Abstract
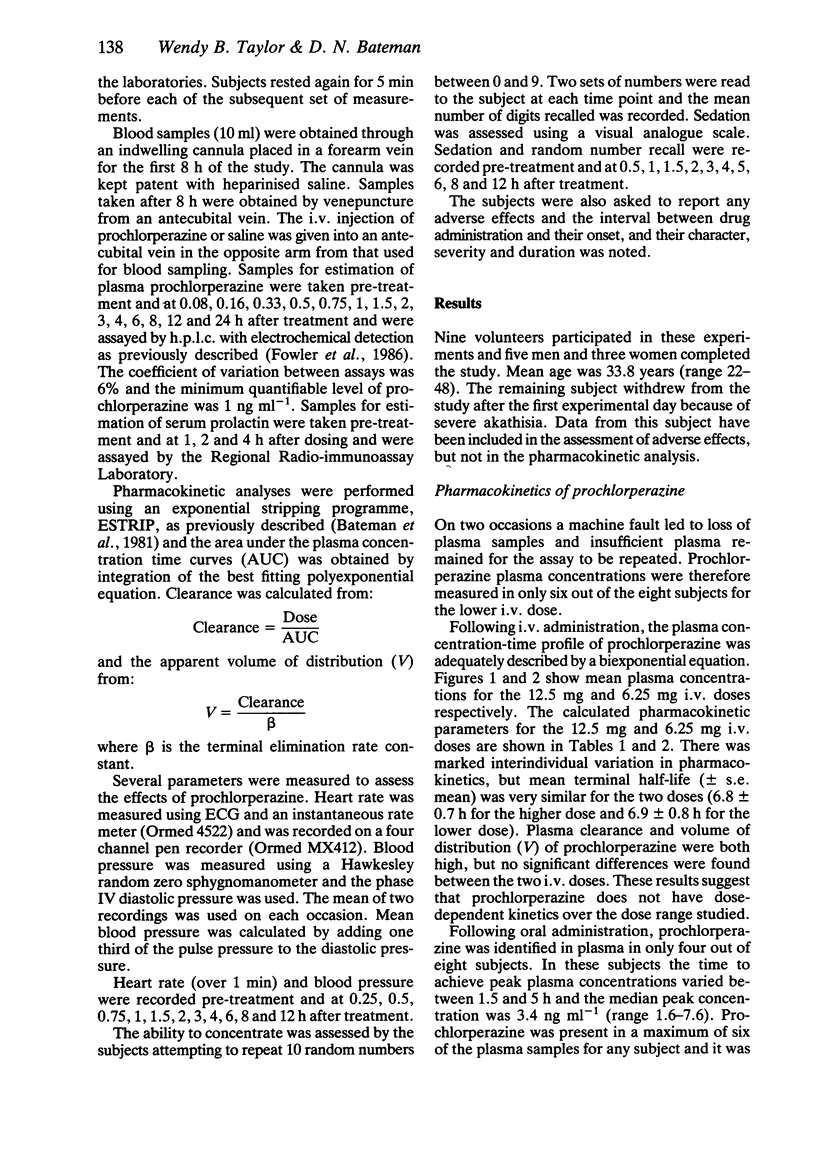
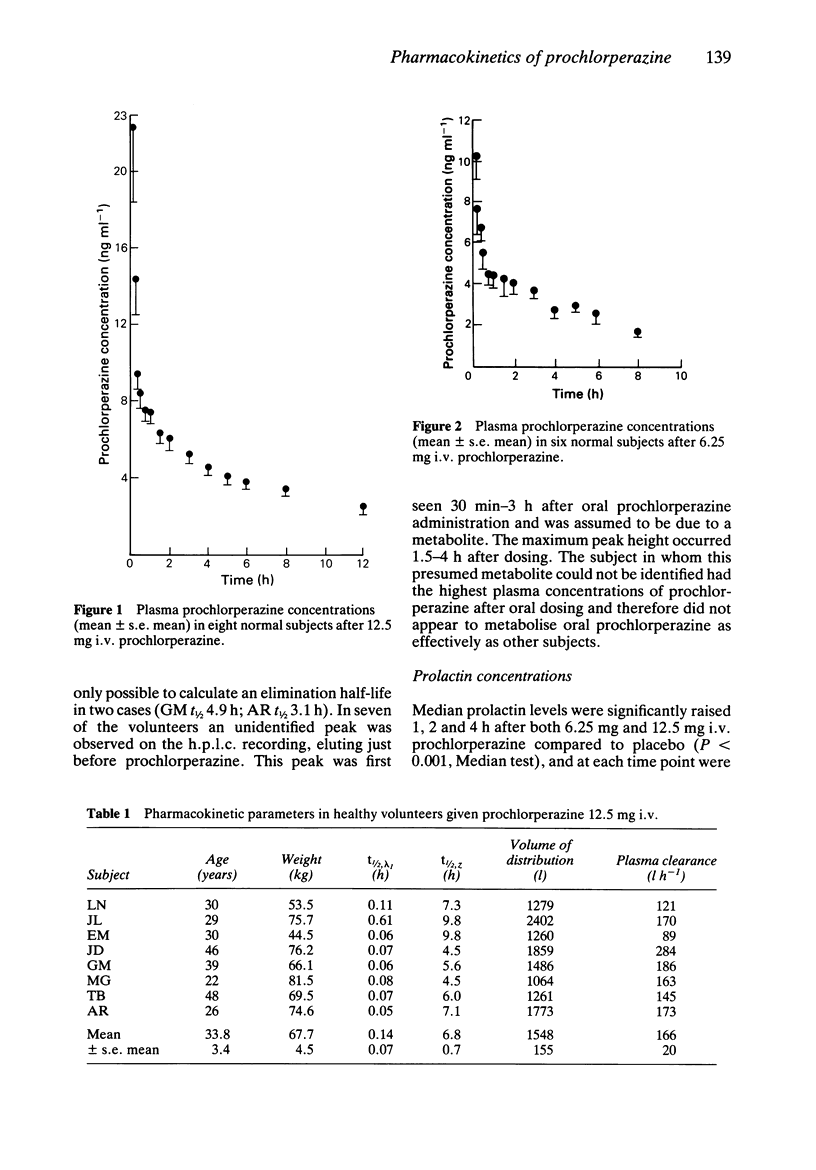
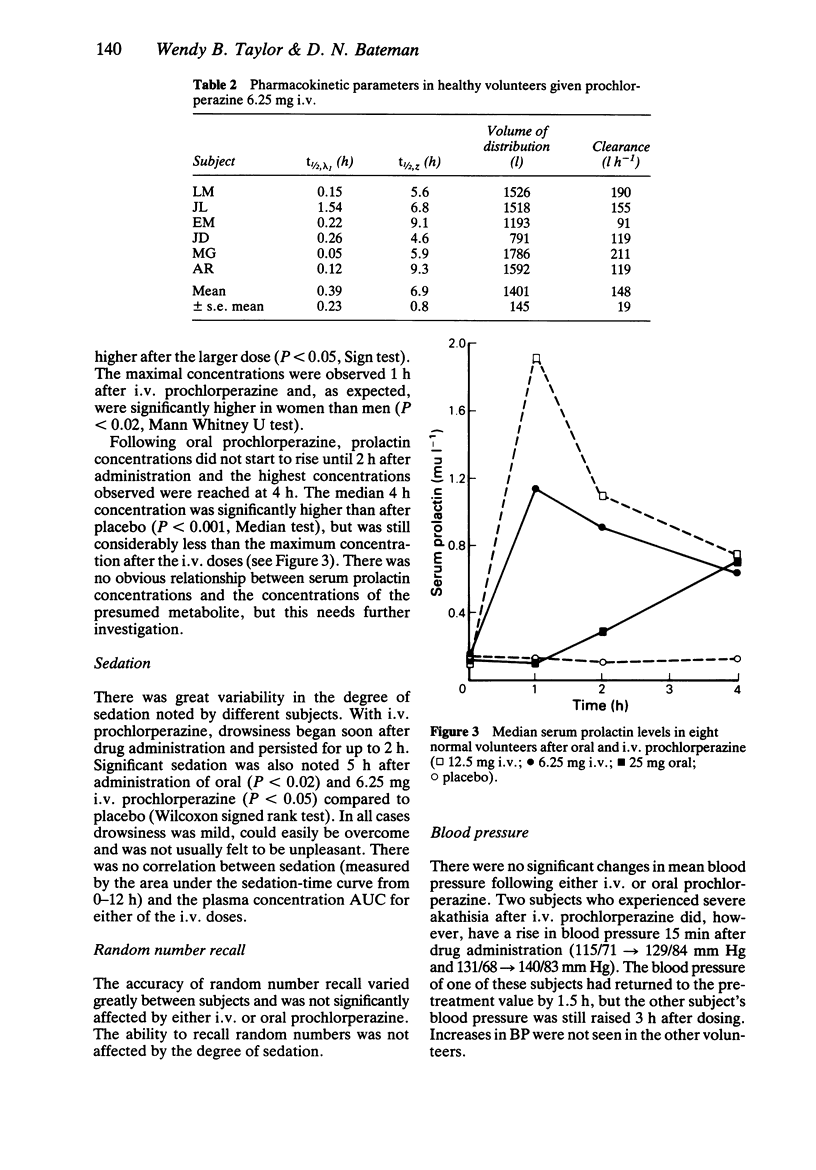
The pharmacokinetics and pharmacodynamics of prochlorperazine were studied in healthy volunteers using a recently developed h.p.l.c. assay. Eight subjects received 12.5 mg and 6.25 mg i.v. doses of prochlorperazine, a 25 mg oral dose and placebo in random order. Plasma half-life (t1/2) of prochlorperazine was 6.8 +/- 0.7 h and 6.9 +/- 0.8 h for the 12.5 mg and 6.25 mg i.v. doses respectively. Apparent volume of distribution and plasma clearance were high and the kinetics did not appear to be dose-related. Absorption of oral prochlorperazine appeared to be slow and bioavailability was very low. A metabolite, possibly prochlorperazine sulphoxide, was noted after oral dosing. Mild sedation was common after i.v. prochlorperazine, but cardiovascular effects were minimal. The main adverse effect was akathisia which was reported by five out of eight subjects after the higher i.v. dose. These results provide preliminary information on the pharmacokinetics of i.v. prochlorperazine which were previously unknown.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYD F. J., Jr A survey of drug-induced extrapyramidal reactions. JAMA. 1961 Mar 25;175:1054–1060. doi: 10.1001/jama.1961.03040120016004. [DOI] [PubMed] [Google Scholar]
- Bardfeld P. A. A controlled double-blind study of trimethobenzamide, prochlorperazine, and placebo. JAMA. 1966 May 30;196(9):796–798. [PubMed] [Google Scholar]
- Bateman D. N., Gokal R., Dodd T. R., Blain P. G. The pharmacokinetics of single doses of metoclopramide in renal failure. Eur J Clin Pharmacol. 1981;19(6):437–441. doi: 10.1007/BF00548588. [DOI] [PubMed] [Google Scholar]
- Bateman D. N., Kahn C., Mashiter K., Davies D. S. Pharmacokinetic and concentration-effect studies with intravenous metoclopramide. Br J Clin Pharmacol. 1978 Nov;6(5):401–407. doi: 10.1111/j.1365-2125.1978.tb04604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl S. G., Strandjord R. E. Pharmacokinetics of chlorpromazine after single and chronic dosage. Clin Pharmacol Ther. 1977 Apr;21(4):437–448. doi: 10.1002/cpt1977214437. [DOI] [PubMed] [Google Scholar]
- FRIEND D. G., MCLEMORE G. A., Jr Antiemetic properties of a new chlorphenothiazine derivative, proclorperazine. AMA Arch Intern Med. 1957 May;99(5):732–735. doi: 10.1001/archinte.1957.00260050060007. [DOI] [PubMed] [Google Scholar]
- Fowler A., Taylor W., Bateman D. N. Plasma prochlorperazine assay by high-performance liquid chromatography--electrochemistry. J Chromatogr. 1986 Jul 11;380(1):202–205. [PubMed] [Google Scholar]
- Graffner C., Lagerström P. O., Lundborg P., Rönn O. Pharmacokinetics of metoclopramide intravenously and orally determined by liquid chromatography. Br J Clin Pharmacol. 1979 Nov;8(5):469–474. doi: 10.1111/j.1365-2125.1979.tb01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla R. J. Metoclopramide. A review of antiemetic trials. Drugs. 1983 Feb;25 (Suppl 1):63–73. doi: 10.2165/00003495-198300251-00007. [DOI] [PubMed] [Google Scholar]
- Sankey M. G., Holt J. E., Kaye C. M. A simple and sensitive H.P.L.C. method for the assay of prochlorperazine in plasma. Br J Clin Pharmacol. 1982 Apr;13(4):578–580. doi: 10.1111/j.1365-2125.1982.tb01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


