Abstract
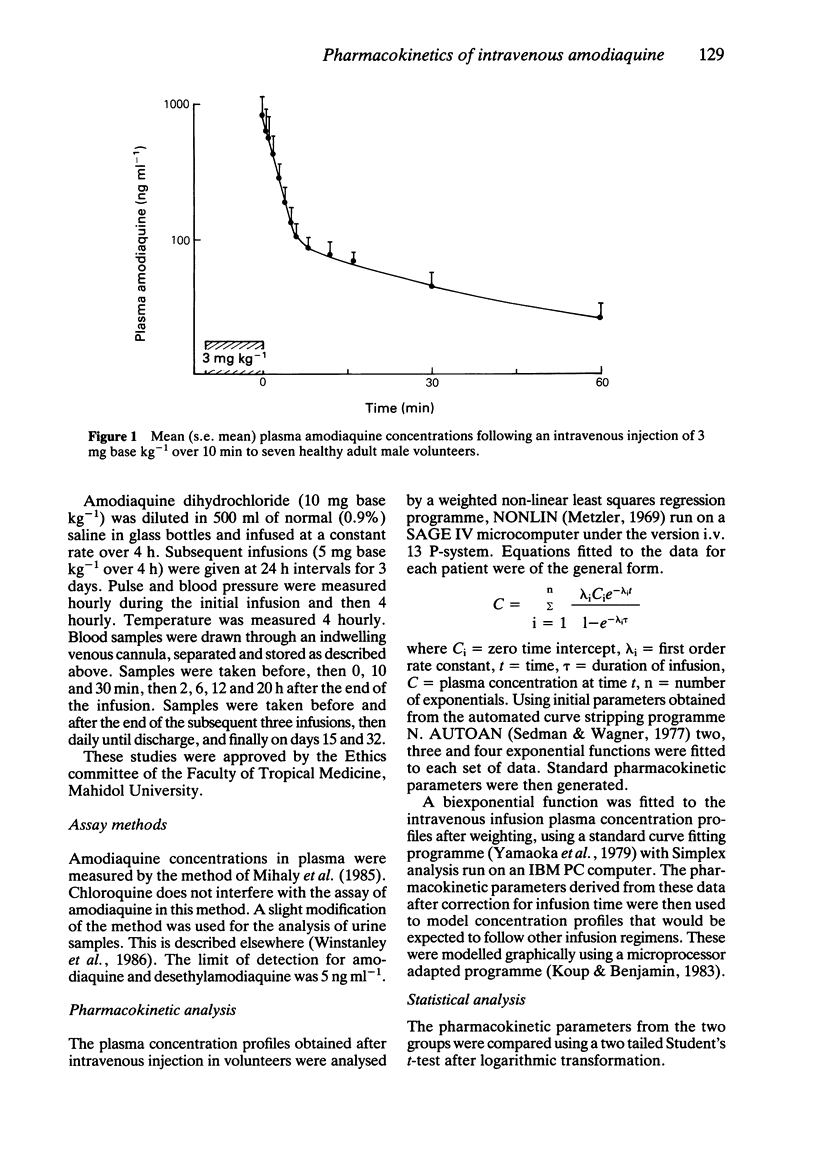
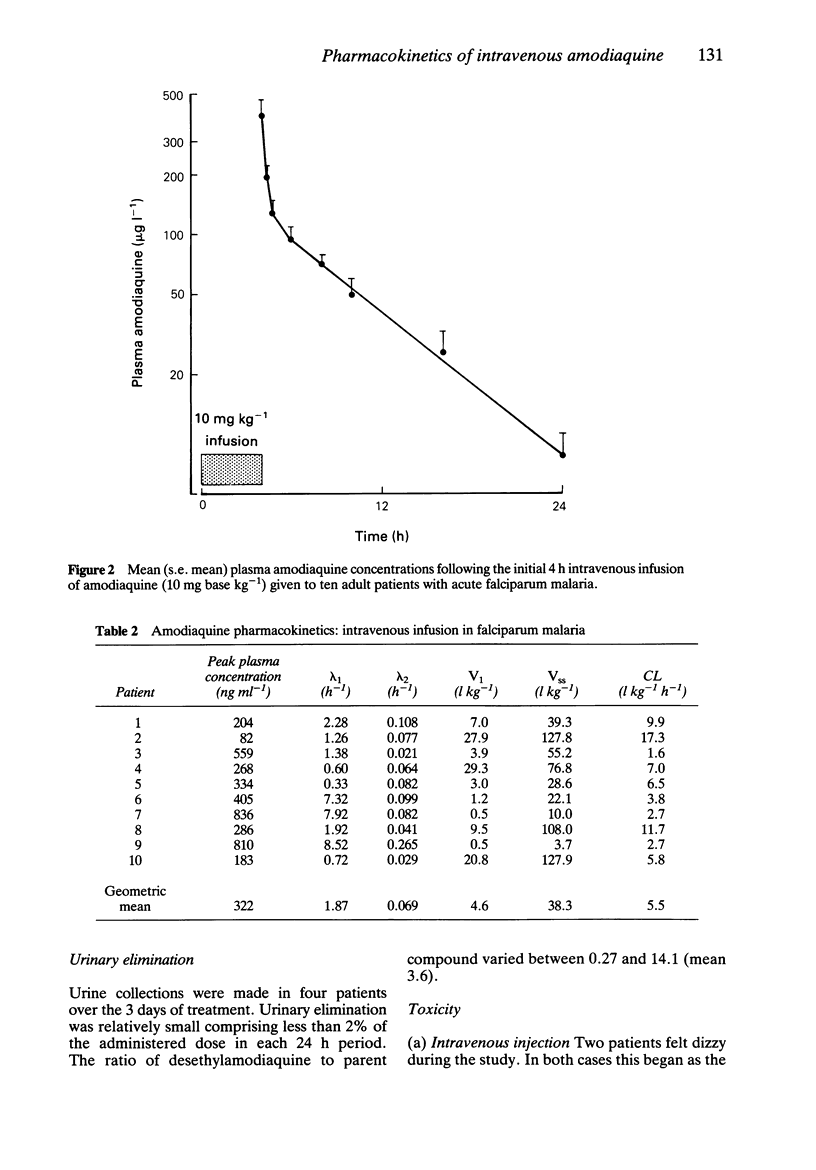
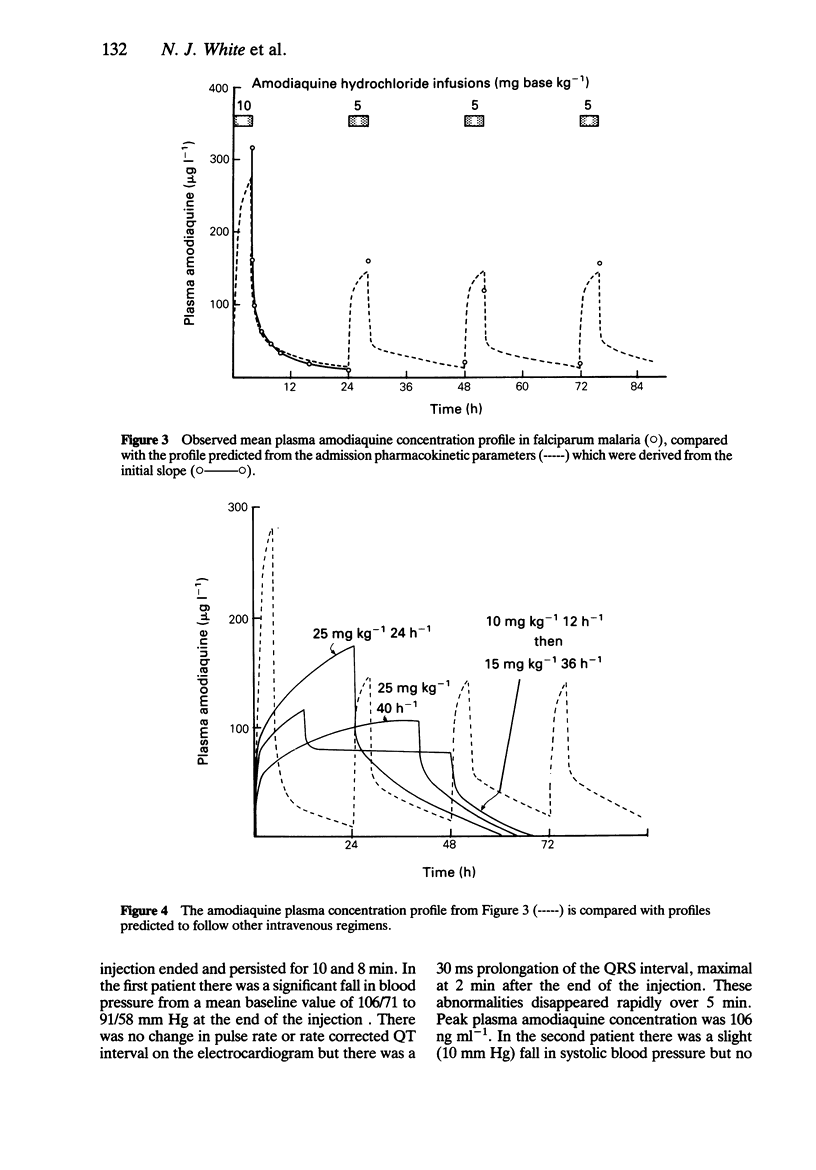
Amodiaquine hydrochloride (3 mg base kg-1) was given by constant rate intravenous injection over 10 min to seven healthy adult male volunteers, and by constant rate infusion (10 mg base kg-1) over 4 h to 10 adult patients admitted to hospital with falciparum malaria. After intravenous injection in volunteers there was considerable variation in plasma concentration profiles between subjects; peak plasma concentrations ranged between 65 and 1921 ng ml-1. A biexponential equation was fitted to the plasma concentration time data and the following estimated pharmacokinetic parameters (geometric mean; range) were derived; lambda 1 = 24.4 (7.6-95.0) h-1, lambda 2 = 0.33 (0.12-0.79) h-1, V1:1.1 (0.3-3.6) 1 kg-1, Vss: 17.4 (2.3-95.9) 1 kg-1 and systemic clearance 13.0 (4.7-56.6) 1 kg-1 h-1. After intravenous infusion there was also considerable variability between patients with post-infusion plasma concentrations ranging between 82 and 836 ng ml-1. The plasma concentration-time profiles were biphasic with the following estimated pharmacokinetic parameters (geometric mean; range) alpha = 1.87 (0.60-8.52) h-1, beta = 0.069 (0.021-0.265) h-1, V1: 4.6 (0.5-29.3) 1 kg-1, Vss: 38.3 (3.7-127.9) 1 kg-1 and systemic clearance CL (1.6-17.3) 1 kg-1 h-1. There was no measurable long terminal elimination phase, and the principal metabolite desethyl amodiaquine was not detected in the plasma samples. There was no serious toxicity in either group. During intravenous injection there was a significant fall in systolic blood pressure in four subjects (mean fall 16 mm Hg) but there was no significant change in heart rate.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTAGNA P. Aperçu général de la documentation relative à l'amodiaquine. Bull World Health Organ. 1951;4(2):267–281. [PMC free article] [PubMed] [Google Scholar]
- Churchill F. C., Patchen L. C., Campbell C. C., Schwartz I. K., Nguyen-Dinh P., Dickinson C. M. Amodiaquine as a prodrug: importance of metabolite(s) in the antimalarial effect of amodiaquine in humans. Life Sci. 1985 Jan 7;36(1):53–62. doi: 10.1016/0024-3205(85)90285-1. [DOI] [PubMed] [Google Scholar]
- Frisk-Holmberg M., Bergqvist Y., Termond E., Domeij-Nyberg B. The single dose kinetics of chloroquine and its major metabolite desethylchloroquine in healthy subjects. Eur J Clin Pharmacol. 1984;26(4):521–530. doi: 10.1007/BF00542151. [DOI] [PubMed] [Google Scholar]
- Hatton C. S., Peto T. E., Bunch C., Pasvol G., Russell S. J., Singer C. R., Edwards G., Winstanley P. Frequency of severe neutropenia associated with amodiaquine prophylaxis against malaria. Lancet. 1986 Feb 22;1(8478):411–414. doi: 10.1016/s0140-6736(86)92371-8. [DOI] [PubMed] [Google Scholar]
- Koup J. R., Benjamin D. R. Numerical integration simulation programs for the microcomputer. Ther Drug Monit. 1980;2(3):243–247. doi: 10.1097/00007691-198007000-00007. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S., Phillips R. E., White N. J., Karbwang J., Benjasurat Y., Attanath P., Warrell D. A. Intravenous amodiaquine and oral amodiaquine/erythromycin in the treatment of chloroquine-resistant falciparum malaria. Lancet. 1985 Oct 12;2(8459):805–808. doi: 10.1016/s0140-6736(85)90796-2. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S., White N. J., Chanthavanich P., Edwards G., Nicholl D. D., Bunch C., Warrell D. A. Cardiovascular toxicity and distribution kinetics of intravenous chloroquine. Br J Clin Pharmacol. 1986 Jul;22(1):31–36. doi: 10.1111/j.1365-2125.1986.tb02876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaly G. W., Nicholl D. D., Edwards G., Ward S. A., Orme M. L., Warrell D. A., Breckenridge A. M. High-performance liquid chromatographic analysis of amodiaquine in human plasma. J Chromatogr. 1985 Jan 11;337(1):166–171. doi: 10.1016/0378-4347(85)80025-6. [DOI] [PubMed] [Google Scholar]
- PAYNE E., VILLAREJOS V. M., SHARP E. A., REINERTSON J. W., WILLE W. S. Intravenous amodiaquin (camoquin) in naturally acquired and induced malaria. Am J Trop Med Hyg. 1951 Nov;31(6):698–702. doi: 10.4269/ajtmh.1951.s1-31.698. [DOI] [PubMed] [Google Scholar]
- Pussard E., Verdier F., Blayo M. C., Pocidalo J. J. Biotransformation de l'amodiaquine et prophylaxie du paludisme à Plasmodium falciparum. C R Acad Sci III. 1985;301(8):383–385. [PubMed] [Google Scholar]
- Salako L. A., Idowu O. R. Failure to detect amodiaquine in the blood after oral administration. Br J Clin Pharmacol. 1985 Oct;20(4):307–311. doi: 10.1111/j.1365-2125.1985.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer H. C., Kipingor T., Agure R., Koech D. K., Chulay J. D. Plasmodium falciparum in Kisumu, Kenya: differences in sensitivity to amodiaquine and chloroquine in vitro. J Infect Dis. 1983 Oct;148(4):732–736. doi: 10.1093/infdis/148.4.732. [DOI] [PubMed] [Google Scholar]
- Watkins W. M., Sixsmith D. G., Spencer H. C., Boriga D. A., Kariuki D. M., Kipingor T., Koech D. K. Effectiveness of amodiaquine as treatment for chloroquine-resistant Plasmodium falciparum infections in Kenya. Lancet. 1984 Feb 18;1(8373):357–359. doi: 10.1016/s0140-6736(84)90410-0. [DOI] [PubMed] [Google Scholar]
- White N. J., Chanthavanich P., Krishna S., Bunch C., Silamut K. Quinine disposition kinetics. Br J Clin Pharmacol. 1983 Oct;16(4):399–403. doi: 10.1111/j.1365-2125.1983.tb02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N. J. Clinical pharmacokinetics of antimalarial drugs. Clin Pharmacokinet. 1985 May-Jun;10(3):187–215. doi: 10.2165/00003088-198510030-00001. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]


