Abstract
An interval timing mechanism in the brain governs reproduction in seasonally breeding mammals by triggering refractoriness to inhibitory short photoperiods during midwinter. The neural mechanisms responsible for the timing and induction of photorefractoriness by this seasonal clock are unknown. Using cDNA microarrays and RT-PCR, we identified a class of genes encoding thyroxine (T4)-binding proteins (transthyretin, T4-binding globulin, albumin) whose expression is associated with reproductive refractoriness to short day lengths. Down-regulation of these genes was associated with reduced hypothalamic T4 uptake, which was reversed by long-day photoperiod treatments that restored responsiveness to short days. Circulating T4 concentrations did not vary with states of photoresponsiveness in euthyroid hamsters, but blockade of thyroid function accelerated the onset of photorefractoriness to short days. These data link changes in gene expression in the hypothalamus to the functional output of a seasonal clock. Reproductive inhibition in short days depends on T4 only late in the nonbreeding season. Down-regulation of genes encoding T4-binding proteins in the hypothalamus during this interval may restrict access of a static T4 signal to hypothalamic target tissues that regulate reproduction, thereby timing annual transitions in reproductive function. Hypothalamic autoregulation of T4 influx may constitute a critical cellular process involved in the generation and expression of seasonal reproductive rhythms and suggests a previously undescribed mechanism by which neural targets gain access to peripheral hormones.
Few animals breed continuously in nature. Instead, virtually all vertebrates and some invertebrate organisms exhibit annual patterns in reproduction that are synchronized to recurring geophysical cycles (1). The reproductive cycles of many seasonally breeding vertebrates include a phase of insensitivity (refractoriness) to environmental stimuli that otherwise inhibit reproductive function (1–3). Day length (photoperiod) is the principal environmental cue used by mammals for timing seasonal reproductive transitions (4, 5), and most photoperiodic mammals exhibit a period of insensitivity to either long or short day lengths that “spontaneously” triggers either regression or regrowth of the reproductive system (5). Long-day breeding Siberian hamsters (Phodopus sungorus) undergo gonadal regression and become reproductively quiescent when exposed to short days (≤13 h of light per day; 13L). Reproductive inhibition is constrained by an endogenous seasonal timing mechanism that triggers photorefractoriness and gonadal recrudescence after ≈20–30 weeks of exposure to short days, after which hamsters are no longer responsive to inhibitory day lengths (2). Only prolonged exposure to long (summer) days thereafter can resensitize the reproductive neuroendocrine system to short day lengths (5, 6). The interval timer mediating photorefractoriness constitutes the endogenous component of an annual timekeeping mechanism that seems to have arisen on multiple occasions over the course of vertebrate evolution and may represent the ancestral form of mammalian seasonal (circannual) clocks (5, 7).
Circadian clocks and seasonal clocks share formal similarities (8), but during the past decade, advances in our understanding of the molecular bases of biological timekeeping mechanisms have accrued disproportionately in favor of circadian clocks. To gain greater insights into seasonal timekeeping mechanisms that govern reproductive photoresponsiveness and photorefractoriness, we have studied gene expression in the brains of photoresponsive and photorefractory Siberian hamsters. Comparisons of hypothalamic gene expression revealed a set of genes, all encoding thyroid hormone-binding proteins (TBPs), the expression of which correlated with the disparate states of reproductive responsiveness and refractoriness to short days. Therefore, we tested the hypothesis that changes in the expression of these genes lead to altered transport of thyroid hormone into a region of the brain that controls reproductive physiology. Lastly, we tested the hypothesis that the availability of thyroid hormones affects the timekeeping properties of the interval timer.
Methods
Animals, Photoperiod Regimes, and Procedures.
Male Siberian hamsters (Phodopus sungorus) used for gene array and RT-PCR experiments were bred in a long-day (14L) colony with ad libitum access to food and water. At 60–120 days of age (week 0), adult hamsters were anesthetized with isoflurane vapors, and estimated testis volumes (ETVs) were calculated as the product of the width2 × length of the left testis. Hamsters were transferred to a short-day photoperiod (10L) on week 0 (SDR; n = 9) or week 20 (SD; n = 16), or remained in long days for the entire 32-week experiment (LD; n = 10). In all photoperiods, the onset of darkness occurred at the same time of day (1400 hours eastern standard time). ETVs were determined at regular intervals. At week 32, hamsters were decapitated between 1100 and 1400 hours, and whole hypothalami were dissected under sterile, RNase-free conditions and snap frozen at −80°C.
cDNA Microarray Analyses.
Total RNA extraction was performed by using Trizol (Life Technologies, Inc., Gaithersburg, MD) according to the manufacturer's instructions. At the time of RNA extraction, whole hypothalami were pooled within each treatment group to obtain sufficient material for hybridization. Poly(A)+ RNA from each of the three samples (LD, SD, and SDR) was purified and labeled with Cy3 and Cy5 fluorescent dyes for microarray hybridization on a mouse UniGEM cDNA microarray (Incyte Genomics, Palo Alto, CA) as described (9). Relative levels of hybridization with each of >9,300 target genes or ESTs on the microarrays were calculated for each of the three microarrays conducted (LD vs. SD, LD vs. SDR, and SD vs. SDR). Twofold or greater differences in hybridization were considered meaningful (10).
RT-PCR Analyses.
Semiquantitative RT-PCR was performed according to standard practices by using ≈0.2 μg of poly(A)+ RNA from LD, SD, and SDR hypothalami as starting material. RT was performed for 50 min at 42°C with Superscript II (Invitrogen). PCR primers were based on conserved sequences of human, rat, and mouse transthyretin (TTR), thyroxine (T4)-binding globulin (TBG), albumin, and β-actin (GenBank accession nos.: TTR, D89076; TBG, M63991, J05329; and albumin, AJ011413). Primers were as follows: TTR forward, TGT-CCT-CTG-ATG-GTC-AAA-GTC-CT; TTR reverse, CTG-CGA-TGG-TGT-AGT-GGC-GAT; TBG forward, GGA-TGA-TGT-CAA-GAC-CCT-CTA-TGA; TBG reverse, GGT-TCC-ACT-TCT-TCA-GTG-TTT-TAG-ATG; albumin forward, GAC-TTG-CTA-AGA-AAT-ATG-AAG-CCA-C; albumin reverse, GGA-AGG-TGA-AGG-TCTCAG-CTT; β-actin forward, GAA-ATC-GTG-CGT-GAC-ATC; β-actin reverse, GCT-TGC-TGA-TCC-ACA-TCT. PCR was performed in a 100-μl volume containing 2 units of Taq polymerase, 1.5 mM MgCl2, and 1–2 μl of cDNA from the RT reaction. The initial denaturation step was at 94°C for 3 min, followed by 20 (TTR), 30 (β-actin), or 36 (TBG, albumin) cycles of denaturation for 25 s at 94°C, annealing for 40 s at either 60°C (TTR, β-actin) or 62°C (TBG, albumin), and extension for 1 min at 72°C. PCR products were electrophoresed on a 1% agarose gel. Each PCR reaction was replicated a minimum of three times. β-actin expression was determined for each sample to equalize cDNA loading and permit semiquantitative comparisons between samples. The product of the TTR reaction was directly sequenced and confirmed as 91% homologous to mouse TTR by sequence analysis (data not shown).
T4 RIA.
Plasma T4 concentrations in trunk blood obtained at autopsy were determined (in duplicate) in a single RIA with a monoclonal anti-human T4 antibody kit (ICN). The lower limit of sensitivity of the assay was 2.4 μg/dl.
125I Tissue Uptake.
Male Siberian hamsters were gestated and raised in 14L and transferred as adults to a progression of short-day photoperiods: 9L from week 0 to week 12 and 6L from week 12 to week 32 (SDR; n = 6), which induced gonadal regression followed by complete recrudescence. Long-day control hamsters were housed in 15L from week 0 to week 32 (LD; n = 6). Photorefractoriness was “broken” in a third group of hamsters that had undergone gonadal regression and recrudescence in 8L over the course of 32 weeks by exposing them to 16L for 12 weeks (6), beginning on week 32 (SDR+LD; n = 5). Testis sizes were determined in all hamsters at regular intervals over the course of photoperiod treatments. On week 32 (LD and SDR hamsters) or week 44 (SDR+LD hamsters), hamsters were injected with 1 μCi (1 Ci = 37 GBq) of 125I[T4] (ICN) at 1400 hours. At 1100 hours on the following day, hamsters were decapitated, whole hypothalami and a portion of the left neocortex were dissected, and a small (150–200 μl) blood sample was obtained. Radioactive tissue and blood samples were centrifuged, and plasma was extracted; all samples were then weighed, and gamma emissions were counted for 1 min per sample. Tissue/plasma uptake ratios for each animal were calculated as cpm/mg of tissue/cpm/μg of plasma (11).
Blockade of Thyroid Function.
Male Siberian hamsters were gestated and raised in 14L and transferred to 16L at 40–80 days of age. At week 0, adult hamsters were transferred to 8L or remained in 16L for the next 27 weeks. Hamsters were rendered hypothyroid (Tx) by the addition of 0.4% or 0.8% thiourea (Sigma) plus 1% sodium saccharin to the drinking water; the drinking water of control hamsters contained 1% sodium saccharin. ETV was determined under light isoflurane anesthesia at 3-week intervals to determine the time course of gonadal regression and recrudescence. The incidence of reproductive nonresponsiveness to short days (failure to exhibit 45% reduction in ETV on weeks 6–12) (12) was comparable between thyroidectomized and control hamsters [12/40 (30%) vs. 7/34 (21%), respectively; P > 0.05], and hamsters that were nonresponsive to 8L were excluded from analyses. Hamsters were bled via the retroorbital sinus on week 9, and plasma T4 concentrations were determined by RIA. Blockade of thyroid function was confirmed by the absence of detectable T4 in the plasma of 10/16 LD and 11/11 SD hamsters, and only these hamsters were used as experimental subjects. Thiourea treatments were equally effective in eliminating plasma T4; thus, data from hamsters receiving 0.4% and 0.8% thiourea were combined within photoperiod treatment for all subsequent analyses. The onset of spontaneous gonadal recrudescence was defined as the first time point at which ETV >150; growth above this value is clearly distinguishable from the completely regressed state (13). Final sample sizes were as follows: LD, n = 16; SD, n = 11; LD-Tx, n = 9; SD-Tx, n = 9.
Statistical Analyses.
Longitudinal changes in ETV were analyzed by using within-subjects ANOVA; paired and unpaired t tests were used to compare mean ETV values. TTR, TBG, and albumin PCR products were expressed as a percentage of β-actin expression for purposes of pairwise comparisons. Tissue/plasma T4 uptake ratios were compared by using one-tailed t tests. Testis volumes and body weights were compared with Fisher's least significant difference test. Plasma T4 concentrations were compared with Mann–Whitney U tests. For all comparisons, differences were considered significant if P < 0.05. All experiments received prior approval from the institutional animal care and use committee.
Results
Photorefractoriness-Induced Changes in Hypothalamic Gene Expression.
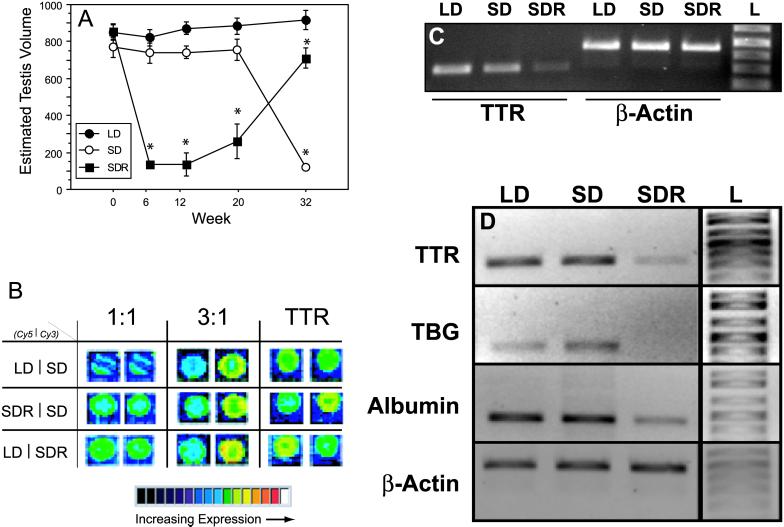
Photosensitive hamsters housed in long days (LD) maintained fully developed gonads, whereas photosensitive hamsters housed in short days (SD) underwent gonadal regression between weeks 20 and 32; photorefractory hamsters (SDR) exhibited gonadal regression, followed by refractoriness and recrudescence after 32 weeks in short days (Fig. 1A). Relative levels of hypothalamic gene expression exhibiting a change of 2-fold or greater as determined in pairwise comparisons between each of the three treatment groups (LD vs. SD, LD vs. SDR, and SD vs. SDR) are listed in Table 1. Candidate “photoresponsiveness genes” were identified based on the a priori criteria that relative expression levels should not differ between equally photoresponsive groups (LD and SD), but that expression should differ in both direction and magnitude between photorefractory (SDR) and photoresponsive (LD and SD) groups. Using these selection criteria, we identified one gene that underwent >2-fold changes in expression between SDR and LD, and between SDR and SD, yet did not differ between LD and SD: transthyretin (TTR) gene expression was down-regulated 2.5-fold in the hypothalamus of SDR hamsters relative to both LD and SD hamsters in this assay (Fig. 1B). Relative quantitative RT-PCR, using primers 100% identical to a conserved sequence in mouse TTR, confirmed the cDNA microarray data: TTR expression was comparable in LD and SD groups (<2-fold change) but was ≈6- and 4-fold higher in the hypothalamus of LD vs. SDR and SD vs. SDR hamsters, respectively (Fig. 1C).
Fig 1.
TBP gene expression in the hypothalamus of photoresponsive and photorefractory hamsters. (A) Mean (±SEM) ETVs of Siberian hamsters exposed to short days for 12 weeks (weeks 20–32; short-day photosensitive, SD) or for 32 weeks (short-day photorefractory, SDR), and of control hamsters exposed to long days for 32 weeks (long-day photosensitive, LD). SDR hamsters initially underwent gonadal regression. In weeks 20–32, SDR hamsters exhibited spontaneous gonadal recrudescence, indicative of short-day photorefractoriness, whereas SD hamsters underwent gonadal regression. LD hamsters sustained fully developed gonads in long days. (*, P < 0.05 vs. LD value.) (B) Pairwise competitive hybridization to a UniGEM cDNA microarray (Incyte Genomics) of fluorescently labeled hypothalamic RNA samples from LD, SD, and SDR hamsters. Control images depict fluorescence indicative of 0-fold (1:1 control; Left) and 3-fold (3:1 control; Center) differences in hybridization between the Cy3 and the Cy5 samples. (Right) Fluorescence of Cy3- and Cy5-labeled samples after hybridization to a probe for mouse TTR in each of the three pairwise microarray experiments. TTR expression was ≈2.5-fold lower in the hypothalamus of SDR hamsters, relative to both LD and SD samples. (C) Relative quantitative RT-PCR assessment of hypothalamic expression of TTR mRNA in photosensitive (LD, SD) and photorefractory (SDR) hamsters confirmed lower expression of TTR in the hypothalamus of SDR hamsters. (D) Expression of TTR, TBG, and albumin mRNAs was lower in the hypothalamus of photorefractory (SDR) hamsters, relative to photosensitive (LD and SD) hamsters.
Table 1.
Comparisons of relative differences (ratios of normalized hybridizations) in hypothalamic gene expression in photosensitive (LD, SD) and photorefractory (SDR) hamsters as indicated by cDNA microarray analyses (see Methods)
| Relative difference, fold Δ | Gene name | Function |
|---|---|---|
| LD vs. SD | ||
| +2.5 | Protein phosphatase 1A | Phosphorylation, intracellular signal transduction |
| −2.2 | GnRH receptor | Neuroendocrine feedback, sex behavior |
| −1.1 | P3/GdX | X-linked gene, housekeeping |
| LD vs. SDR | ||
| +2.5 | Transthyretin | Thyroid hormone transport |
| +2.4 | Protein phosphatase 1A | Phosphorylation, intracellular signal transduction |
| 1.0 | P3/GdX | X-linked gene, housekeeping |
| SD vs. SDR | ||
| +2.7 | GnRH receptor | Neuroendocrine feedback, sex behavior |
| +2.5 | Transthyretin | Thyroid hormone transport |
| +2.2 | Solute carrier, family 1, member 2 | Energy transfer |
| −2.3 | Protein phosphatase 1A | Phosphorylation, intracellular signal transduction |
| −1.1 | P3/GdX | X-linked gene, housekeeping |
In each pairwise competitive hybridization experiment, positive values indicate relative up-regulation of gene expression in the treatment group listed first, and negative values indicate relative down-regulation of gene expression in the treatment group listed first. For each hybridization experiment, value is also listed for the transcriptionally active “housekeeping” (23) genes P3 and GdX (value reflects mean hybridization ratio for three separate P3/GdX probes included on the microarray; GenBank accession nos. AA655428, AI550986, and AA061673).
TTR is a TBP that, together with TBG and albumin, binds T4 and establishes a T4 pool in the plasma and cerebrospinal fluid (14). Because the microarray experiments did not include probes for TBG and albumin, we also quantified expression of these genes by using RT-PCR. TBG was expressed in the hypothalamus of photosensitive (LD and SD) hamsters but was undetectable in the hypothalamus of photorefractory (SDR) hamsters (Fig. 1D). In common with TTR and TBG, albumin expression was down-regulated substantially (2- to 3-fold) in the hypothalamus of refractory, relative to photosensitive, hamsters and did not differ between groups of photoresponsive hamsters (Fig. 1D).
Changes in TBP Gene Expression Are Unlikely to Be Masking Effects.
It is noteworthy that down-regulation of TTR, TBG, and albumin gene expression is not simply a consequence of masking by short days, because groups housed in the same short photoperiod (i.e., SD and SDR) exhibited different amounts of gene expression. Nor are these differences in expression likely to be consequences of changes in the endocrine milieu associated with the maintenance of, or the transition to, reproductive competence, because groups with fully developed gonads (i.e., LD and SDR) exhibited substantial differences in expression of TBP genes. Rather, we propose that changes in hypothalamic expression of TBPs contribute to the expression of photorefractoriness.
Expression of TBPs in the mammalian hypothalamus is previously undocumented; thus, the functional significance of changes in hypothalamic TBP gene expression is unclear. TTR synthesis at circumventricular sites (i.e., choroid plexus) is the principal mechanism by which T4 is transported into the cerebrospinal fluid (14); synthesis of TTR within the brain has been reported only in the amygdala, but its function is not known (15). If down-regulation of TBP gene expression in the hypothalamus contributes to the loss of reproductive responsiveness to short days, then it may do so either via direct (i.e., acting as signaling molecules) (16, 17) or indirect (via binding of T4) mechanisms.
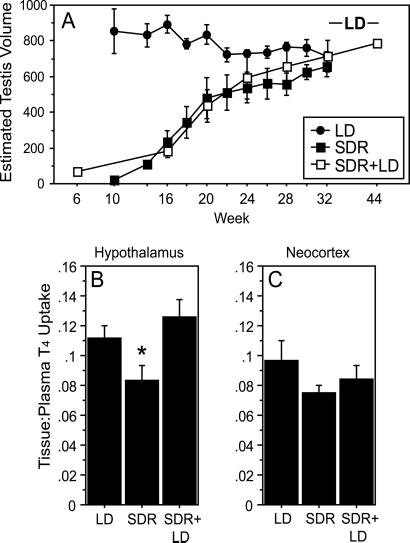
Photorefractoriness Reduces Hypothalamic T4 Uptake.
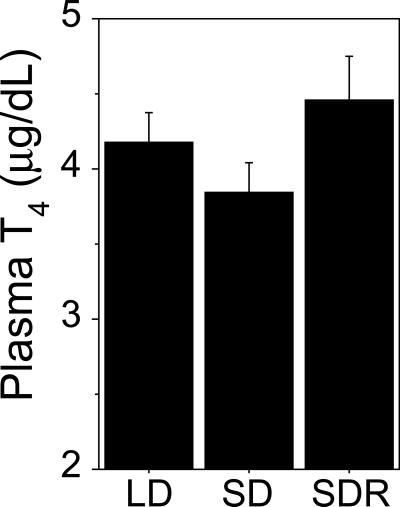
In the present experiment hamsters manifested only modest differences in circulating T4 at distinct phases of the seasonal reproductive cycle (Fig. 2; cf. 18); however, considering the role of choroid plexus TBP synthesis in the establishment of a T4 gradient between the blood and cerebrospinal fluid, we hypothesized that local synthesis of TBPs in the hypothalamus might regulate access of peripheral T4 to neural targets. Therefore, we tested the hypothesis that down-regulation of TBP gene expression in photorefractory hamsters decreases thyroid hormone transport into the hypothalamus and thereby impinges on thyroid–hypothalamus signaling. Hamsters rendered photorefractory by exposure to short days for 32 weeks (Fig. 3A) exhibited significantly lower hypothalamic T4 uptake relative to photosensitive (long-day control) hamsters (Fig. 3B). Moreover, resensitizing photorefractory hamsters to short days (by exposure to long days for 12 weeks) (6) increased hypothalamic T4 uptake to values comparable to those of photosensitive hamsters that had never been exposed to short days (Fig. 3B). Importantly, the decrease in hypothalamic uptake of T4 associated with photorefractoriness was not observed in a control region of the brain (neocortex; Fig. 3C). These data suggest a functional role for changes in TBP expression in the brain, indicating that down-regulation of TBP gene expression in the hypothalamus is associated with reduced hypothalamic concentrations of T4. The results also suggest that hypothalamic T4 concentrations correlate with and thus may contribute to central states of reproductive responsiveness to short days. Despite comparable concentrations of T4 in the periphery, lower concentrations of T4 exist in the hypothalamus when photorefractoriness is manifest.
Fig 2.
Mean (+SEM) total plasma T4 concentrations of LD, SD, and SDR hamsters obtained after 32 weeks of photoperiod treatments. T4 concentrations in the plasma did not differ significantly in response to any of the photoperiod treatments. (ANOVA, P > 0.05.)
Fig 3.
Diminished T4 uptake in the hypothalamus of photorefractory hamsters. (A) Mean ETV (±SEM) over the 44-week experiment for hamsters that had been exposed to short or long day lengths for 32 weeks (SDR and LD, respectively), and in hamsters exposed to short day lengths for 32 weeks followed by long day lengths for 12 weeks (SDR+LD; a photoperiod treatment that restores photosensitivity in photorefractory hamsters) (6). (B and C) Tissue/plasma ratios (+SEM) of 125I-L-T4 uptake in the hypothalamus and neocortex of photosensitive (LD and SDR+LD) and photorefractory (SDR) hamsters. SDR hamsters exhibited lower T4 uptake relative to LD hamsters; breaking of refractoriness by exposure to LD restored elevated hypothalamic T4 uptake. (*, P < 0.05 vs. all other groups.)
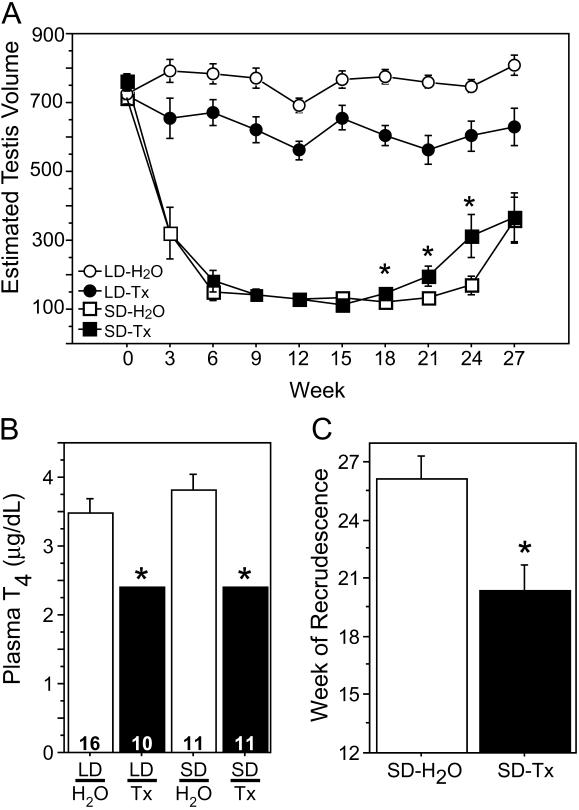
Blockade of Thyroid Function Accelerates the Onset of Photorefractoriness.
Together, gene-expression and hormone-uptake data suggest that cells in the brain may regulate influx of thyroid hormone into the hypothalamus via expression of TBPs. To examine in further detail the role of thyroid hormone in the expression of hamster photorefractoriness, we inhibited thyroid gland function in adult male hamsters by adulterating their drinking water with thiourea, which eliminated T4 in the plasma (Fig. 4B). Hypothyroidism did not interfere with gonadal regression upon exposure to short days; however, thiourea-treated hamsters became photorefractory and initiated gonadal recrudescence ≈3–6 weeks earlier than did euthyroid hamsters (Fig. 4 A and C). These data suggest that normal plasma thyroid hormone concentrations are not necessary for the decrease in gonadotrophin secretion that occurs when hamsters are first exposed to short days, but that the presence of endogenous thyroid hormone is required for the prolonged maintenance of reproductive inhibition in short days.
Fig 4.
Blockade of thyroid function accelerates spontaneous gonadal recrudescence in short days. (A) Mean (± SEM) ETV over the 27-week experiment for hamsters exposed to long (LD) or short (SD) photoperiods and provided with a solution of either 0.4% or 0.8% thiourea/saccharin (Tx) or a control solution of 1% sodium saccharin (H2O) as their source of drinking water. SD-Tx hamsters exhibited a normal pattern of gonadal regression, but they exhibited spontaneous recrudescence earlier than did SD-H2O hamsters. (B) Mean (+SEM) total plasma T4 concentrations on week 9 in Tx and H2O hamsters exposed to LD and SD. Adulteration of the drinking water with thiourea rendered plasma T4 concentrations undetectable (<2.4 μg/dl). (C) Mean (+SEM) time of onset of gonadal recrudescence of Tx and H2O hamsters exposed to LD and SD. (*, P < 0.05.)
The effect of thiourea treatments on photorefractoriness in Siberian hamsters bears formal similarity to effects of surgical or radioactive thyroidectomy observed in other seasonally breeding vertebrates. Sheep and starlings, for example, require thyroid hormones for the expression of seasonal reproductive transitions (19, 20). In ewes, thyroidectomy uncouples the reproductive axis from photoperiodic influences and blocks the photorefractoriness-induced spontaneous transition to reproductive inhibition in winter (19, 21); in starlings, thyroidectomy abolishes photorefractoriness-induced gonadal regression in long days (20). Like sheep (19), hamsters require thyroid hormones for photoperiodic control of the reproductive system only during a restricted phase of the seasonal cycle (19, 21, 22). The present data are compatible with the conjecture that reproductive photoresponsiveness in Siberian hamsters is T4-independent for the majority of the annual cycle, but that sustained reproductive inhibition becomes T4-dependent after prolonged exposure to short days. Under normal (i.e., euthyroid) conditions, if a “spontaneous” down-regulation of hypothalamic TBP expression coincided with such a T4-dependent phase, the resulting reduction in T4 availability to hypothalamic target tissues (potentially critical to the maintenance of gonadal inhibition) could be responsible for the manifestation of photorefractoriness.
Discussion
These data link gene expression in the hypothalamus to the output of a seasonal clock. Prolonged exposure to short days results in a down-regulation of TBP synthesis in the hypothalamus and a reduction in hypothalamic T4 concentrations. The hypothesis that hypothalamic T4 concentrations serve a functional role in photoresponsiveness is supported by the observations that T4 uptake was diminished in the hypothalamus of photorefractory hamsters and that exposure to long photoperiods sufficient to resensitize the reproductive neuroendocrine system to short days restored hypothalamic T4 concentrations. The parallel changes in gene-expression and hormone-uptake data suggest that cells in the hypothalamus may regulate influx of thyroid hormone via expression of TTR, TBG, and albumin. Absent pronounced photoperiodic changes in peripheral T4 concentrations, regional-specific expression of TBPs in the brain may establish local differences in available T4 in discrete brain nuclei. The observation that gonadal recrudescence occurred several weeks earlier in hypothyroid hamsters suggests that photoresponsiveness may be T4-dependent late during the interval of gonadal involution. A diminished T4 signal in the hypothalamus may effectively “thyroidectomize” only this region of the brain. Coincidence of a T4-dependent interval for reproductive inhibition with a TBP-mediated spontaneous reduction in hypothalamic T4 concentrations may uncouple the reproductive axis from photic inhibition and drive the recovery of reproductive function in mid- to late winter.
The availability of thyroid hormones influences the expression of seasonal timekeeping mechanisms. Hamsters require the absence of T4 for initiation of the breeding season (i.e., triggering refractoriness); in contrast, sheep require the presence of T4 for termination of the breeding season. Hamsters, unlike sheep, do not exhibit endogenous rhythms in reproduction; rather, changes in photoperiod drive seasonal reproductive transitions (5). It remains to be determined whether neural expression of TBPs figure prominently in biological timekeeping mechanisms of species such as sheep and ground squirrels, in which changes in photoperiod only entrain an otherwise self-sustaining circannual rhythm of reproductive transitions (5). For example, to terminate estrus, sheep require T4 during a restricted interval that begins in late breeding season and ends ≈6 months later; T4 treatments delivered after this interval (i.e., early summer) lose the ability to terminate estrus but phase-delay the subsequent breeding season (19, 22). If, like hamsters, sheep exhibit seasonal changes in neural expression of TBPs, up- and down-regulation of TBP expression could amplify and diminish, respectively, access of T4 to targets in the brain, ensuring T4 terminates estrus during winter and fine-tuning its effects on the circannual clock (22).
Hypothalamic synthesis of TTR, TBG, and albumin provides a mechanism whereby the brain may locally regulate T4 concentrations and thereby permit or prevent reproductive photoresponsiveness. In nature, down-regulation of hypothalamic TBP expression after prolonged exposure to short days may diminish hypothalamic T4 concentrations, and thereby initiate the recovery of reproductive competence in mid- to late winter; exposure to long day lengths during the summer months may “break” refractoriness in part by restoring a level of thyroid–hypothalamus signaling sufficient to permit sustained reproductive responses to short days during the following autumn. The neural mechanisms responsible for triggering the spontaneous down-regulation of TBP expression await specification, as do the mechanism(s) by which thyroid hormones interact with melatonin-mediated inhibition of gonadotrophin secretion. The present data suggest a novel neuroendocrine mechanism, whereby the brain may exert control over the local availability of circulating hormones and are consistent with the hypothesis that changes in hypothalamic expression of TBPs constitute an important link between the thyroid gland and the hypothalamic-pituitary-gonadal axis.
Acknowledgments
We thank Fran Adamski and Johnathan Pevsner for expert technical assistance. We also thank Irving Zucker, David Freeman, and two anonymous reviewers for advice and discussion. This investigation was supported by National Research Service Award 12875 from the National Institute of Mental Health and National Institutes of Health Grants MH 57535 and NS 40254.
Abbreviations
L, h of light per day
TBP, thyroid hormone-binding protein
ETV, estimated testis volume
TTR, transthyretin
T4, thyroxine
TBG, T4-binding globulin
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bronson F. H., (1989) Mammalian Reproductive Biology (Univ. of Chicago Press, Chicago).
- 2.Watson-Whitmyre K. & Stetson, M. H. (1988) in Processing of Environmental Information in Vertebrates, ed. Stetson, M. H. (Springer, New York), pp. 203–218.
- 3.Licht P. (1979) Annu. Rev. Physiol. 41, 337-351. [DOI] [PubMed] [Google Scholar]
- 4.Goldman B. D. (2001) J. Biol. Rhythms 16, 283-301. [DOI] [PubMed] [Google Scholar]
- 5.Prendergast B. J., Nelson, R. J. & Zucker, I. (2002) in Hormones, Brain and Behavior, eds. Pfaff, D., Arnold, A. P., Etgen, A. M., Fahrbach, S. E. & Rubin, R. T. (Academic, San Diego), Vol. 2, pp. 93–156. [Google Scholar]
- 6.Kauffman, A. S., Freeman, D. A. & Zucker, I. (2003) J. Neuroendocrinol., in press. [DOI] [PubMed]
- 7.Farner D. S. (1985) Annu. Rev. Physiol. 47, 65-82. [DOI] [PubMed] [Google Scholar]
- 8.Gorman M. R., Goldman, B. D. & Zucker, I. (2001) in Handbook of Behavioral Neurobiology, eds. Takahashi, J. S., Turek, F. W. & Moore, R. Y. (Plenum, New York), Vol. 12, pp. 481–508. [Google Scholar]
- 9.Shiffman D., Mikita, T., Tai, J. T., Wade, D. P., Porter, J. G., Seilhamer, J. J., Somogyi, R., Liang, S. & Lawn, R. M. (2000) J. Biol. Chem. 275, 37324-37332. [DOI] [PubMed] [Google Scholar]
- 10.Colantuoni C., Purcell, A. E., Bouton, C. M. & Pevsner, J. (2000) J. Neurosci. Res. 59, 1-10. [DOI] [PubMed] [Google Scholar]
- 11.Schadlow A. R. (1972) Science 176, 1252-1254. [DOI] [PubMed] [Google Scholar]
- 12.Freeman D. A. & Zucker, I. (2001) Proc. Natl. Acad. Sci. USA 98, 6447-6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorman M. R. (2001) Am. J. Physiol. 281, R1613-R1623. [DOI] [PubMed] [Google Scholar]
- 14.Schreiber G., Richardson, S. J. & Prapunpoj, P. (2001) Microsc. Res. Tech. 52, 21-30. [DOI] [PubMed] [Google Scholar]
- 15.Stork O., Stork, S., Pape, H. C. & Obata, K. (2001) Learn. Mem. 8, 209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuchler-Bopp S., Dietrich, J. B., Zaepfel, M. & Delaunoy, J. P. (2000) Brain Res. 870, 185-194. [DOI] [PubMed] [Google Scholar]
- 17.Sousa M. M. & Saraiva, M. J. (2001) J. Biol. Chem. 276, 14420-14425. [DOI] [PubMed] [Google Scholar]
- 18.O'Jile J. R. & Bartness, T. J. (1992) Physiol. Behav. 52, 267-270. [DOI] [PubMed] [Google Scholar]
- 19.Thrun L. A., Dahl, G. E., Evans, N. P. & Karsch, F. J. (1997) Endocrinology 138, 3402-3409. [DOI] [PubMed] [Google Scholar]
- 20.Bentley G. E., Dawson, A. & Goldsmith, A. R. (2000) J. Exp. Zool. 287, 74-79. [DOI] [PubMed] [Google Scholar]
- 21.Dahl G. E., Evans, N. P., Moenter, S. M. & Karsch, F. J. (1994) Endocrinology 135, 10-15. [DOI] [PubMed] [Google Scholar]
- 22.Billings H. J., Viguié, C., Karsch, F. J., Goodman, R. L., Connors, J. M. & Anderson, G. M. (2002) Endocrinology 143, 2618-2625. [DOI] [PubMed] [Google Scholar]
- 23.Thellin O., Zorzi, W., Lakaye, B., De Borman, B., Coumans, B., Hennen, G., Grisar, T., Igout, A. & Heinen, E. (1999) J. Biotechnol. 75, 291-295. [DOI] [PubMed] [Google Scholar]