Abstract
The plant hormone abscisic acid (ABA), synthesized in response to water-deficit stress, induces stomatal closure via activation of complex signaling cascades. Recent work has established that nitric oxide (NO) is a key signaling molecule mediating ABA-induced stomatal closure. However, the biosynthetic origin of NO in guard cells has not yet been resolved. Here, we provide pharmacological, physiological, and genetic evidence that NO synthesis in Arabidopsis guard cells is mediated by the enzyme nitrate reductase (NR). Guard cells of wild-type Arabidopsis generate NO in response to treatment with ABA and nitrite, a substrate for NR. Moreover, NR-mediated NO synthesis is required for ABA-induced stomatal closure. However, in the NR double mutant, nia1, nia2 that has diminished NR activity, guard cells do not synthesize NO nor do the stomata close in response to ABA or nitrite, although stomatal opening is still inhibited by ABA. Furthermore, by using the ABA-insensitive (ABI) abi1–1 and abi2–1 mutants, we show that the ABI1 and ABI2 protein phosphatases are downstream of NO in the ABA signal-transduction cascade. These data demonstrate a previously uncharacterized signaling role for NR, that of mediating ABA-induced NO synthesis in Arabidopsis guard cells.
Increased biosynthesis and subsequent action of the hormone abscisic acid (ABA) is a key plant response to water-deficit stress. ABA initiates several processes, including stomatal closure, thereby leading to water conservation. The intracellular signaling cascades by which ABA effects guard cell shrinkage resulting in stomatal closure are complex, with several new signaling intermediates having been identified recently (1, 2). One such molecule is nitric oxide (NO), a signal molecule of increasing importance in plants (3, 4). Recent work has demonstrated that NO is an essential signaling intermediate in ABA-induced stomatal closure in Pisum sativum and Vicia faba (5, 6). However, despite these emerging new roles for NO, its biosynthetic origins in plants have not yet been resolved. Elucidation of the biosynthetic route(s) for NO, particularly during stomatal responses to ABA, is an important research goal, because it may facilitate the production of plants with enhanced drought tolerance.
Two potential enzymatic sources of NO in plants are NO synthase (NOS) and nitrate reductase (NR). NOS is a family of well characterized enzymes in mammalian cells that catalyze the conversion of l-arginine to l-citrulline and NO. NOS-like activity has been demonstrated in various plant tissues by using biochemical and pharmacological approaches (7). However, in Arabidopsis thaliana, NOS inhibitors have variable effects (8–10), and no obvious NOS-like sequences have been located in the Arabidopsis genome (11).
NR is a central enzyme of nitrogen assimilation in plants, catalyzing the transfer of two electrons from nicotinamide-adenine dinucleotide phosphate [NAD(P)H] to nitrate to produce nitrite (12). NR also catalyzes the NAD(P)H-dependent reduction of nitrite to NO (13), and this NO-generating capacity of NR has been demonstrated both in vitro and in vivo (14–16). However, a physiological role for NR-mediated NO synthesis has not yet been established.
In this article, we provide genetic evidence that NR-mediated NO synthesis is required for ABA-induced stomatal closure in Arabidopsis. Guard cells in epidermal peels of wild-type Arabidopsis generate NO in response to ABA and nitrite, such synthesis being essential for stomatal closure. However, in the NR double mutant nia1, nia2 that has greatly diminished NR activity (17), guard cells do not synthesize NO, nor do the stomata close in response to ABA or nitrite, although they still respond to exogenous NO. These data reveal a previously uncharacterized signaling role for NR in Arabidopsis, that of mediating ABA-induced stomatal closure.
Materials and Methods
Plant Material.
Wild type and various mutants of the Landsberg erecta (Ler) and Columbia (Col-O) ecotypes of A. thaliana were sown in Levington's F2 compost and grown under a 16-h photoperiod (250–300 μE·m−2·s−1) and 80% humidity in plant growth chambers (Sanyo Gallenkamp, Loughborough, U.K.) for 3–4 weeks before being used. The nia1, nia2 double mutant seeds (background Col-O) were obtained from the Nottingham Arabidopsis Stock Centre (Nottingham, U.K.); abi1–1 seeds (background Ler) were obtained from Peter Morris (Heriot-Watt University, Edinburgh); and abi2–1 seeds (background Ler) were obtained from Maarten Koornneef (Wageningen University and Research Centre, Wageningen, The Netherlands). abi1–1 and abi2–1 genotypes were confirmed by diagnostic PCR (18). For all experiments using mutants, the appropriate background was used for wild-type controls.
Stomatal Bioassays.
Stomatal assays were performed with epidermal peels and leaves, as indicated in the figures. Stomatal bioassays using leaves and epidermal fragments were carried out essentially as described (1). For experiments using epidermal peels, leaves were fixed onto cellotape with the abaxial side stuck down. The mesophyll cells were subsequently peeled off by using another strip of cellotape, and peels left stuck to the cellotape were incubated in CO2-free Mes/KCl buffer (5 mM KCl/10 mM Mes/50 μM CaCl2, pH 6.15) for 3 h. Once the stomata were fully open, peels were treated with ABA or various compounds and incubated in the same buffer for a further 3 h. Stomatal apertures were measured by using a light microscope (20 stomata per treatment) with a calibrated micrometer scale. Data are presented as the mean of three independent experiments.
Confocal Microscopy.
NO measurement was performed by using the fluorescent NO indicator dye DAF-2DA (diaminofluorescein diacetate, Calbiochem). Epidermal strips were prepared by homogenizing leaves in a Waring blender for 20 s, and the strips were collected on a 100-μm nylon mesh (SpectraMesh, BDH-Merck) and incubated for 2–3 h in Mes/KCl buffer. After this step, the strips were loaded with 10 μM DAF-2DA for 10 min, followed by a wash step (with Mes/KCl buffer) for 20 min. The strips were subsequently incubated in buffer alone or treated with ABA, nitrite, or other compounds for various times as indicated in the text, before imaging with confocal microscopy (excitation 488 nm, emission 515–560 nm; Nikon PCM2000). Data acquired from the confocal microscope were analyzed by using scion image software (Scion, Frederick, MD). Data are presented either as a percentage of fluorescence intensities of control-treated guard cells or as average intensities from several guard cells analyzed in different experiments.
Results
ABA and Nitrite Induce NO Synthesis in Arabidopsis Guard Cells.
To determine the effects of ABA on NO synthesis in Arabidopsis guard cells, epidermal fragments prepared from Arabidopsis leaves were treated with 50 μM ABA, and NO synthesis was observed by using the fluorescent dye DAF-2DA and confocal microscopy (Fig. 1a). ABA induced a rapid increase in NO synthesis; a maximum response was reached within 10 min and persisted for at least 60 min (data not shown). Fluorescence was apparent throughout the cytoplasm and, in some cells, was particularly enhanced in and around the chloroplasts, in a manner similar to the ABA-induced DAF-2DA fluorescence previously observed in P. sativum and V. faba guard cells (5, 6). NO synthesis induced by ABA preceded stomatal closure (data not shown).
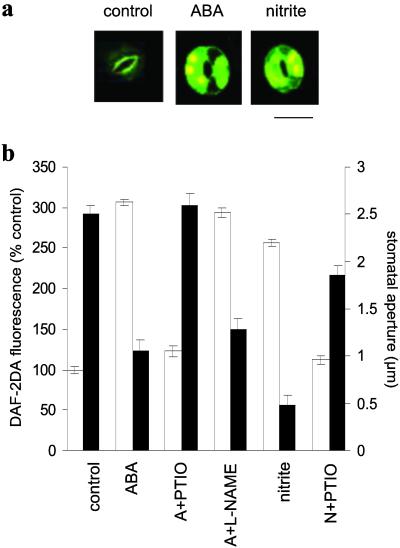
Fig. 1.
ABA- and nitrite-induced NO generation correlate with stomatal closure. (a) ABA and nitrite induce NO synthesis in Arabidopsis guard cells. NO synthesis in wild-type epidermal fragments was monitored by DAF-2DA fluorescence (after 30 min) in the absence (control) or presence of ABA (50 μM) or sodium nitrite (1 mM). (Bar = 14 μm.) (b) NO generation (white bars) was determined by DAF-2DA fluorescence of wild-type guard cells either without (control) or with ABA (A, 50 μM) or sodium nitrite (N, 1 mM) in the presence or absence of PTIO (200 μM) or l-NAME (25 μM). Fluorescence intensities were determined 1 h after treatment (n = 43–252 for various treatments ± SE). Stomatal apertures (black bars) were measured for the same treatments in wild-type epidermal peels. Data are from three independent experiments (n = 60 stomata per treatment ± SE).
Nitrite is a substrate for NO synthesis mediated by NR both in vitro and in vivo (14, 15). To determine the potential role of NR in ABA-induced NO synthesis in Arabidopsis guard cells, epidermal fragments were incubated in sodium nitrite (1 mM) and DAF-2DA-fluorescence monitored. Nitrite induced NO synthesis in a manner similar to ABA (Fig. 1a), suggesting that guard cell NO synthesis might be mediated by NR. At this concentration, within the physiological range used in vitro (14) and measured in vivo (15), nitrite was not toxic to the cells (data not shown). Epidermal fragments also were loaded with 4AF-DA to serve as a negative control for NO-induced DAF-2DA fluorescence, as described (6). ABA treatment did not induce any increase in 4AF-DA fluorescence (data not shown).
NO Is Required for ABA- and Nitrite-Induced Stomatal Closure.
Induction of NO synthesis is required for ABA-induced stomatal closure in pea and V. faba (5, 6). To determine the involvement of NO in the regulation of stomatal aperture in Arabidopsis, the effects of two NO donors, sodium nitroprusside (SNP) and S-nitrosoglutathione (GSNO), were assessed. Both SNP and GSNO induced stomatal closure (data not shown), similar to their effects in pea (5).
The relationship between NO synthesis and stomatal closure was investigated further by assessing these responses to the same treatments. A striking correlation between NO generation and stomatal closure was observed (Fig. 1b). Generation of NO after treatment of epidermal fragments with either ABA or nitrite correlated with stomatal closure. On the other hand, removal of NO by coincubation with the NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO) reduced both stomatal closure and DAF-2DA fluorescence induced by ABA or nitrite (Fig. 1b). Moreover, incubation in NaCl (as a negative control for sodium nitrite) did not induce closure (data not shown), indicating that the nitrite effects were not simply ionic. These data indicate that NO synthesis is required for stomatal closure in response to ABA or nitrite.
Various compounds have been used to inhibit NO synthesis in plants (7). The NOS inhibitors Nω-nitro-l-arginine methyl ester (l-NAME) and NG-monomethyl-l-arginine monoacetate (l-NMMA) have been used to conclude that NOS is a potential source of NO in several plant species (5, 19, 20), including Arabidopsis (8, 10). However, l-NAME had little effect on ABA-induced stomatal closure or NO synthesis in Arabidopsis epidermal peels (Fig. 1b), implying that ABA-induced NO synthesis in Arabidopsis guard cells proceeds via a NOS-independent route. Taken together, the induction by nitrite of NO synthesis and stomatal closure and the lack of inhibition by l-NAME indicate NR as the likely source of NO in the ABA signaling cascade in Arabidopsis guard cells.
Nitrate Reductase Mediates ABA-Induced NO Synthesis and Stomatal Closure in Guard Cells.
Currently, there are no specific inhibitors of NR available. Consequently, we adopted a genetic approach to provide evidence of a signaling role for NR in ABA-induced stomatal closure. Two genes in the Arabidopsis genome, NIA1 and NIA2, encode nitrate reductase. The double mutant, nia1, nia2, that has <1% of the NR activity of the wild type (17) did not emit NO under conditions in which the wild type did (21), although wounding-induced NO production was still observed (10). It is not yet known which of the two NIA genes is expressed in guard cells; therefore, the double mutant was used to determine the role of NR in ABA-mediated NO synthesis and stomatal closure.
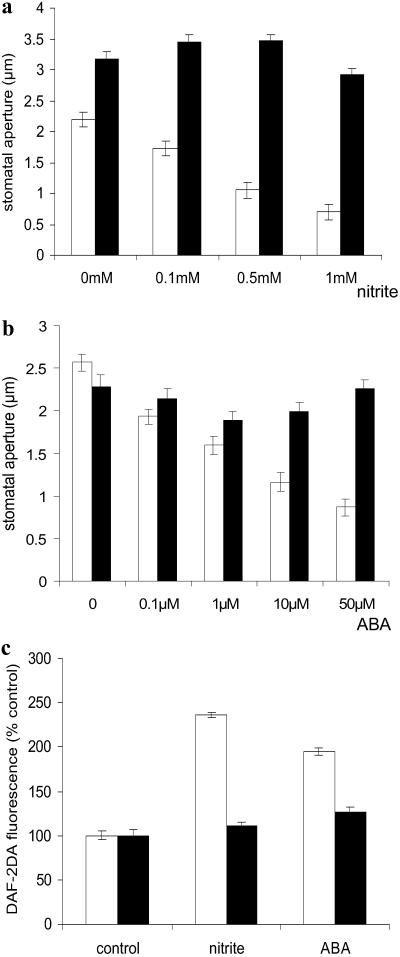
Nitrite failed to induce stomatal closure in epidermal peels of the nia1, nia2 mutant, whereas there was a clear dose–response in the wild type (Fig. 2a). Similarly, nia1, nia2 stomata were far less sensitive to ABA (Fig. 2b). This insensitivity of nia1, nia2 stomata to nitrite and ABA correlated with their inability to generate NO: both ABA and nitrite, when used at concentrations that induce NO synthesis in wild-type guard cells, did not induce NO synthesis in nia1, nia2 guard cells (Fig. 2c). These data demonstrate that NR is required to generate NO that subsequently mediates stomatal responses to ABA.
Fig. 2.
Nitrite and ABA do not induce stomatal closure and NO synthesis in the nia1, nia2 NR-deficient mutant. (a) Nitrite-induced stomatal closure. Epidermal peels from leaves of either wild type (white bars) or nia1, nia2 (black bars) were treated with various concentrations of sodium nitrite, and stomatal apertures were measured after 3 h. (b) ABA-induced stomatal closure. Epidermal peels from leaves of either wild type (white bars) or nia1, nia2 (black bars) were treated with various concentrations of ABA, and stomatal apertures were measured after 3 h. Data for a and b are from three independent experiments (n = 60 stomata per treatment ± SE). (c) ABA- and nitrite-induced NO synthesis. DAF-2DA fluorescence was determined (30 min) from control, nitrite-treated (1 mM), or ABA-treated (50 μM) guard cells of either wild type (white bars) or nia1, nia2 (black bars; n = 44–124 ± SE).
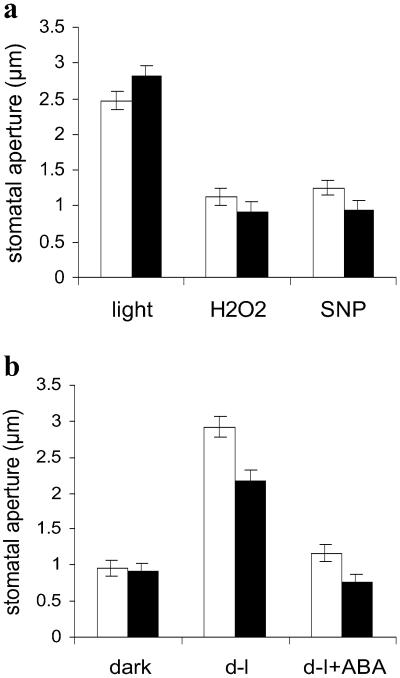
To confirm that the insensitivity of nia1, nia2 stomata to ABA and nitrite reflects their reduced NR activity, epidermal peels were exposed to NO via incubation in SNP. nia1, nia2 stomata showed a response similar to wild-type stomata (Fig. 3a), indicating that NR lies upstream of NO in the signaling pathway leading to closure. Stomatal closure was also similarly induced in nia1, nia2 as in the wild type by hydrogen peroxide (H2O2; Fig. 3a), another key signaling intermediate in the stomatal closure response activated by ABA (1), as well as by incubation in darkness (Fig. 3b), demonstrating that the failure of nia1, nia2 stomata to close in response to ABA or nitrite is not caused by a general malfunction in the mechanisms of closure. The effect of ABA on inhibition of stomatal opening, a process differing from closure (22), was also determined. ABA inhibited stomatal opening in nia1, nia2 as in the wild type (Fig. 3b), revealing that not all stomatal responses to ABA are reduced in nia1, nia2.
Fig. 3.
Stomatal movements per se are not impaired in nia1, nia2 plants. (a) Stomatal closure response. Epidermal peels of either wild type (white bars) or nia1, nia2 (black bars) were kept in the light in the absence (control) or presence of H2O2 (100 μM) or SNP (50 μM), and stomatal apertures were measured after 3 h. (b) Stomatal opening response. Epidermal peels previously kept in the light to induce stomatal opening were transferred to the dark for 3 h (dark). Epidermal peels incubated in the dark to induce stomatal closure were transferred to the light and incubated for 3 h to induce stomatal opening in the absence (d-l) or presence of 50 μM ABA (d-l+ABA). Data from three independent experiments (n = 60 stomata per treatment ± SE).
Guard Cells of abi1-1 and abi2-1 Synthesize NO in Response to ABA but Do Not Respond to NO.
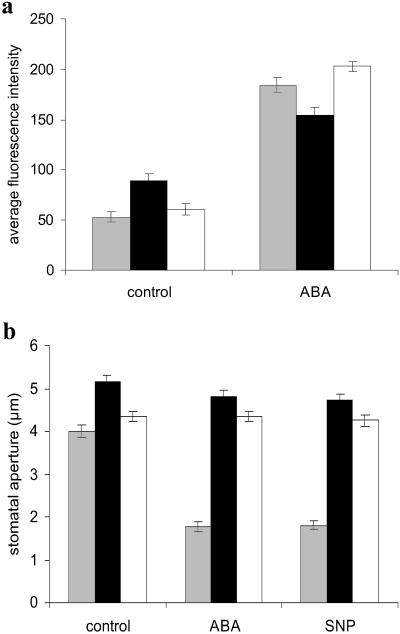
Recently, the protein phosphatase 2C enzymes ABI1 and ABI2 have been placed in the H2O2 signaling pathway induced by ABA that leads to stomatal closure (23). To establish their potential role in ABA-induced NO signaling during stomatal responses, we investigated NO synthesis and stomatal closure in abi1-1 and abi2-1 guard cells. Guard cells of both abi1-1 and abi2-1 synthesized NO in response to ABA (Fig. 4a), but their stomata did not close in response to treatment with exogenous ABA or NO, even at concentrations that induced nearly complete closure in wild-type plants (Fig. 4b, data not shown). These data position abi1-1p and abi2-1p downstream of NO synthesis (and hence NR) in the ABA signal-transduction pathway in guard cells.
Fig. 4.
ABA and NO signaling in ABA-insensitive mutants. (a) Guard cells of both abi1-1 and abi2-1 generate NO in response to ABA. DAF-2DA fluorescence was determined (30 min) from either control or ABA-treated (50 μM) guard cells of wild type (gray bars), abi1-1 (black bars), or abi2-1 (white bars) (n = 16–41 ± SE). (b) Stomata of abi1-1 or abi2-1 do not close in response to exogenous NO or ABA. Arabidopsis leaves of wild type (gray bars), abi1-1 (black bars), or abi2-1 (white bars) were treated either without (control) or with ABA (50 μM) or SNP (50 μM), and stomatal apertures were measured after 3 h (n = 40 stomata per treatment ± SE).
Discussion
The data reported in this paper have considerable significance for both fundamental and applied plant biology. They demonstrate a previously uncharacterized signaling role for NR, that of mediating ABA-induced NO synthesis in Arabidopsis guard cells, required for ABA-induced stomatal closure. This conclusion is based on (i) pharmacological and physiological evidence showing that ABA and the NR substrate nitrite induce NO synthesis in guard cells and that removal of this NO inhibits ABA-induced stomatal closure, and on (ii) genetic evidence obtained through use of the NR-deficient nia1, nia2 mutant. nia1, nia2 guard cells do not generate NO in response to ABA or nitrite, nor do nia1, nia2 stomata close in response to these stimuli, although they still close in response to NO itself or to other closing stimuli. Other studies, including the use of NR-deficient mutants (13, 16, 21, 24), have shown that NR can generate NO in plant cells, but this article reports a physiological role for such NO generation. Whether or not induction of NR-mediated NO synthesis by ABA is a general response of all plant cells remains to be determined, although a recent report that ABA failed to induce NO synthesis in tobacco cell suspension cultures (25) suggests that it may be restricted to specialized ABA target cells such as guard cells. It is also possible that more than one route to NO production exists, and that different stimuli induce different biosynthetic pathways in different cells and species. For example, mechanical stress-induced NO formation in Arabidopsis was recently reported to be inhibited by NOS inhibitors but not reduced in the NR-deficient mutant (10).
It will be important to elucidate the mechanisms by which ABA rapidly activates the NO-generating capacity of NR in guard cells and how NO fits into the complex signaling web by which ABA influences stomatal movements (2, 22). NR activity is known to reflect the NR phosphorylation state; dephosphorylation, by a protein phosphatase (PP), is required to activate NR (13). Therefore, it may be that ABA activates NR via the activation of a PP enzyme. This possibility would accord with the known inhibition by okadaic acid of ABA-induced stomatal closure in Arabidopsis (22, 26).
The action of the two PP2C enzymes, ABI1-1 and ABI2-1, do not seem to be required for NO synthesis, because guard cells of both abi1-1 and abi2-1 were able to generate NO in response to ABA. However, stomata of neither mutant closed in response to NO, indicating that the action of these two enzymes is downstream of NO synthesis. Both ABI1 and ABI2 can act as direct targets for in vitro modification by H2O2 (27, 28). However, whether or not these enzymes are also modified by NO, either in vitro or in vivo, remains to be determined.
Drought stress is a major environmental constraint on crop production. Thus, the elucidation of previously uncharacterized signaling pathways mediating water stress tolerance provides new opportunities to enhance the water use efficiency of plants. However, under well watered conditions, the nia1, nia2 mutant does not have a wilty phenotype, despite the ABA insensitivity of guard cells in epidermal peels. This observation might reflect the involvement of other ABA-signaling intermediates, such as H2O2 (1, 23), or complex cellular interactions in the whole plant. In fact, preliminary data indicate that nia1, nia2 guard cells do synthesize H2O2 in response to ABA (data not shown). Given the complexity of ABA signaling in guard cells (2) and the interactions between H2O2 and NO (4), it is likely that H2O2 and NO interact to effect stomatal closure in response to ABA.
Abbreviations
ABA, abscisic acid
ABI, ABA-insensitive
DAF-2DA, diaminofluorescein diacetate
l-NAME, Nω-nitro-l-arginine methyl ester
NOS, NO synthase
NR, nitrate reductase
SNP, sodium nitroprusside
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pei Z.-M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G. J., Grill, E. & Schroeder, J. I. (2000) Nature 406, 731-734. [DOI] [PubMed] [Google Scholar]
- 2.Hetherington A. M. (2001) Cell 107, 711-714. [DOI] [PubMed] [Google Scholar]
- 3.Beligni M. V. & Lamattina, L. (2001) Plant Cell Environ. 24, 267-278. [Google Scholar]
- 4.Neill S. J., Desikan, R., Clarke, A., Hurst, R. D. & Hancock, J. T. (2002) J. Exp. Bot. 53, 1237-1247. [PubMed] [Google Scholar]
- 5.Neill S. J., Desikan, R., Clarke, A. & Hancock, J. T. (2002) Plant Physiol. 128, 13-16. [PMC free article] [PubMed] [Google Scholar]
- 6.Mata C. G. & Lamattina, L. (2002) Plant Physiol. 128, 790-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wendehenne D., Pugin, A., Klessig, D. F. & Durner, J. (2001) Trends Plant Sci. 6, 177-183. [DOI] [PubMed] [Google Scholar]
- 8.Delledonne M., Xia, Y., Dixon, R. A. & Lamb, C. (1998) Nature 394, 585-588. [DOI] [PubMed] [Google Scholar]
- 9.Clarke A., Desikan, R., Hurst, R., Hancock, J. T. & Neill, S. J. (2000) Plant J. 24, 667-677. [DOI] [PubMed] [Google Scholar]
- 10.Garces H., Durzan, D. & Pedroso, M. C. (2001) Ann. Bot. (London) 87, 567-574. [Google Scholar]
- 11.The Arabidopsis Genome Initiative (2000) Nature 408, 796-815. [DOI] [PubMed] [Google Scholar]
- 12.Lea P. J. (1999) in Plant Biochemistry and Molecular Biology, eds. Lea, P. J. & Leegood, R. C. (Wiley, Chichester, U.K.), pp. 163–192.
- 13.Kaiser W. M., Weiner, H., Kandlbimder, A., Tsai, C.-B., Rockel, P., Sonoda, M. & Planchet, E. (2002) J. Exp. Bot. 53, 875-882. [DOI] [PubMed] [Google Scholar]
- 14.Yamasaki H. & Sakihama, Y. (2000) FEBS Lett. 468, 89-92. [DOI] [PubMed] [Google Scholar]
- 15.Rockel P., Strube, F., Rockel, A., Wildt, J. & Kaiser, W. M. (2002) J. Exp. Bot. 53, 103-110. [PubMed] [Google Scholar]
- 16.Dean J. V. & Harper, J. E. (1986) Plant Physiol. 82, 718-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson J. Q. & Crawford, N. M. (1993) Mol. Gen. Genet. 239, 289-297. [DOI] [PubMed] [Google Scholar]
- 18.Leung J., Merlot, S. & Giraudat, J. (1997) Plant Cell 9, 759-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foissner I., Wendehenne, D., Langebartels, C. & Durner, J. (2000) Plant J. 23, 817-824. [DOI] [PubMed] [Google Scholar]
- 20.Pedroso M. C., Magalhaes, J. R. & Durzan, D. (2000) J. Exp. Bot. 51, 1027-1036. [DOI] [PubMed] [Google Scholar]
- 21.Magalhaes J. R., Monte, D. C. & Durzan, D. (2000) Physiol. Mol. Biol. Plants 2, 117-127. [Google Scholar]
- 22.Schroeder J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M. & Waner, D. (2001) Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627-658. [DOI] [PubMed] [Google Scholar]
- 23.Murata Y., Pei, Z.-M., Mori, I. C. & Schroeder, J. (2001) Plant Cell 13, 2513-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakihama Y., Nakamura, S. & Yamasaki, H. (2002) Plant Cell Physiol. 43, 290-297. [DOI] [PubMed] [Google Scholar]
- 25.Tun N. N., Holk, A. & Scherer, G. F. E. (2001) FEBS Lett. 509, 174-176. [DOI] [PubMed] [Google Scholar]
- 26.Pei Z.-M., Kuchitsu, K., Ward, J. M., Schwarz, M. & Schroeder, J. I. (1997) Plant Cell 9, 409-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meinhard M., Rodriguez, P. L. & Grill, E. (2002) Planta 214, 775-782. [DOI] [PubMed] [Google Scholar]
- 28.Meinhard M. & Grill, E. (2001) FEBS Lett. 508, 443-446. [DOI] [PubMed] [Google Scholar]