Abstract
gACRP30, the globular subunit of adipocyte complement-related protein of 30 kDa (ACRP30), improves insulin sensitivity and increases fatty acid oxidation. The mechanism by which gACRP30 exerts these effects is unknown. Here, we examined if gACRP30 activates AMP-activated protein kinase (AMPK), an enzyme that has been shown to increase muscle fatty acid oxidation and insulin sensitivity. Incubation of rat extensor digitorum longus (EDL), a predominantly fast twitch muscle, with gACRP30 (2.5 μg/ml) for 30 min led to 2-fold increases in AMPK activity and phosphorylation of both AMPK on Thr-172 and acetyl CoA carboxylase (ACC) on Ser-79. Accordingly, concentration of malonyl CoA was diminished by 30%. In addition, gACRP30 caused a 1.5-fold increase in 2-deoxyglucose uptake. Similar changes in malonyl CoA and ACC were observed in soleus muscle incubated with gACRP30 (2.5 μg/ml), although no significant changes in AMPK activity or 2-deoxyglucose uptake were detected. When EDL was incubated with full-length hexameric ACRP30 (10 μg/ml), AMPK activity and ACC phosphorylation were not altered. Administration of gACRP30 (75 μg) to C57 BL/6J mice in vivo led to increased AMPK activity and ACC phosphorylation and decreased malonyl CoA concentration in gastrocnemius muscle within 15–30 min. Both in vivo and in vitro, activation of AMPK was the first effect of gACRP30 and was transient, whereas alterations in malonyl CoA and ACC occurred later and were more sustained. Thus, gACRP30 most likely exerts its actions on muscle fatty acid oxidation by inactivating ACC via activation of AMPK and perhaps other signal transduction proteins.
Keywords: diabetes, insulin sensitivity, obesity, malonyl CoA
The hormone ACRP30, also referred to as adiponectin and adipo Q (1), is exclusively secreted by differentiated adipocytes (2). It circulates in serum as three distinct oligomers: trimer, hexamer, and an even higher molecular weight species (3). Early studies suggested that ACRP30 might participate in energy homeostasis because its mRNA was decreased in obese mice (4) and humans (5). In addition, a negative correlation between plasma ACRP30 levels and both insulin resistance and obesity has been described (6), as have polymorphisms in the ACRP30 locus in patients with the metabolic (insulin resistance) syndrome (7, 8) and some populations with type 2 diabetes (9). A proteolytic cleavage product of ACRP30 that includes its globular head group, gACRP30, has been found to circulate in human plasma (10). Recent studies have shown that bacterially expressed and purified gACRP30 is pharmacologically active and induces free fatty acid oxidation in incubated mouse muscles and cultured muscle cells (10). In addition, when administered to intact mice with a fat meal, it modestly lowers plasma-free fatty acid and glucose levels, and when administered chronically, it causes weight loss without diminishing food intake (10). In contrast, full-length ACRP30 injection had no effect on plasma-free fatty acid levels and muscle fatty acid oxidation (10). We previously reported that the hexamer and high molecular weight species of ACRP30 can activate transcription factor NF-κB following serine phosphorylation and degradation of inhibitory ΙκΒ-α (3). However, the signaling events that mediate the actions of gACRP30 are not known.
AMP-activated protein kinase (AMPK) is a fuel-sensing enzyme present in most mammalian tissues (11). In response to a decrease in the energy state of a cell, as reflected by an increase in the AMP/ATP ratio, AMPK is phosphorylated and activated by a still uncharacterized upstream AMPK kinase. In addition, it can be activated allosterically by increases in the AMP/ATP and creatine/creatine-P ratios. Activated AMPK phosphorylates a variety of intracellular proteins to increase ATP generation and decrease ATP utilization for processes not immediately essential for survival (11). The actions of AMPK have perhaps been best studied in exercising skeletal muscle, where its activation seems to contribute to increased glucose transport (12) and fatty acid oxidation (11, 13). The latter occurs because AMPK phosphorylates (Ser-79) and inhibits acetyl CoA carboxylase (ACC) and phosphorylates and activates malonyl CoA decarboxylase, leading to a decrease in the concentration of malonyl CoA (14). Malonyl CoA is an allosteric inhibitor of carnitine palmitoyl transferase, the enzyme that controls the transfer of long chain fatty acyl CoA molecules into mitochondria where they are oxidized. Thus, a decrease in its concentration increases fatty acid oxidation in all tissues so far studied (11, 13, 15). In addition, administration of the AMPK activator 5-amino 4-imidazolecarboxamide riboside (AICAR) has been shown to diminish adiposity in rats independent of its effects on food intake (16). Because of the similarity of these effects to those of gACRP30, the possibility that gACRP30 activates AMPK was examined. We describe here the effects of gACRP30 on AMPK activity and related parameters, such as malonyl CoA concentration and ACC phosphorylation, in incubated rat muscle and mouse muscle in vivo. In addition, the effect of gACRP30 on glucose transport was examined.
Materials and Methods
Animals.
Male Sprague–Dawley rats (50–65 g) were purchased from Charles River Breeding Laboratories, and 10-week-old female C57BL/6J mice (20 g) were purchased from The Jackson Laboratory. They were maintained on a 12-h light/dark cycle in a temperature-controlled (19–21°C) room and were allowed free access to water and standard rodent chow. Food was withdrawn 16–20 h before the rats were killed.
Protein Preparation and Antibodies.
Purified globular portion (gACRP30) and full-length hexameric ACRP30 were prepared from Escherichia coli as described previously (3). AMPK and Phospho-AMPK (Thr-172) antibodies were purchased from Cell Signaling Technology (Beverly, MA), and antiphospho-ACC (Ser-79) antibody was purchased from Upstate Biochemicals (Waltham, MA). AMPK antibody suitable for blotting and immunoprecipitating assayable AMPK activity in mice was kindly provided by Dr. Laurie Goodyear (Joslin Diabetes Center, Boston). Fatty acid synthase for the malonyl CoA assay was prepared by a modification of the method of McGarry as described (17).
Muscle Incubations.
On the experimental day, rats were anesthetized with pentobarbital sodium (60 mg/kg i.p.), and either soleus or extensor digitorum longus (EDL) muscles strips were prepared and tied to stainless steel clips as described previously (17). Muscles were preincubated for 20 min at 37°C in oxygenated (95% O2/5% CO2) Krebs–Henseleit solution containing 6 mM glucose and then incubated for 15, 30, or 60 min in the same medium in the absence or presence of gACRP30 (2.5 μg/ml) or full-length ACRP30 hexamer (10 μg/ml) for 30 min. At the end of this incubation, muscles were either used directly for analyses or for a second incubation, during which glucose transport was evaluated (see below).
In Vivo Studies in Mice.
On the day of the experiment, gACRP30 (75 μg/animal) or PBS was injected retroorbitally into female C57BL/6J mice (weighing ≈20 g each). After 15 or 30 min, the mice were anesthetized with pentobarbital sodium (60 mg/kg i.p.), and the gastrocnemius muscle was quickly removed and frozen in liquid N2. The muscles were stored at −80°C until analyzed.
Glucose Transport.
Glucose transport was assessed on the basis of 2-deoxyglucose uptake as described (18). Briefly, after the 30-min incubation with or without gACRP30, rat EDL muscles were washed for 2 min in fresh Krebs–Henseleit solution (6 mM glucose) and then incubated for 20 min in the same medium containing 2-[1,2-3H]deoxy-D-glucose. No insulin was added. At the end of the incubation, muscles were quickly removed from the medium, blotted, frozen in liquid N2, and stored at −80°C until analyzed.
AMPK Activity and Malonyl–CoA Concentration.
AMPK activity was assayed in α2 AMPK immunoprecipitates of the muscle homogenate as described (19). Malonyl CoA was measured radioenzymatically in neutralized perchloric acid extracts (17) as described elsewhere.
Western Analysis.
Fifty micrograms of crude muscle homogenate was electrophoresed and transferred to poly(vinylidene difluoride) membrane (Bio-Rad). After transfer, the membranes were blocked with 5% BSA in TBS (25 mM Tris/135 mM NaCl/2.5 mM KCl)/0.05% Tween 20 (TBST) for 1 h at room temperature (blots of phospho-AMPK and AMPK) or with 5% nonfat dry milk in TBST overnight at 4°C (blots of phospho-ACC). The membranes were incubated with the specific antibodies and then with secondary antibodies conjugated to horseradish peroxidase from Amersham Pharmacia or Santa Cruz Biotechnology (AMPK, PACC). Bands were visualized by enhanced chemiluminescence and were quantitated by laser densitometry. Immunoblots were performed under conditions in which autographic detection was in the linear response range.
Statistical Analysis.
All data are expressed as means ± SE. Statistical significance between groups was assessed by Student's t test.
Results
Effect of gACRP30 on AMPK Activity and Phosphorylation and AMPK-Mediated Events in the Incubated EDL Muscle.
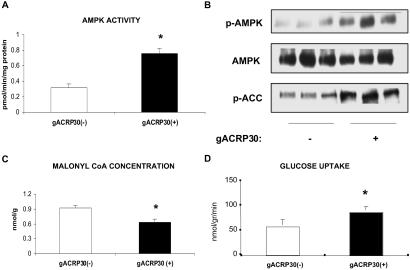
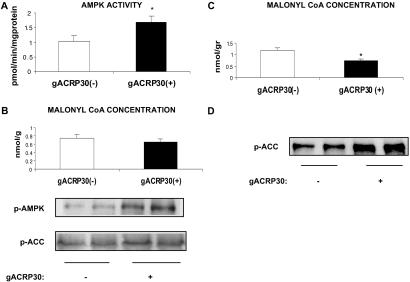
Incubation of the EDL, a muscle containing predominantly fast-twitch white fibers (type 2b), with gACRP30 (2.5 μg/ml or 50 nM globular trimer) for 30 min led to a 2-fold increase in the activity of the α-2 isoform of AMPK (Fig. 1A). This was associated with a comparable increase in the phosphorylation of Thr-172 of AMPK but no change in total AMPK abundance (Fig. 1B). Consistent with the results of prior studies in which AMPK activity in muscle was increased by exercise, electrically induced contractions, or exposure to AICAR, phosphorylation of ACC on Ser-79 was increased nearly 2-fold (Fig. 1B), and the concentration of malonyl CoA was diminished to 68% of control (Fig. 1C) (11, 19). In addition, after 30 min of incubation with gACRP30, glucose transport in the absence of added insulin, assessed by 2-deoxyglucose uptake, was increased by 50% (Fig. 1D).
Fig 1.
Effect of incubation with gACRP30 on AMPK and related parameters. EDL muscles from 60-g rats were incubated for 30 min in the presence (+) or absence (−) of gACRP30 (2.5 μg/ml) and then frozen in liquid N2 until analyzed. (A) AMPK activity in immunoprecipitates obtained with antibody to the α2 isoform. (B) Western blots of AMPK phosphorylated at Thr-172 (active enzyme; p-AMPK), total AMPK abundance (anti-α1 and anti-α2 AMPK antibodies; AMPK), and ACC phosphorylated at Ser-79 (inhibited enzyme; p-ACC). (C) Concentration of malonyl CoA. Blots are representative of muscles of three to five animals. Measurements of AMPK activity and malonyl CoA concentration are means ± SE (n = 5). (D) Effect of gACRP30 on glucose transport in rat EDL. Muscles were incubated with (+) or without (−) gACRP30 (2.5 μg/ml) for 30 min. Glucose transport was then assessed in the absence of insulin based on the uptake of [3H]-2-deoxyglucose. Measurements of glucose transport are means ± SE (n = 6). Additional details are described in Materials and Methods. *, P < 0.05 vs. gACRP30 (−) group.
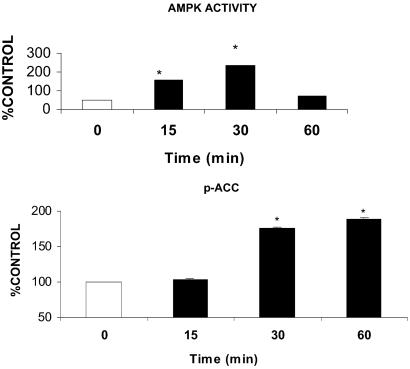
Time Course of Changes Induced by gACRP30 in the EDL.
As already noted, AMPK activity and phosphorylation, ACC phosphorylation, and the concentration of malonyl CoA were all significantly altered after 30 min of incubation with gACRP30. The time of onset and the duration of these effects did not seem to be the same, however (Fig. 2). After 15 min of incubation with gACRP30, the activity of AMPK was already increased (1.5 fold), albeit somewhat less than at 30 min (2-fold); however, ACC phosphorylation was only slightly increased above control muscles. Likewise, after 60 min of incubation with gACRP30, ACC phosphorylation was increased, as it was at 30 min; however, AMPK had returned to baseline values. Thus, AMPK activation seemed to be the earliest event altered by gACRP30, but it was relatively short-lived. In contrast, the changes in ACC phosphorylation occurred later and were more sustained.
Fig 2.
Time course of changes in AMPK activity and ACC phosphorylation in rat EDL incubated with and without gACRP30. AMPK activity is increased at 15 and 30 min and then returns to baseline values, whereas significant increases in ACC phosphorylation first become significant at 30 min and persist at 60 min. Results are means ± SE (n = 5) and are expressed as value for gACRP30 (−) group at each time point. *, P < 0.05 vs. 0 min.
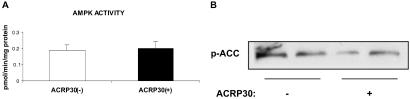
Effect of ACRP30 on the EDL.
In contrast to gACRP30, full-length ACRP30 hexamer at 10 μg/ml (67 nM) had no effect on either AMPK activity or ACC phosphorylation in the incubated EDL (Fig. 3). No changes in malonyl CoA concentration were detected in muscles incubated with 5 μg/ml ACRP30 (data not shown).
Fig 3.
Lack of effect of incubation with full-length ACRP30 on AMPK activity (A) and ACC phosphorylation (B). EDL were incubated for 30 min in the presence (+) or absence (−) of ACRP30 hexamer (10 μg/ml). Blots are representative of muscles of two to four animals. Measurements of AMPK activity are means ± SE (n = 4).
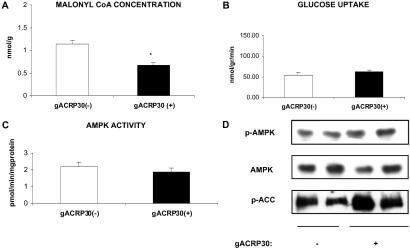
Effect of gACRP30 on AMPK Activity and Related Parameters in the Incubated Soleus Muscle.
Incubation with gACRP30 (2.5 μg/ml) for 30 min increased ACC phosphorylation and decreased the concentration of malonyl CoA (Fig. 4 A and D) in the incubated soleus, a muscle containing predominantly slow-twitch red (type 1) fibers. On the other hand, it did not activate AMPK or stimulate glucose transport (Fig. 4 B and C), as it did in the EDL (Fig. 1). Nevertheless, fatty acid oxidation was increased by 60% (control, 50 ± 2.5 nmol/h per g; gACRP30, 80 ± 3 nmol/h per g). The similarities and differences in the responses of the two muscles are identical to those previously reported by some (20) but not all (21) investigators when they are incubated with AICAR. Perhaps our experimental conditions did not permit detection of AMPK activation because, as shown in Fig. 2, activation of AMPK by gACRP30 is transient. Perhaps AMPK activity had already returned to baseline when we first measured it. Alternatively, other kinases may be responsible for phosphorylation and deactivation of ACC in the soleus.
Fig 4.
Effect of gACRP30 on AMPK activity and related parameters in the incubated rat soleus. Muscles were incubated for 30 min in the presence (+) and absence (−) of gACRP30 (2.5 μg/ml). (A) Malonyl CoA concentration. (B) Uptake of 2-deoxyglucose. (C) AMPK activity. (D) Phosphorylation of AMPK and ACC and AMPK abundance. Similar differences between the soleus and EDL (see Fig. 1) have been observed when these muscles are incubated with the AMPK activator AICAR (20). See the legend to Fig. 1 for additional details. Blots are representative of muscles of two to five animals. Measurements of AMPK activity and malonyl CoA concentration are means ± SE (n = 5). *, P < 0.05 vs. gACRP30 (−) group.
Effects of gACRP30 Administered in Vivo on AMPK Activity and Related Parameters.
To determine whether gACRP30 has similar effects on AMPK activity in vivo, female C57 BL/6J mice (20 g) were fasted overnight and then injected retroorbitally with 75 μg of gACRP30. AMPK activity and related parameters were assayed in the gastrocnemius, a mixed red and white muscle at 15 and 30 min later. As shown in Fig. 5, AMPK activity and phosphorylation were substantially increased at the 15-min time point; however, ACC phosphorylation and malonyl CoA concentration were not significantly altered (Fig. 5 A and B). At 30 min, substantial increases in ACC phosphorylation and a 60% decrease in malonyl CoA concentration were observed (Fig. 5 C and D).
Fig 5.
Effect of gACRP30 administered in vivo on AMPK activity and related parameters in mouse gastrocnemius muscle. Ten-week-old female C57BL/6J mice were fasted for 16 h, and then gACRP30 (75 μg per animal) (+) or saline [gACRP30 (−)] was injected into the retroorbital sinus. The rats were anesthetized with pentobarbital and muscle frozen in situ 15 or 30 min later. (A) AMPK activity (mean ± SE, n = 5) and malonyl CoA (n = 4). (B) Representative Western blots (n = 5) at 15 min. (C) Malonyl CoA concentration (mean ± SE, n = 5). (D) Representative Western blots of phosphorylated ACC at 30 min (n = 4). *, P < 0.05 vs. control.
Discussion
Our results suggest that gACRP30 stimulates fatty acid oxidation in skeletal muscle by inactivating ACC and activating AMPK. The evidence is as follows. First, gACRP30 increased the phosphorylation and activity of AMPK in EDL and gastrocnemius skeletal muscle when administered in vivo or in vitro. Second, increases in AMPK activity caused by gACRP30 were associated with increases in ACC phosphorylation and decreases in the concentration of malonyl CoA. Third, like other stimuli that increase AMPK, such as AICAR, muscle contraction, and hypoxia (12), gACRP30 enhanced insulin-independent glucose transport in incubated EDL muscle. In addition to these findings, prior studies showed that the administration of gACRP30 in vivo increases insulin sensitivity in muscle and causes a loss of body weight without altering food intake in mice (10). These effects have also been observed in rats following the administration of AICAR (22–24).
The results also reveal that the activation of AMPK and inhibition of ACC by gACRP30 are sequential events that can be distinguished temporally in muscles with high fast-twitch fiber content. Phosphorylation and activation of AMPK were evident at 15 min, whereas the phosphorylation of ACC and decrease in malonyl CoA concentration predominantly occurred later, both under in vivo and in vitro incubation conditions. Also of note, the activation of AMPK was relatively transient and had disappeared by 60 min, whereas ACC phosphorylation was still markedly increased at this time. This suggests that brief periods of AMPK activation have a more sustained effect on target proteins and presumably the processes that they regulate. A similar pattern of events has been observed in rat skeletal muscle after short bouts of high intensity exercise (25).
gACRP30 increased ACC phosphorylation and fatty acid oxidation and decreased the concentration of malonyl CoA in incubated rat soleus muscle but failed to increase either AMPK activity or glucose transport, as it did in the EDL. Similar differences between the two muscles have been observed when they are incubated with AICAR (20). This probably reflects some property of the rat soleus muscle when it is incubated, as this disparity was not observed when AMPK is activated by AICAR treatment in vivo (26). On the other hand, the possibility that signaling mechanisms not including AMPK led to ACC phosphorylation following treatment of the soleus with gACRP30 has not been ruled out.
Previous studies have shown that amounts of full-length ACRP30 equimolar to gACRP30 do not increase fatty acid oxidation in incubated muscle (10). Consistent with these findings, incubation of the EDL with ACRP30 at a similar or even higher molarity than that used in the gACRP30 experiments failed to increase either AMPK activity or ACC phosphorylation. In contrast to its lack of effect on muscle, ACRP30, but not gACRP30, has been shown to inhibit hepatic glucose production (27), at least in part by inhibiting the synthesis of the gluconeogenic enzymes phosphoenolpyruvate carboxykinase and glucose 6-phosphatase at the level of transcription (28). Activation of AMPK in liver in vivo by AICAR (29) has been shown to have a similar effect. Direct evidence that AMPK mediates the effects of full-length ACRP30 in liver has recently been reported (30).
Independent of the studies reported here, Yamauchi et al. (30) have carried out a series of investigations in which the effects of gACRP30 and ACRP30 on AMPK activation were examined. In agreement with our findings, they observed that gACRP30 increased AMPK activity in cultured C2C12 myocytes and, when administered in vivo, in mouse soleus muscle. In addition, they found that ACRP30, but not gACRP30, activated AMPK and decreased the expression of the mRNAs for phosphoenolpyruvate carboxykinase and glucose 6-phosphatase in liver. The data reported here, together with the findings of Yamauchi et al. (30) and those by Lopaschuck's laboratory in isolated cardiomyocytes (31), add gACRP30/ACRP30 to leptin (32), α-adrenergic (32), and β-adrenergic (33) agonists as endocrine or neuroendocrine factors that can activate AMPK in vivo. Whether these agents work by altering the energy state of a cell or by some other mechanism remains to be determined.
Despite many similarities, the studies of Yamauchi et al. (30) differ from ours in one important aspect. Yamauchi et al. reported that both gACRP30 and full-length ACRP30 are able to activate AMPK in muscle. They also observed that full-length ACRP30 administered in vivo (50 μg/10 g) caused a modest increase in AMPK phosphorylation and activity in the mouse soleus. In contrast, we did not observe AMPK activation using the purified hexameric isoform of full-length ACRP30. As described previously, ACRP30 produced in E. coli by recombinant DNA technology is a mixture of hexamer and two types of trimers: trimer A and trimer B (3). Trimer A contains three full-length ACRP30 polypeptides, whereas trimer B is a heterotrimer containing one N-terminally truncated ACRP30 monomer and two full-length monomers (3). Unable to form a rigid collagen triple helix, trimer B may be functionally similar to gACRP30. Thus, in the study reported by Yamauchi et al., activation of AMPK by full-length ACRP30 may be due to the presence of trimer B and/or trimer A in their preparation. Alternatively, variations in protein expression and preparation methods may have contributed to the different results between the two studies.
A variety of data suggest that ACRP30/gACRP30 enhances insulin actions in vivo under physiological conditions. ACRP30-deficient mice develop insulin resistance when fed a high fat diet (34), and polymorphisms of the ACRP30 gene in humans are linked to increased risk for type 2 diabetes and the metabolic syndrome (8–10). In addition, a number of studies have shown that thiazolidinediones, a class of insulin-sensitizing drugs used to improve glucose tolerance in patients with type 2 diabetes, stimulate ACRP30 production by adipocytes and increase its concentration in plasma (1, 35). Finally, prior exercise (36, 37) and the administration of AICAR (24) and the anti-diabetic agent metformin (38), all activate AMPK and enhance insulin action in rodents and humans. Thus, the insulin-sensitizing action of ACRP30 could be mediated by AMPK.
A final question about ACRP30 and gACRP30 relates to their structural homology to TNFα (39). TNFα has been shown to alter the expression of a wide variety of proinflammatory and other genes in adipocytes by activating NF-κB (40). The high molecular weight and hexameric isoforms of ACRP30 also activate NF-κB (3). On the other hand, recent studies by Ido and his coworkers (41, 42) have shown that AMPK activation by AICAR or expression of a constitutively active AMPK can inhibit NF-κB-mediated gene expression in both cultured human umbilical vein endothelial cells and COS7 cells. Thus, the interaction of gACRP30 and the various oligomeric forms of ACRP30 in regulating NF-κB activity, and in turn its relevance to their biological actions, clearly requires further study.
In conclusion, gACRP30 seems to stimulate muscle fatty acid oxidation by inactivating ACC and decreasing malonyl CoA concentration. These events and increased glucose transport may be explained by AMPK activation. Whether gACRP30/ACRP30 enhances insulin action in vivo by activating AMPK remains to be determined.
Acknowledgments
We thank Scarlet Constant and Murwarid M. Assifi for technical assistance. This work was supported in part by U.S. Public Health Service Grants DK 19514 and 19417 (to N.B.R. and A.K.S.) and R37DK47618 (to H.F.L.), by a grant from Genset Corporation (to H.F.L.), and by mentor-based grants from the American Diabetes Association (to N.B.R. and H.F.L.). T.-S.T. is supported by fellowships from the Ares-Serono Foundation and the American Diabetes Association.
Abbreviations
AMPK, AMP-activated protein kinase
EDL, extensor digitorum longus
ACC, acetyl CoA carboxylase
AICAR, 5-amino 4-imidazolecarboxamide riboside
References
- 1.Tsao T. S., Lodish, H. F. & Fruebis, J. (2002) Eur. J. Pharmacol. 440, 213-221. [DOI] [PubMed] [Google Scholar]
- 2.Scherer P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. (1995) J. Biol. Chem. 270, 26746-26749. [DOI] [PubMed] [Google Scholar]
- 3.Tsao T. S., Murrey, H. E., Hug, C., Lee, D. H. & Lodish, H. F. (2002) J. Biol. Chem. 277, 29359-29362. [DOI] [PubMed] [Google Scholar]
- 4.Arita Y., Kihara, S., Ouchi, N., Takahashi, M., Meada, K., Miyagawa, J., Hotta, K., Shimomura, I., Nakamura, I., Miyaoka, K., et al. (1999) Biochem. Biophys. Res. Commun. 257, 79-83. [DOI] [PubMed] [Google Scholar]
- 5.Hotta K., Funahashi, T., Arita, Y., Takahashi, M., Matsuda, M., Okamoto, Y., Iwahashi, H., Kuriyama, H., Ouchi, N., Maeda, K., et al. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 1595-1599. [DOI] [PubMed] [Google Scholar]
- 6.Hotta K., Funahashi, T., Bodkin, N. L., Ortmeyer, H. K., Arita, Y., Hansen, B. C. & Matsuzawa, Y. (2001) Diabetes 50, 1126-1133. [DOI] [PubMed] [Google Scholar]
- 7.Kissebah A. H., Sonnenberg, G. E., Myklebust, J., Goldstein, M., Broman, K., James, R. G., Marks, J. A., Krakower, G. R., Jacob, H. J., Weber, J., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 14478-14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menzaghi C., Ercolino, T., Di Paola, R., Berg, A. H., Warram, J. H., Scherer, P. E., Trischitta, V. & Doria, A. (2002) Diabetes 51, 2306-2312. [DOI] [PubMed] [Google Scholar]
- 9.Hara K., Boutin, P., Mori, Y., Tobe, K., Dina, C., Yasuda, K., Yamauchi, T., Otabe, S., Okada, T., Eto, K., et al. (2002) Diabetes 51, 536-540. [DOI] [PubMed] [Google Scholar]
- 10.Fruebis J., Tsao, T. S., Javorschi, S., Ebbets-Reed, D., Erickson, M. R., Yen, F. T., Bihain, B. E. & Lodish, H. F. (2001) Proc. Natl. Acad. Sci. USA 98, 2005-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winder W. W. & Hardie, D. G. (1999) Am. J. Physiol. 277, E1-E10. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T., Hirshman, M. F., Fujii, N., Habinowski, S. A., Witters, L. A. & Goodyear, L. J. (2000) Diabetes 49, 527-531. [DOI] [PubMed] [Google Scholar]
- 13.Ruderman N. B., Saha, A. K., Vavvas, D. & Witters, L. A. (1999) Am. J. Physiol. 276, E1-E18. [DOI] [PubMed] [Google Scholar]
- 14.Park H., Kaushik, V., Constant, S., Prentki, M., Przybytkowski, E., Ruderman, N. B. & Saha, A. K. (2002) J. Biol. Chem. 277, 32571-32577. [DOI] [PubMed] [Google Scholar]
- 15.McGarry J. D. & Brown, M. F. (1997) Eur. J. Biochem. 244, 1-14. [DOI] [PubMed] [Google Scholar]
- 16.Winder W. W. (2000) Diabetes Technol. Ther. 2, 441-448. [DOI] [PubMed] [Google Scholar]
- 17.Saha A. K., Kurowski, T. G. & Ruderman, N. B. (1995) Am. J. Physiol. 269, E283-E289. [DOI] [PubMed] [Google Scholar]
- 18.Kurowski T. G., Lin, Y., Luo, Z., Tsichlis, P. N., Buse, M. G., Heydrick, S. J. & Ruderman, N. B. (1999) Diabetes 48, 658-663. [DOI] [PubMed] [Google Scholar]
- 19.Vavvas D., Apazidis, A., Saha, A. K., Gamble, J., Patel, A., Kemp, B. E., Witters, L. A. & Ruderman, N. B. (1997) J. Biol. Chem. 272, 13255-13261. [DOI] [PubMed] [Google Scholar]
- 20.Kaushik V. K., Young, M. E., Dean, D. J., Kurowski, T. G., Saha, A. K. & Ruderman, N. B. (2001) Am. J. Physiol. 281, E335-E340. [DOI] [PubMed] [Google Scholar]
- 21.Fryer L. G., Hajduch, E., Rencurel, F., Salt, J. P., Hundal, H. S., Hardie, D. G. & Carling, D. (2000) Diabetes 49, 1978-1985. [DOI] [PubMed] [Google Scholar]
- 22.Winder W. W., Holmes, B. F., Rubink, D. S., Jensen, E. B., Chen, M. & Holloszy, J. O. (2000) J. Appl. Physiol. 88, 2219-2226. [DOI] [PubMed] [Google Scholar]
- 23.Fisher J. S., Gao, J., Han, D. H., Holloszy, J. O. & Nolte, L. A. (2002) Am. J. Physiol. 282, E18-E23. [DOI] [PubMed] [Google Scholar]
- 24.Iglesias M. A., Ye, J., Frangioudakis, G., Saha, A. K., Tomas, E., Ruderman, N. B., Cooney, G. J. & Kraegen, E. W. (2002) Diabetes 51, 2886-2894. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen B. B., Hancock, C. R. & Winder, W. W. (1998) J. Appl. Physiol. 85, 1629-1634. [DOI] [PubMed] [Google Scholar]
- 26.Bergeron R., Russell, R. R., III, Young, L. H., Ren, J. M., Marcucci, M., Lee, A. & Shulman, G. I. (1999) Am. J. Physiol. 276, E938-E944. [DOI] [PubMed] [Google Scholar]
- 27.Berg A. H., Combs, T. P., Du, X., Brownlee, M. & Scherer, P. E. (2001) Nat. Med. 7, 947-953. [DOI] [PubMed] [Google Scholar]
- 28.Combs T. P., Berg, A. H., Obici, S., Scherer, P. E. & Rossetti, L. J. (2001) Clin. Invest. 108, 1875-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lochhead P. A., Salt, I. P., Walker, K. S., Hardie, D. G. & Sutherland, C. (2000) Diabetes 49, 896-903. [DOI] [PubMed] [Google Scholar]
- 30.Yamauchi T., Kamon, J., Minokoshi, Y., Ito, Y., Waki, H., Uchida, S., Yamashita, S., Noda, M., Kita, S., Ueki, K., et al. (2002) Nat. Med. 8, 1288-1295. [DOI] [PubMed] [Google Scholar]
- 31.Altarejos J. Y., Liew, E. & Lopaschuk, G., (2002) Abstracts of AMPK Symposium II, Dundee, Scotland.
- 32.Minokoshi Y., Kim, Y. B., Peroni, O. D., Fryer, L. G., Muller, C., Carling, D. & Kahn, B. B. (2002) Nature 415, 339-343. [DOI] [PubMed] [Google Scholar]
- 33.Moule S. K. & Denton, R. M. (1998) FEBS Lett. 439, 287-290. [DOI] [PubMed] [Google Scholar]
- 34.Maeda N., Shimomura, I., Kishida, K., Nishizawa, H., Matsuda, M., Nagaretani, H., Furuyama, N., Kondo, H., Takahashi, M., Arita, Y., et al. (2002) Nat. Med. 8, 731-737. [DOI] [PubMed] [Google Scholar]
- 35.Yu J. G., Javorschi, S., Hevener, A. L., Kruszynska, Y. T., Norman, R. A., Sinha, M. & Olefsky, J. (2002) Diabetes 51, 2968-2974. [DOI] [PubMed] [Google Scholar]
- 36.Oakes N. D., Bell, K. S., Furler, S. M., Camilleri, S., Saha, A. K., Ruderman, N. B., Chisholm, D. J. & Kraegen, E. W. (1997) Diabetes 46, 2022-2028. [DOI] [PubMed] [Google Scholar]
- 37.Tomas E., Zorzano, A. & Ruderman, N. B. (2002) J. Appl. Physiol. 93, 765-772. [DOI] [PubMed] [Google Scholar]
- 38.Zhou G., Myers, R., Li, Y., Chen, Y., Shen, X., Fenyk-Melody, J., Wu, M., Ventre, J., Doebber, T., Fujii, N., et al. (2001) J. Clin. Invest. 108, 1167-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shapiro L. & Scherer, P. E. (1998) Curr. Biol. 8, 335-338. [DOI] [PubMed] [Google Scholar]
- 40.Ruan H., Hacohen, N., Golub, T. R., Van Parijs, L. & Lodish, H. F. (2002) Diabetes 51, 1319-1336. [DOI] [PubMed] [Google Scholar]
- 41.Ido Y., Zou, M., Chen, K., Cohen, R. A., Keaney, J. F., Jr. & Ruderman, N. B. (2002) Diabetes 51, Suppl. 2, A396. [Google Scholar]
- 42.Ruderman, N. B., Cacicedo, J., Itani, S., Yagihashi, N., Saha, A. K., Ye, J., Chen, K., Zou, M., Carling, D., Cohen, R. A., et al. (2003) Biochem. Soc. Trans., in press. [DOI] [PubMed]