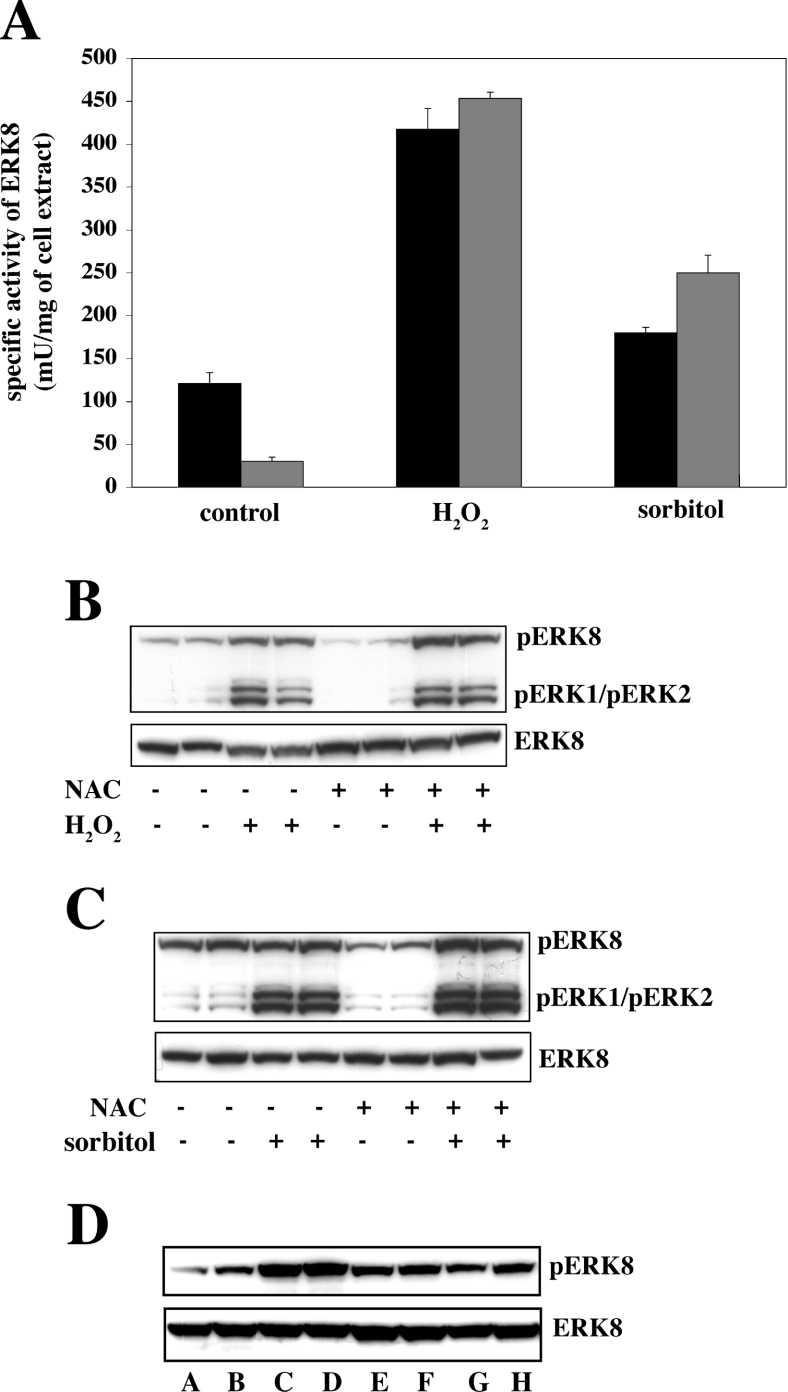
Since hydrogen peroxide is an oxidative stress, it was of interest to examine the effect of the antioxidant N-acetyl-L-cysteine on the activity of ERK8 in cells. Interestingly, incubation of cells with this compound reduced the basal level of ERK8 activity (Figure 6A) and phosphorylation of the Thr-Glu-Tyr motif (Figure 6B) considerably, without affecting subsequent activation by hydrogen peroxide (Figures 6A and 6B). After lowering the basal level of ERK8 activity with N-acetyl-L-cysteine, activation of ERK8 by osmotic shock could now be detected, which was not significant in cells not treated with antioxidant (Figures 6A and 6C). The hydrogen peroxide-induced phosphorylation of ERK8 was reversible, because washing the cells free of hydrogen peroxide followed by incubation for 30 min led to some dephosphorylation (Figure 6D). ERK8 could not be activated by IGF-1, EGF or anisomycin, even in cells pre-treated with antioxidant (results not shown).
Figure 6. Effect of N-acetyl-L-cysteine on the activity of ERK8 and its phosphorylation at the Thr-Glu-Tyr motif in response to hydrogen peroxide or osmotic shock in HEK293 cells.
(A) The cells were transfected with a vector encoding wild-type HA–ERK8 as in Figure 4(A) and at 36 h post-transfection incubated for 15 min with (grey bars) or without (black bars) 10 mM N-acetyl-L-cysteine (NAC). The cell media were aspirated, the cells were washed at 21 °C with PBS and fresh media was added. Cells were then exposed for 10 min to either 1 mM hydrogen peroxide or 0.5 M sorbitol and lysed. ERK8 was immunoprecipitated from cell extracts using an anti-HA antibody and its activity was measured using MBP as substrate. (B and C) The cell lysates from (A) were analysed by immunoblotting, as in Figure 4(A). (D) The cells were first transfected as in (A). At 36 h post-transfection they were then incubated for 10 min without (lanes A and B) or with (lanes C–H) 1 mM hydrogen peroxide. Either the cells were lysed (lanes A–D) or the culture medium was aspirated and the cells washed at 21 °C with PBS and fresh media was added (lanes E–H). The cells were then incubated for 30 min without (lanes E and F) or with (lanes G and H) 10 mM N-acetyl-L-cysteine.
ERK8 has a substrate specificity distinct from ERK2
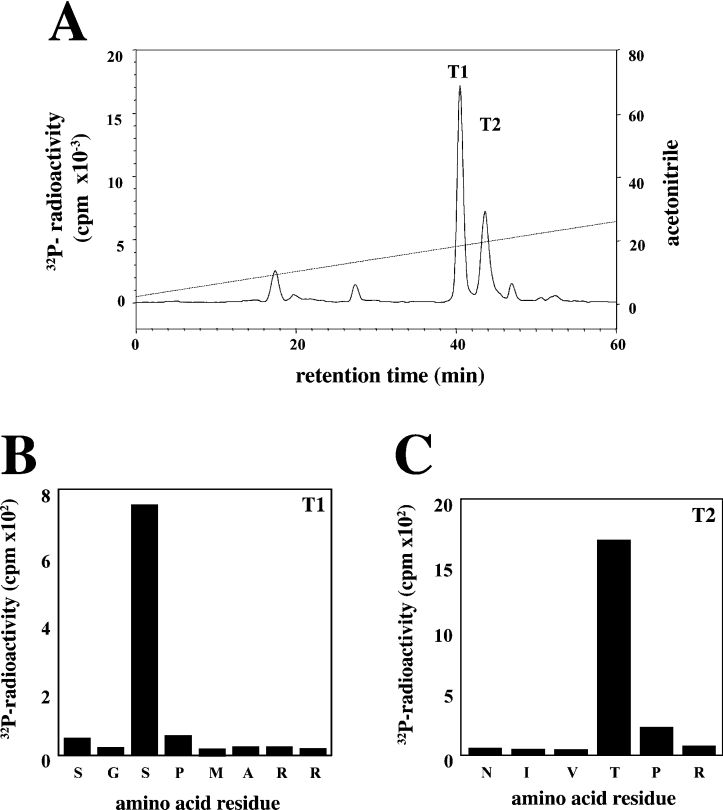
MBP was maximally phosphorylated by incubation with MgATP and either ERK8 or ERK2. The phosphorylated MBP was digested with trypsin, and the resulting phosphopeptides were separated by chromatography on a C18 column. The major phosphopeptide T1 and the minor phosphopeptide T2 phosphorylated by ERK8 were subjected to MS (results not shown), Edman and solid-phase sequencing. These analyses revealed that peptide T1 comprised residues 124–131 of MBP phosphorylated at Ser-126, whereas T2 comprised residues 91–96 of MBP phosphorylated at Thr-94 (Figure 7). In contrast, the major residue phosphorylated by ERK2 was Thr-97, while Thr-94 was phosphorylated less extensively (results not shown), as reported previously by others [16].
Figure 7. Identification of the sites on MBP phosphorylated by ERK8.
Bovine MBP (0.33 mg/ml) was phosphorylated by incubation for 10 min at 30 °C with Mg[γ-32P]ATP (106 c.p.m./nmol) and ERK8 (2 units/ml). Reactions were denatured in lauryl dodecyl sulphate and subjected to SDS/PAGE. After staining with Coomassie Blue, the MBP was excised from the gel, digested with trypsin and the resulting peptides were chromatographed on a Vydac C18 column equilibrated in 0.1% (v/v) trifluoroacetic acid (A). The acetonitrile gradient is indicated by the diagonal line. Peptides T1 (B), T2 (C) from A were subjected to Edman degradation to determine the amino acid sequence, and to solid phase sequencing to identify the sites of phosphorylation, as in [24].
Identification of additional phosphorylation sites on ERK8
During the MS analysis of bacterially expressed ERK8, we observed that ERK8 was phosphorylated not only at the Thr-Glu-Tyr motif, but also at Ser-192, Ser-331, Thr-352, Ser-362, Ser-378 and Thr-380 (results not shown). These residues were not phosphorylated in preparations of catalytically inactive ERK8 mutants, indicating that they arise from autophosphorylation. Residues Ser-362 and Thr-380 are followed by proline, but the other four residues are not, indicating that ERK8 is intrinsically capable of phosphorylating residues that lie in sequences distinct from Ser-Pro and Thr-Pro.
DISCUSSION
The activation of many MAPK family members, such as ERK1, ERK2 and p38 MAPKs, requires their phosphorylation on both the threonine and the tyrosine residues of the Thr-Xaa-Tyr motif present in the ‘activation loops’ of these enzymes. In the case of ERK1/ERK2, the phosphorylation of both residues can be catalysed by either MKK1 or MKK2, whereas, for p38 MAPKs, phosphorylation is catalysed by MKK3 or MKK6. However, not all MAPK family members are regulated in this way. For example, JNK isoforms are phosphorylated at the threonine residue of the Thr-Xaa-Tyr motif by MKK7, and phosphorylated preferentially at the tyrosine residue by MKK4 [17,18]. For this reason, and because phosphorylation of the threonine residue of the JNK3 isoform by MKK7 accelerates the rate at which MKK4 phosphorylates the tyrosine residue [19], the two MKKs activate JNK isoforms synergistically. The phosphorylation of JNK isoforms at the threonine residue by MKK7 is sufficient to generate 10–15% of the activity that is attained when both the threonine and the tyrosine residues are phosphorylated [17,18]. Similar to MKK7, MKK5 phosphorylates the threonine of the Thr-Xaa-Tyr motif of ERK5 preferentially, and phosphorylation of this residue generates 10% of the activity towards MBP observed with the dually phosphorylated kinase. Moreover, ERK5 phosphorylated at only the threonine residue autophosphorylates at a similar rate to the dually phosphorylated enzyme [9]. In the present study, we have demonstrated that phosphorylation of the threonine of the Thr-Glu-Tyr motif was the major determinant of ERK8 activity towards an exogenous substrate (MBP), while phosphorylation of the tyrosine had only a minor effect (Figures 1B and 4B).
ERK8 expressed in bacteria was active and largely present as the diphosphorylated species, phosphorylated at both residues of the Thr-Glu-Tyr motif (Figure 3A, top panel), but catalytically inactive ERK8 was not phosphorylated at either residue (Figure 1A), indicating that in bacteria the phosphorylation of ERK8 was catalysed by ERK8 itself. However, when bacterially expressed ERK8 was dephosphorylated, it was only capable of rephosphorylation at the tyrosine and not the threonine residue in vitro. Interestingly, mutation of either the threonine or the tyrosine of the Thr-Glu-Tyr motif to alanine or phenylalanine respectively decreased by more than 99.9% the activity of bacterially expressed ERK8 towards MBP (Figure 1F), despite the fact that Thr-175 or Tyr-177 were still heavily phosphorylated in the ERK8[Y177F] and ERK8[T175A] mutants, respectively (Figure 1E). Since bacterially expressed wild-type ERK8 phosphorylated at Thr-175 alone was almost fully active (Figure 1B), but ERK8[Y177F] phosphorylated at Thr-175 was essentially inactive, these observations suggest that ERK8 may need to be phosphorylated on both the threonine and the tyrosine residue of the Thr-Glu-Tyr motif to lock the enzyme into a conformation capable of phosphorylating exogenous substrates in vitro and in vivo.
The observations we have made with ERK8 are reminiscent of those described recently by Cleghon and co-workers [20] with DYRK (dual tyrosine-regulated phosphorylated kinase) 1A. DYRK1A, like ERK8 and GSK3 (glycogen synthase kinase 3) [21], autophosphorylates the activation loop tyrosine residue in vivo, yet only phosphorylates exogenous substrates on serine and threonine residues. These investigators presented evidence that the autophosphorylation of DYRK1A at tyrosine was catalysed by a transitional intermediate, only formed transiently while the enzyme was being synthesized, and that tyrosine phosphorylation was essential to trigger conversion into the mature conformation that phosphorylates exogenous substrates. For this reason, the mature form of DYRK1A (and probably GSK3) was unable to rephosphorylate the tyrosine residue to a significant extent after it had been dephosphorylated. The situation we have found with ERK8 appears to be analogous except that, after dephosphorylation of the purified enzyme in vitro, it is the threonine and not the tyrosine of the Thr-Glu-Tyr motif that cannot be rephosphorylated. It is possible that the presence of a molecular chaperone(s) is needed to induce the conformation that is capable of autophosphorylating Thr-175 in vivo and that the dual phosphorylation of the Thr-Glu-Tyr motif is required to induce the mature conformation capable of phosphorylating exogenous substrates at serine and threonine residues.
Several lines of evidence suggest that the activation of ERK8 in mammalian cells, as in E. coli, is catalysed by ERK8 itself, rather than by a distinct MKK(s). First, two different catalytically inactive mutants (ERK8[D154A] and ERK8[K42A]) were not phosphorylated at the Thr-Glu-Tyr motif (Figure 4A). Secondly, Ro 318220, a potent inhibitor of ERK8 (Figure 4D) that does not inhibit MKK1, ERK1, ERK2, p38 MAPKs or JNK1 [11], prevented both the basal and hydrogen peroxide-induced phosphorylation of the Thr-Glu-Tyr motif of ERK8 in HEK-293 cells (Figure 4C). Thirdly, we were unable to induce any phosphorylation of the Thr-Glu-Tyr motif of catalytically inactive ERK8 mutants when transfected HEK-293 cells were stimulated with hydrogen peroxide (Figure 4A) or osmotic shock, EGF, IGF-1, PMA or anisomycin (results not shown).
When overexpressed in HEK-293 cells, ERK8 had a far lower specific activity than ERK8 expressed in E. coli and insect Sf21 cells, presumably because it was phosphorylated at the tyrosine and not the threonine residue (Figure 4B). The low basal activity of ERK8 in unstimulated cells could be reduced further by an antioxidant, suggesting that this treatment reduces the basal level of tyrosine phosphorylation, because the cysteine residue required for the activity of PTPases is known to be extremely sensitive to oxidation [22]. The lack of threonine phosphorylation in unstimulated cells and the induction of threonine phosphorylation and activation upon treatment with hydrogen peroxide (Figure 4B) or okadaic acid (Figure 5), a specific inhibitor of the PPP family of protein serine/threonine phosphatases [23], implies that one or more members of the PPP family protein phosphatases is responsible for suppressing the activity of transfected ERK8 in HEK-293 cells. In contrast with okadaic acid, sodium orthovanadate, which inhibits the distinct family of protein phosphatases that includes conventional PTPases and the ‘dual specificity’ MKPs, had only a minor effect on ERK8 activity (Figure 5), perhaps because the tyrosine residue was already nearly maximally phosphorylated.
In summary, our results indicate that the activity of ERK8 in mammalian cells is determined by the relative rates of ERK8 autophosphorylation and its dephosphorylation at Thr-175 catalysed by one or more members of the PPP family of protein phosphatases. It will clearly be important to identify this (these) protein phosphatase(s), and to investigate whether the activity of this phosphatase(s) is decreased when cells are exposed to agonists that activate ERK8. Alternatively, or in addition, agonists that activate ERK8 may increase the ability of ERK8 to autoactivate by a mechanism that has yet to be identified. The role of tyrosine phosphorylation in the regulation of ERK8 activity is unclear but, as discussed earlier, it may be required to induce the mature conformation of the enzyme.
Acknowledgments
We thank the Protein Production and Antibody Purification Teams (co-ordinated by Dr Hilary McLauchlan and Dr James Hastie) in the Division of Signal Transduction Therapy (School of Life Sciences, University of Dundee) for ERK2, His6–ERK8, PP2A and the anti-ERK8 antibody. DNA sequencing was performed by The Sequencing Service (School of Life Sciences, University of Dundee, Scotland; www.dnaseq.co.uk). The MS facilities used in this study are part of the RASOR project (http://www.gla.ac.uk/rasor/) funded by the IRColl for proteomics [BBSRC (Biotechnology and Biological Sciences Research Council)/EPSRC (Engineering and Physical Sciences Research Council)]. This study was supported by the UK Medical Research Council, The Royal Society, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck and Co., Merck KGaA and Pfizer.
References
- 1.Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353-361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- 2.Cohen P. Protein kinases – the major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002;1:309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 3.Abe M. K., Kuo W. L., Hershenon M. C., Rosner M. R. Extracellular signal-regulated kinase (ERK7), a novel ERK with a C-terminal domain that regulates its activity, its cellular localisation, and cell growth. Mol. Cell. Biol. 1999;19:1301–1312. doi: 10.1128/mcb.19.2.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abe M. K., Kahle K. T., Saelzer M. P., Orth K., Dixon J. E., Rosner M. R. ERK7 is an autoactivated member of the MAPK family. J. Biol. Chem. 2001;276:21272–21279. doi: 10.1074/jbc.M100026200. [DOI] [PubMed] [Google Scholar]
- 5.Kuo W. L., Duke C. J., Abe M. K., Kaplan E. L., Gomes S., Rosner M. R. ERK7 expression and kinase activity is regulated by the ubiquitin–proteasome pathway. J. Biol. Chem. 2004;279:23073–23081. doi: 10.1074/jbc.M313696200. [DOI] [PubMed] [Google Scholar]
- 6.Henrich L. M., Smith J. A., Kitt D., Errington T. M., Nguyen B., Traish A. M., Lannigan D. A. Extracellular signal-regulated kinase 7, a regulator of hormone-dependent oestrogen receptor destruction. Mol. Cell. Biol. 2003;23:5979–5988. doi: 10.1128/MCB.23.17.5979-5988.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian Z., Okuhara D., Abe M. K., Rosner M. R. Molecular cloning and characterisation of a mitogen-activated protein kinase-associated intracellular chloride channel. J. Biol. Chem. 1999;274:1621–1627. doi: 10.1074/jbc.274.3.1621. [DOI] [PubMed] [Google Scholar]
- 8.Abe M. K., Saelzler M. P., Espinosa R., III, Kahle K. T., Hershenon M. B., Le Beau M. M., Rosner M. R. ERK8, a new member of the mitogen-activated protein kinase family. J. Biol. Chem. 2002;277:16733–16743. doi: 10.1074/jbc.M112483200. [DOI] [PubMed] [Google Scholar]
- 9.Mody N., Campbell D. G., Morrice N., Peggie M., Cohen P. An analysis of the phosphorylation and activation of extracellular-signal-regulated protein kinase 5 (ERK5) by mitogen-activated protein kinase kinase 5 (MKK5) in vitro. Biochem. J. 2003;372:567–575. doi: 10.1042/BJ20030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNeill H., Knebel A., Arthur J. S. C., Cuenda A., Cohen P. A novel UBA and UBX domain protein that binds polyubiquitin and VCP and is substrate for SAPKs. Biochem. J. 2004;384:391–400. doi: 10.1042/BJ20041498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies S. P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuenda A., Cohen P., Buee-Scherrer V., Goedert M. Activation of stress activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6): comparison of the specificities of SAPK3 and SAPK2 (RK/p38) EMBO J. 1997;16:295–305. doi: 10.1093/emboj/16.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durocher Y., Perret S., Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30:E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auld G. C., Campbell D. G., Morrice N., Cohen P. Identification of calcium-regulated heat stable protein (CRHSP24) as a physiological substrate for PKB and RSK using KESTREL. Biochem. J. 2005;389:775–783. doi: 10.1042/BJ20050733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson B. L., Marchese J., Morrice N. A. Automated identification and quantification of protein phosphorylation sites by LC/MS on a hybrid triple quadrupole linear ion trap mass spectrometer. Mol. Cell. Proteomics. 2005 doi: 10.1074/mcp.M500210-MCP200. in the press. [DOI] [PubMed] [Google Scholar]
- 16.Erickson A. K., Payne D. M., Martino P. A., Rossomando A. J., Shabanowitz J., Weber M. J., Hunt D. F., Sturgill T. W. Identification by mass spectrometry of threonine 97 in bovine myelin basic protein as a specific phosphorylation site for mitogen-activated protein kinase. J. Biol. Chem. 1990;265:19728–19735. [PubMed] [Google Scholar]
- 17.Lawler S., Cuenda A., Goedert M., Cohen P. SKK4, a novel activator of stress-activated protein kinase-1 (SAPK1/JNK) FEBS Lett. 1997;414:153–158. doi: 10.1016/s0014-5793(97)00990-3. [DOI] [PubMed] [Google Scholar]
- 18.Fleming Y., Armstrong C. G., Morrice N., Paterson A., Goedert M., Cohen P. Synergistic activation of stress-activated protein kinase 1/c-Jun N-terminal kinase (SAPK1/JNK) isoforms by mitogen-activated protein kinase kinase 4 (MKK4) and MKK7. Biochem. J. 2000;352:145–154. [PMC free article] [PubMed] [Google Scholar]
- 19.Lisnock J. M., Griffin P., Calaycay J., Frantz B., Parsons J., O'Keffe S. J., LoGrasso P. Activation of JNK3α1 requires both MKK4 and MKK7; kinetic characterisation of in vitro phosphorylated JNK3α1. Biochemistry. 2000;39:3141–3148. doi: 10.1021/bi992410+. [DOI] [PubMed] [Google Scholar]
- 20.Lochhead P. A., Sibbet G., Morrice N., Cleghon V. Activation-loop autophosphorylation is mediated by a novel transitional intermediate form of DYRKs. Cell. 2005;121:925–936. doi: 10.1016/j.cell.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 21.Cole A., Frame S., Cohen P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem. J. 2004;377:249–255. doi: 10.1042/BJ20031259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salmeen A., Andersen J. N., Myers M. P., Meng T. C., Hinks J. A., Tonks N. K., Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature (London) 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 23.Cohen P., Holmes C. F. B., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem. Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- 24.Stokoe D., Campbell D. G., Nakielny S., Hidaka H., Marshall C., Cohen P. MAPKAP kinase-2; a novel protein kinase activated by mitogen activated protein kinase. EMBO J. 1992;11:3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]