Abstract
Intercellular attachment is an essential process in the morphogenesis of multicellular organisms. A unique mutant, nolac-H18 (nonorganogenic callus with loosely attached cells), generated by T-DNA transformation using leaf-disk cultures of haploid Nicotiana plumbaginifolia, lost the ability to form tight intercellular attachments and adventitious shoots. The gene tagged with T-DNA, named NpGUT1 (glucuronyltransferase 1), was similar to the gene for the catalytic domains of animal glucuronyltransferases and was expressed predominantly in shoot and root apical meristems. The transformation of NpGUT1 complemented the nolac-H18 mutation, and the expression of antisense NpGUT1 RNA produced crumbled shoots. The mutation caused defects in the glucuronic acid of rhamnogalacturonan II of pectin, which drastically reduced the formation of borate cross-linking of rhamnogalacturonan II. NpGUT1, which encodes a unique glucuronyltransferase, is a glycosyltransferase gene identified in pectin biosynthesis and is essential for intercellular attachment in plant meristems and tissues.
Spatially and temporally controlled intercellular attachment and communication are indispensable for the organization of plant tissues, making them critical for normal development and morphogenesis in every multicellular organism. Plant cell walls are composed primarily of cellulose microfibrils, hemicellulose, pectic polysaccharides, and small amounts of structural proteins (1–3). Pectin is believed to be involved in intercellular attachment because it is localized mainly in the primary cell wall, middle lamella, and cell corners. Pectin consists mostly of three structurally well-characterized polysaccharides: homogalacturonans (HGs) and highly branched rhamnogalacturonans I and II (RG-I and RG-II). Compared with cellulose and hemicellulose, little is known about the synthesis and assembly of pectins. The biosynthesis of HG, RG-I, and RG-II likely requires at least 41 unique glycosyltransferases (1). The activities of several transferases involved in the biosynthesis of pectin have been identified (1). However, none of these enzymes have been purified, and their genes have never been identified.
Recently, we established a system for producing mutants called nolac (nonorganogenic callus with loosely attached cells) by T-DNA transformation, which involves in vitro cultures of leaf disks of haploid Nicotiana plumbaginifolia (4). These mutants are defective in intercellular attachment, which results in the failure of organogenesis. Haploid N. plumbaginifolia plants (5) are suitable for generating and studying such mutants, because mutations have a direct effect on phenotype and because cells with embryo-lethal mutations can be maintained in tissue culture as unorganized callus, which enables us to analyze mutant cell walls. We identified 199 lines of callus with loosely attached cells from cultures of 2,970 leaf disks that had been transformed with T-DNA. Although, only 25 of these continued to grow on the medium, nolac-H18 had a growth rate that was similar to that of normal callus.
In this study, we identified a glycosyltransferase gene (NpGUT1: glucuronyltransferase 1) for the biosynthesis of pectin by using a system of mutant production, and the gene was found to be indispensable for plant intercellular attachment.
Materials and Methods
Plant Material.
Haploid N. plumbaginifolia plants (5), a gift from the Institut National de la Recherche Agronomique Centre de Versailles, Versailles, France, were maintained on Murashige and Skoog's basal medium containing 1/2 strength macronutrients (4), 30 g/liter sucrose, and 1 g/liter agar. N. plumbaginifolia and Nicotiana tabacum were maintained under short-day conditions (50 μmol photons m−2⋅s−1, 8 h light/16 h dark).
Production and Screening of the nolac Mutant.
Leaf disks of haploid N. plumbaginifolia were transformed by using a modified version of the leaf-disk method (4). The pBI121-revised vector that included the 35S promoter-driven hygromycin phosphotransferase gene was introduced into Agrobacterium tumefaciens strain LBA4404. Agrobacterium-infected leaf disks were cultured on Murashige and Skoog's agar-solidified medium containing 1 mg/liter benzyladenine for shoot induction, and the formation of nolac was evaluated after 1 month. In normal callus, compact clusters of cells were generated with multiple shoots. However, a paste-like callus with weak intercellular attachments formed on the Agrobacterium-infected leaf disks. We confirmed the characteristics of the paste-like callus by touching it with tweezers to test the hardness of the callus (4).
Scanning Electron Microscopy.
Cells were fixed in 2.5% (vol/vol) glutaraldehyde in 25 mM sodium phosphate buffer (pH 6.8) for 2 h at room temperature. They were then washed with the same buffer for 10 min. After dehydrating the cells by passing them through an ethanol series, the ethanol was replaced by isoamyl acetate. Then, samples were dried in a critical-point dryer (HCP-2; Hitachi, Tokyo). Finally, the cells were coated with platinum/palladium in an ion-sputtering system (E-102; Hitachi) and observed with a scanning electron microscope (S-2500; Hitachi).
Light Microscopy.
Clumps of callus were fixed in 2.5% (vol/vol) glutaraldehyde in 25 mM sodium phosphate buffer (pH 6.8) for 2 h at room temperature. Then, the clumps were washed with the same buffer for 10 min, dehydrated by passage through an ethanol series, and embedded in resin as described by Iwai et al. (4). Thin sections (2 μm) were cut with a glass knife on an ultramicrotome (Reichert EM-ULTRACUT; Leica, Wetzlar, Germany). The sections were stained with 0.5% toluidine blue. Stained samples were then observed under a light microscope (VANOX-T; Olympus, Tokyo).
Cloning and Transformation of NpGUT1.
To identify insertion sites, the genomic DNA flanking the T-DNA border in nolac-H18 was amplified by thermal asymmetric interlaced-PCR (6). NpGUT1 cDNA was obtained from the seedling mRNA by RT-PCR (7) and cloned downstream from the 35S promoter in plasmid pBI121 in both sense and antisense constructs. These plasmids were introduced into A. tumefaciens C58C1 by using the freeze-thaw method. The sense construct was used to complement nolac-H18. Antisense NpGUT1-transgenic N. tabacum was produced by the leaf disk method as described above.
RNA Gel Blot Analysis.
RNA (10 μg) was extracted from the shoot apex, root, leaf, and stem of N. plumbaginifolia and fractionated by electrophoresis on a formaldehyde agarose gel; the bands of RNA were transferred to a Gene Screen Plus membrane (DuPont). The RNA on the filter was allowed to hybridize with 32P-labeled NpGUT1 cDNA in hybridization solution containing 50% formamide, 5× SSPE (0.18 M NaCl/10 mM phosphate, pH 7.4/1 mM EDTA), 5× Denhardt's solution, 0.1% SDS, and 150 μg⋅ml−1 salmon sperm DNA at 42°C for 20 h. The filter was first washed with 2× SSC at room temperature and then with 2× SSC and 0.1% SDS at 42°C. For visualization on the filter, we used a bio-imaging analyzer (BAS-5000; Fuji).
Preparation of Cell Wall Material.
The methods used to prepare and fractionate cell walls were based on Selvendran's procedure (8). Calli were suspended in 80% (vol/vol) ethanol and homogenized for 10 min at 1,500 rpm by using a Polytron blender (Physco-Troller NS-610, Nichion Rikakikai, Tokyo). The suspensions were centrifuged at 2,500 × g for 5 min. The insoluble residue was then washed with 80% (vol/vol) ethanol, 95% (vol/vol) ethanol, 100% ethanol, chloroform/methanol (1:1 vol/vol), resuspended in acetone, collected on a glass-fiber filter (Whatman GF/F), air-dried, and used as cell wall material. The cell wall materials were hydrolyzed with 72% (vol/vol) H2SO4 for 2 h at room temperature. Then, the acid concentration was diluted to 3% and the mixture was incubated for 2 h at 100°C (8). The cell walls were extracted sequentially with 50 mM Na2CO3 at room temperature for 2 h (pectic fraction), 1 M KOH at room temperature for 2 h, and 4 M KOH at room temperature for 2 h (hemicellulose fractions). The supernatants were neutralized with acetic acid to pH 6.0, dialyzed, and freeze-dried. The glycosyl-residue compositions were determined by gas chromatography-MS of the alditol acetate and trimethylsilyl methyl glycoside derivatives (8).
Isolation and in Vitro Dimer Formation of RG-II.
The cell wall material was treated for 4 h at 4°C with 0.1 M NaOH to saponify the methyl and acetyl esters. The suspensions were adjusted to pH 5.0 with 10% (vol/vol) glacial acetic acid and then treated for 16 h at 30°C with a homogeneous preparation of endo-polygalacturonase (EPG) from Aspergillus niger [2.5 units, Megazyme, Wicklow, Ireland; 1 unit releases 1 μmol of reducing sugar min−1 from a 1% (wt/vol) solution of polygalacturonate at pH 5.0 and 25°C]. The suspensions were centrifuged, and the insoluble residues were washed with water. The EPG-soluble fractions were dialyzed (1-kDa cutoff) against water and freeze-dried. RG-II was purified from the EPG-solubilized material by size-exclusion chromatography on a Superdex 75 HR 10/30 column (Amersham Pharmacia Biotech) equipped with a refractive index detector (9, 10). Similar amounts (≈3% of the wall) of RG-II were released from normal and nolac-H18 cell walls. The monomeric RG-II (mRG-II) and dimeric RG-II-B (dRG-II-B) in the EPG digests were confirmed by comparing their retention times with those of the authentic mRG-II and dRG-II-B from sugar beet and red wine. The EPG-soluble fraction (2 mg) was treated for 1 h at 30°C with 1 M HCl solution (0.5 ml). The acid-treated sample was dialyzed (1-kDa cutoff) and freeze-dried. The acid-treated sample (1 mg) was kept for 16 h in K+ hydrogen phthalate/HCl, pH 3.7, (200 μl) containing boric acid (6 mM) and lead acetate (4 mM) (11). This solution was then dialyzed (1-kDa cutoff) and freeze-dried. The relative amounts of the RG-II dimer and monomer were determined by size-exclusion chromatography/refractive index detector (9, 10).
In Situ Hybridization of mRNA and β-Glucuronidase (GUS) Assay for Promoter Activity.
In situ hybridization was performed as described (12) with minor modifications. For the GUS assay, a 680-bp DNA fragment of the NpGUT1 genomic sequence upstream from the putative initiation codon (PNpGUT1) was amplified by PCR and cloned upstream from the GUS gene in plasmid pBI-121. This plasmid was introduced into A. tumefaciens C58C1, used to transform N. plumbaginifolia, and subjected to GUS activity assays (13).
Results
Light and Scanning Electron Microscopy of nolac-H18.
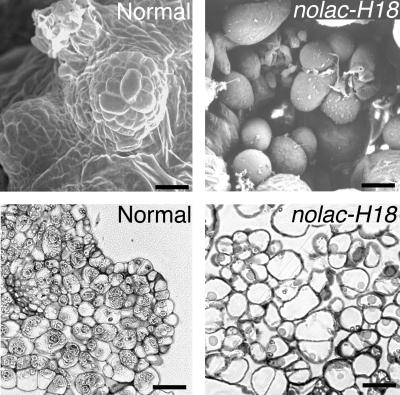
The cells of nolac-H18 callus, a mutant cell line, were somewhat larger than those of normal organogenic callus and were loosely attached, and the morphology of the cell clusters was irregular. In normal callus, the cells were tightly attached to one another and formed shoot meristems at the surface of the callus (Fig. 1). The green color of the callus became pale in nolac-H18 (see Fig. 3A). No nolac mutant calli appeared on T-DNA-transformed leaf disks from diploid plants or from nontransformed haploid and diploid plants.
Fig 1.
Morphological features of normal callus (Left) and nolac-H18 (Right) formed on leaf disks, observed by scanning electron (Upper) and light (Lower) microscopy. The bars represent 50 μm.
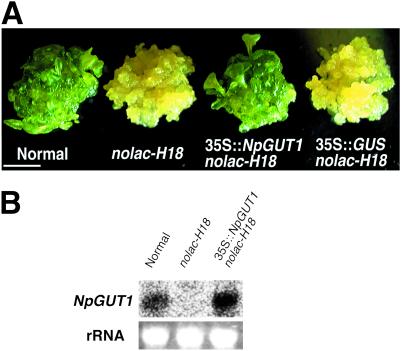
Fig 3.
Complementation of the nonorganogenic nature in nolac-H18 transformed with 35S promoter-driven NpGUT1. (A) Morphological features of normal, nolac-H18, nolac-H18 transformed with 35S promoter-driven NpGUT1 (35S:NpGUT1 nolac-H18), and 35S:GUS nolac-H18. The bar represents 1 cm. (B) NpGUT1 mRNA levels in normal, nolac-H18, and 35S:NpGUT1 nolac-H18.
Identification of NpGUT1 with T-DNA Tagging.
Gel blot analysis of nolac-H18 genomic DNA showed the presence of four copies of T-DNA (data not shown). To characterize the insertion sites, the genomic DNA flanking the right T-DNA border in nolac-H18 was amplified by thermal asymmetric interlaced-PCR. Three copies of T-DNA were inserted into retrotransposons and appeared not to be responsible for the nolac-H18 mutation. A gene was identified in the fourth flanked DNA that had 60% homology in the glycosyltransferase domain of animal EXT2 (exostoses) genes (Fig. 2A). Because EXT2s are glucuronyltransferases involved in heparan sulfate synthesis (14), we named this gene NpGUT1 (GenBank accession no. AB08676). This nolac-H18 mutant contains a T-DNA insertion in exon 1 of the NpGUT1 gene between amino acids Y89 and Q90.
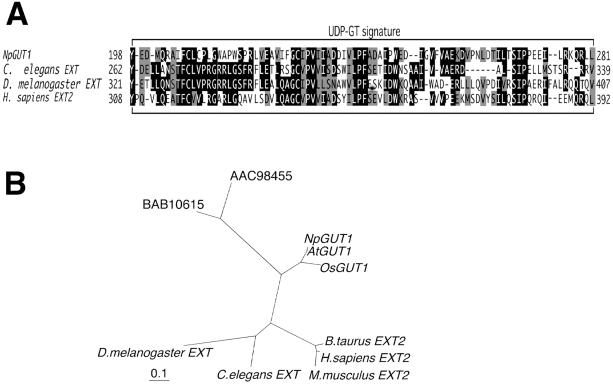
Fig 2.
(A) Alignment of the deduced amino acid sequences of NpGUT1 and animal EXTs in the UDP-glycosyltransferase (GT) signature. (B) The phylogenetic tree for NpGUT1 (AB080676), AtGUT1 (AB080693), OsGUT1 (AB080694), animal EXTs (Homo sapiens, AAB62718; Drosophila melanogaster, AAF58236; Bos taurus, AAC35386; Mus musculus, AAB17006; and Caenorhabditis elegans, AAC47509), and Arabidopsis DNA sequences (BAB10615 and AAC98455). Numbers are GenBank accession numbers.
The sequence analysis of NpGUT1 revealed that the protein encoded by this gene consists of transmembrane, stem, and catalytic domains, matching the structure of animal and yeast glycosyltransferases (15). In addition, the UDP-glycosyltransferase signature in the catalytic domain is conserved within the NpGUT1 and EXT gene families (16) (Fig. 2A). We found sequences with 90% and 87% identity to NpGUT1 in the Arabidopsis (AAC80624.1) and rice (OSJNBa0095J15.1) genome databases, and designated them AtGUT1 and OsGUT1, respectively (Fig. 2B).
DNA gel blot analysis of the N. plumbaginifolia genome indicated that NpGUT1 is a single-copy gene. AtGUT1 is also a single-copy gene that is identical to the EST clone APZ07g09F of Arabidopsis. In the phylogenetic tree, NpGUT1, AtGUT1, and OsGUT1 clustered separately from the animal genes (Fig. 2B).
Complementation of nolac-H18 with NpGUT1.
When the nolac-H18 callus was transformed with 35S promoter-driven NpGUT1 (35S:NpGUT1), the nonorganogenic and weak intercellular attachment phenotypes of nolac-H18 were complemented (Fig. 3A). Namely, ≈80% of the transformed nolac-H18 calli formed adventitious shoots as normal calli, whereas only ≈3% of the nolac-H18 calli transformed with 35S:GUS formed adventitious shoots (Fig. 3A). The transformation of nolac-H18 with 35S:NpGUT1 also rescued the level of NpGUT1 transcription, as found in mRNA gel blot analysis (Fig. 3B).
Effects of the Expression of Antisense NpGUT1.
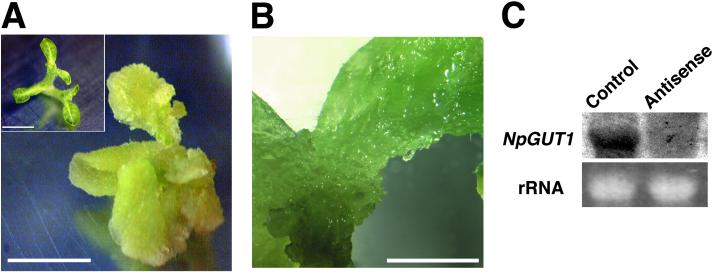
Transformation of normal leaf disks of N. tabacum with the 35S promoter-driven antisense NpGUT1 gene resulted in the formation of crumbled shoots (Fig. 4A) in accord with the reduction in the level of NpGUT1 mRNA (Fig. 4C). In these transformants, the leaf and stem tissues were ragged and brittle, and callus-like cell clusters were seen on the shoot apex (Fig. 4B).
Fig 4.
The morphology of 35S:GUS-transgenic (control; A Inset) and antisense NpGUT1-transgenic N. tabacum shoots. (A) Crumbled shoot in the severe phenotype. (B) Callus-like structure on the apical shoot. (C) NpGUT1 mRNA levels in normal and antisense NpGUT1-transgenic plants. The bars represent 5 mm (A) and 1 cm (B).
Sugar Composition of Cell Wall Matrix Polysaccharide.
The sugar composition analysis of the cell walls from normal and nolac-H18 calli is shown in Table 1. The glucuronic acid (GlcA) content in nolac-H18 callus was drastically reduced to ≈14% of that of normal callus, whereas the levels of the other sugars were reduced by as much as 60%. When the cell wall materials were subjected to sequential extraction with 50 mM Na2CO3, 1 M KOH, and 4 M KOH, the GlcA levels were markedly reduced only in the 50 mM Na2CO3-soluble pectic fraction of nolac-H18 (data not shown).
Table 1.
Sugar content and glycosyl composition analysis of whole cell wall prepared from normal and nolac-H18
| Glycosyl residue
|
Sugar amount, mg/g fresh cell | ||
|---|---|---|---|
| Normal ± SD | nolac-H18 ± SD | % | |
| Ara | 1,745 ± 85 | 997 ± 151 | 57 |
| Rha | 196 ± 31 | 240 ± 27 | 122 |
| Fuc | 64 ± 1 | 46 ± 5 | 72 |
| Xyl | 220 ± 3 | 138 ± 30 | 63 |
| Man | 133 ± 11 | 154 ± 50 | 116 |
| Gal | 935 ± 119 | 597 ± 173 | 64 |
| Glc | 3,306 ± 57 | 2,612 ± 24 | 79 |
| GalA | 520 ± 57 | 532 ± 46 | 102 |
| GlcA | 132 ± 21 | 19 ± 2 | 14 |
The data are the mean ± SD of at least three independent experiments.
Percentages of sugars (nolac-H18/normal callus).
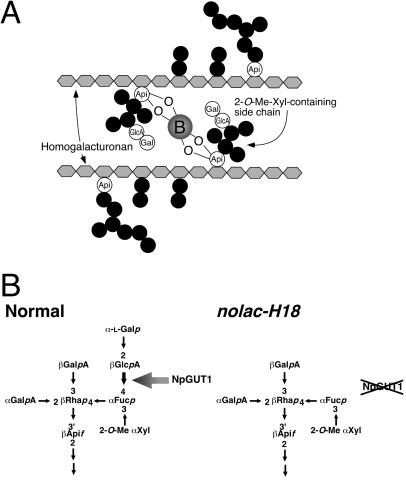
Pectins are a family of complex polysaccharides that are characterized by a backbone of α-1,4-linked polygalacturonic acid. In the primary cell wall, they have three domains: the HG, RG-I, and RG-II domains (1, 2, 11, 17). Because GlcA occurs predominantly in the RG-II domain of pectin and exists in 2-O-Me-xylosyl-containing side chains (see Fig. 7), which are indispensable for cross-linking the RG-II dimers via borate-esters (9, 10, 18), we analyzed the sugar composition of the RG-II domain prepared from the pectin fraction (Table 2). The monosaccharide composition was determined by gas chromatography-MS. We did not detect any GlcA in nolac-H18, but the nolac-H18 callus transformed with 35S:NpGUT1 had the same GlcA levels as seen in normal callus. The Gal level in RG-II of nolac-H18 was half that in normal callus and 35S:NpGUT1-transformed nolac-H18, suggesting that the defect is in the terminal disaccharide, α-L→Galp-(1→2)-β-GlcpA in the 2-O-Me-xylosyl-containing side chain of RG-II of nolac-H18 (see Fig. 7).
Fig 7.
(A) Two RG-II borate molecules are covalently cross-linked by a borate ester formed between the apiosyl residues in the 2-O-Me-Xyl-containing side chain of each RG-II domain of pectin. Api, apiose; Gal, galactose. (B) Models of the structure of 2-O-Me-Xyl-containing side chains of the RG-II domain of pectins. The nolac-H18 lacks the terminal α-L→Galp-(1→2)-β-GlcpA disaccharide in the side chain, and this defect drastically affects the ability to form RG-II dimer. NpGUT1 should transfer GlcA to Fucp in 2-O-Me-Xyl-containing side chains of RG-II domain of pectin.
Table 2.
Glycosyl residue composition of RG-II prepared from the cell walls of normal, nolac-H18, and 35S:NpGUT1 nolac-H18
| Glycosyl residue, mol% | Normal | nolac-H18 | 35S:NpGUT1 nolac-H18 |
|---|---|---|---|
| Ara | 18 | 28 | 20 |
| Rha | 22 | 20 | 20 |
| Gal | 10 | 5 | 12 |
| Fuc | 3 | 3 | 3 |
| Apiose | 10 | 9 | 9 |
| 2MeFuc | 5 | 5 | 5 |
| 2MeXyl | 4 | 4 | 4 |
| GalA | 26 | 26 | 26 |
| GlcA | 3 | ND | 3 |
ND, not detected. Aceric acid, 3-deoxy-d-manno-2-octulosonic acid, and 3-deoxy-d-lyxo-2-heptulosaric acid were detected but not quantified.
In Vitro Formation of RG-II Dimer.
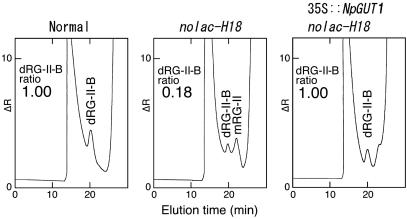
Because mRG-II accounted for 56% of the total RG-II in the cell walls of nolac-H18, whereas the borate ester cross-linked RG-II dimer (dRG-II-B) accounted for >95% of the RG-II in normal callus and 35S:NpGUT1 nolac-H18 (data not shown), we examined the in vitro dimer formation ability of RG-II isolated from the mutant. The in vitro formation of dRG-II-B with mRG-II prepared from nolac-H18 was only ≈18% (Fig. 5). In contrast, the mRG-II from the normal callus and 35S:NpGUT1 nolac-H18 was completely converted to dRG-II-B in vitro. These results showed that the lack of GlcA in pectin RG-II drastically affected the formation of borate ester cross-linking of RG-II.
Fig 5.
In vitro dimer formation of RG-II prepared from normal, nolac-H18, and 35S:NpGUT1 nolac-H18. The inset number represents the relative proportion of dRG-II-B to total RG-II, which was determined by size exclusion chromatography and a refractive index detector.
Expression of NpGUT1.
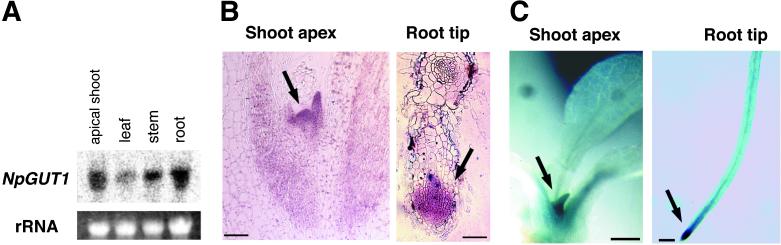
NpGUT1 mRNA was abundant in apical shoots and roots, but not in leaves (Fig. 6A). In situ hybridization showed that NpGUT1 mRNA specifically accumulated in the shoot apical meristem, the uppermost region of the leaf primordia, and the root tip (Fig. 6B). In the GUS assay for promoter activity of the upstream DNA region of NpGUT1, GUS activity in transgenic shoots with PNpGUT1:GUS was detected in both the shoot apex and young leaves that were in the early stages of shoot development (Fig. 6C). GUS activity was also detected in root tips (Fig. 6C).
Fig 6.
(A) The organ-specific expression of NpGUT1. (B) The localization of NpGUT1 transcripts in longitudinal sections of the shoot apex (Left) and root tip (Right). The bars represent 50 μm. (C) GUS activity in the shoot apex (Left) and root tip (Right) of PNpGUT1:GUS-transgenic plant. The bar represents 1 mm.
Discussion
Identification of NpGUT1 and Its Responsibility for nolac Mutation.
We have established a method to produce mutants of haploid N. plumbaginifolia plants with defects in intercellular attachment (5). Haploid plants, which directly demonstrate phenotypic alteration caused by a recessive mutation, provide a system for the production and selection of altered cell wall mutants. In recent years, Arabidopsis has become a preferred experimental species, because it is amenable to molecular study. However, mutations that cause defects in cell wall polysaccharides, such as cellulose and matrix polysaccharides, sometimes have embryonic lethal phenotypes, making the biochemical analysis of the cell wall polymers in such mutant cells very difficult (19, 20). To overcome this difficulty, a temperature-sensitive Arabidopsis mutant for cellulose biosynthesis has been successfully isolated and the biochemical phenotype of its cell wall has been analyzed (21). The present system for mutant production using cultured haploid N. plumbaginifolia permits subsequent in vitro culture of lethal mutants as nonorganogenic callus, thus enabling biochemical analysis of the matrix polysaccharides of the cell wall (4).
In the presence of a single cytokinin plant hormone in the culture medium, cells of normal calli formed organized clusters on which shoot meristems were formed and the cells were tightly attached to one another (Figs. 1 and 3). Clusters of nolac-H18 cells were disorganized with random morphology and weak intercellular attachment (Figs. 1 and 3). The T-DNA-tagged gene, NpGUT1, which is similar to animal heparan sulfate polymerases (glucuronyltransferases) in the conserved domain of UDP-glycosyltransferase (Fig. 2A), was thought responsible for the mutation, because the transformation of nolac-H18 with 35S:NpGUT1 induced the normal formation of adventitious buds (Fig. 3A), and the expression of antisense NpGUT1 caused a nonorganogenic, loosely attached phenotype similar to nolac-H18 on normal leaf disks (Fig. 4).
When the effect of antisense NpGUT1 expression was mild (Fig. 4B), morphogenesis was relatively normal and the shoot apex formed a light green callus. When the effect of antisense NpGUT1 expression was severe (Fig. 4A), shoot development and intercellular attachment were drastically depressed and the color became pale. Because even those transformants with the mild phenotype could not form the second node, a high level of NpGUT1 expression appears essential for shoot development.
Involvement of NpGUT1 in the Biosynthesis and Function of Pectin.
The sugar analysis of the cell wall of normal and nolac-H18 revealed a drastic reduction in the amount of pectic GlcA to ≈14% that in normal calli (Table 1). The GlcA residue is one of the glycosyl residues in RG-II, which is usually located in 2-O-Me-xylosyl-containing side chains of the RG-II domain of pectin, although RG-I also contains a few GlcA residues (22). This side chain is the borate-binding site of borate cross-linked RG-II dimers (1). No GlcA and almost half the amount of Gal were detected in RG-II isolated from nolac-H18 (Table 2). Another side chain, the 2-O-Me-fucosyl-containing side chain of RG-II, also contains a Gal residue (1, 11). These results indicate that nolac-H18 lacks the terminal α-L→Galp-(1→2)-β-GlcpA disaccharide in the 2-O-Me-xylosyl-containing side chain of RG-II (Fig. 7). Namely, the terminal Gal could not be transferred because of the absence of GlcA. In the mutant RG-II, an increase in the level of Ara was observed (Table 2). This finding may be caused by the substitution of Ara for GlcA in the RG-II side chain. We previously reported the importance of the arabinan side chain of the pectin RG-I domain in intercellular attachment (4), but the additional Ara in RG-II may not affect pectin function. The fact that the transformation of nolac-H18 with 35S:NpGUT1 completely complemented the sugar phenotype (Table 2) clearly shows that NpGUT1 encodes a glucuronyltransferase that transfers GlcA to the RG-II domain of pectin (Fig. 7).
Cross-Linking of RG-II via Borate Ester.
Pectin consists of HG, RG-I, and RG-II domains and is associated with cell wall polymers through covalent and noncovalent bonds via these domains (3, 4, 11, 17, 23). The presence of divalent calcium ions allows ionic cross-linking between the galacturonic acid residues in the HG domains, resulting in the formation of pectin gel. The side chains, composed of arabinan and arabinogalactan in the RG-I domains, are also thought to be involved in the intercellular attachment (4, 23–26). The RG-II domain is rich in primary walls, which are composed of four structurally different oligosaccharide side chains, formed from galacturonic acid, rhamnose, apiose, GlcA, 2-O-Me-xylose, 2-O-Me-fucose, galactose, arabinose, aceric acid, 3-deoxy-d-manno-2-octulosonic acid, 3-deoxy-d-lyxo-2-heptulosaric acid, and fucose. RG-II preferentially forms a dimer that is cross-linked by a 1:2 borate-diol ester (11, 27). The cross-link is formed between the two 2-O-Me-xyl-containing apioses of the side chains via borate. Borate cross-linking of RG-II generates complex pectin networks.
In nolac-H18, the rate of dRG-II-B formation in vitro was only ≈18%, whereas it was 100% in normal callus (Fig. 5). Therefore, the lack of GlcA in the RG-II domain of pectin in nolac-H18 drastically affects formation of the RG-II dimer and hence pectin–pectin interactions. Because this phenotype of nolac-H18 was completely complemented by the transformation of 35S:NpGUT1, NpGUT1 expression appears to be essential for the pectin network.
The substitution of 2-O-Me galactose for 2-O-Me fucose in the RG-II of the Arabidopsis mur1 mutant also reduces the rate of formation and the stability of the RG-II dimer (10). The mutant phenotypes of nolac-H18 and mur1–1 indicate that the entire structure of the side chain of RG-II is essential for borate cross-linking of the RG-II dimer. The functions of GlcA and 2-O-Me fucose likely differ, because the addition of excess borate could not rescue the nolac phenotype (data not shown), whereas the mur1–1 phenotype was rescued by the addition of borate (9).
Requirement of NpGUT1 in the Meristems.
NpGUT1 was specifically expressed in the shoot and root meristems and younger tissues (Fig. 6 B and C). This pattern of NpGUT1 expression is associated with the plant parts where the phenotype of antisense NpGUT1 expression was observed (Fig. 4 A and B). These tissues must require particularly strong intercellular attachment for intercellular communication and organization. Recently, it was determined that cell signaling pathways via plasmodesmata play roles in regulating shoot meristem development and organ formation (28, 29). Disconnection of the plasmodesmata between cells may be one of the main causes of disordered shoot apex morphology in both nolac-H18 and the antisense NpGUT1-transformed shoots.
Because boron deficiency causes structurally abnormal walls and abnormal meristem morphology (10), boron is considered essential for the meristems and cells of growing plants (9–11, 30). Our study expands on this understanding by showing that the glucuronate linkage of pectin, catalyzed by the product of NpGUT1, is essential for intercellular attachment in the meristematic regions of higher plants.
In conclusion, we succeeded in identifying a glycosyltransferase gene for pectin biosynthesis. This study also demonstrated that the loss of one unit of GlcA in the pectin molecule can cause drastic morphological abnormalities, particularly in the meristem, and indicates that the glucuronyltransferase gene is essential for intercellular organization in plant meristems and tissues.
Acknowledgments
We thank Professor Kiyotaka Okada (University of Kyoto, Kyoto) for critical reading of the manuscript. We are also grateful to the Institut National de la Recherche Agronomique Centre de Versailles in France for the gift of haploid N. plumbaginifolia plants. This work was supported in part by Grants-in-Aid for Scientific Research on Priority Area (nos. 11163204 and 14036204). It was also supported in part by Research Fellowship 0206093 from the Japan Society for the Promotion of Science by Young Scientists (to H.I.).
Abbreviations
HG, homogalacturonan
RG, rhamonogalacturonan
mRG, monomeric RG
dRG, dimeric RG
NpGUT1, glucuronyltransferase 1
nolac, nonorganogenic callus with loosely attached cells
EPG, endo-polygalacturonase
GUS, β-glucuronidase
EXT, exostose
GlcA, glucuronic acid
References
- 1.Ridley B. L., O'Neill, M. A. & Mohnen, D. (2001) Phytochemistry 57, 929-967. [DOI] [PubMed] [Google Scholar]
- 2.Willats W. G., McCartney, L., Mackie, W. & Knox, J. P. (2001) Plant Mol. Biol. 47, 9-27. [PubMed] [Google Scholar]
- 3.Carpita N. C. & Gibeaut, D. M. (1993) Plant J. 3, 1-30. [DOI] [PubMed] [Google Scholar]
- 4.Iwai H., Ishii, T. & Satoh, S. (2001) Planta 213, 907-915. [DOI] [PubMed] [Google Scholar]
- 5.Marion-Poll A., Marin, E., Bonnefoy, N. & Pautot, V. (1993) Mol. Gen. Genet. 238, 209-217. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y. G. & Whittier, R. F. (1995) Genomics 25, 674-681. [DOI] [PubMed] [Google Scholar]
- 7.Frohman M. A. (1993) Methods Enzymol. 218, 340-356. [DOI] [PubMed] [Google Scholar]
- 8.Selvendran R. R. & O'Neill, M. A. (1987) Methods Biochem. Anal. 32, 25-153. [DOI] [PubMed] [Google Scholar]
- 9.O'Neill M. A., Eberhard, S., Albersheim, P. & Darvill, A. G. (2001) Science 294, 846-849. [DOI] [PubMed] [Google Scholar]
- 10.Ishii T., Matsunaga, T. & Hayashi, N. (2001) Plant Physiol. 126, 1698-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Neill M. A., Warrenfeltz, D., Kates, K., Pellerin, P., Doco, T., Darvill, A. G. & Albersheim, P. (1996) J. Biol. Chem. 271, 22923-22930. [DOI] [PubMed] [Google Scholar]
- 12.Demura T. & Fukuda, H. (1994) Plant Cell 6, 967-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jefferson R. (1987) Plant Mol. Biol. Rep. 5, 387-405. [Google Scholar]
- 14.Duncan G., McCormick, C. & Tufaro, F. (2001) J. Clin. Invest. 108, 511-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keegstra K. & Raikhel, N. (2001) Curr. Opin. Plant Biol. 4, 219-224. [DOI] [PubMed] [Google Scholar]
- 16.Altschul S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W. & Lipman, D. J. (1997) Nucleic Acids Res. 25, 3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii T. & Matsunaga, T. (2001) Phytochemistry 57, 969-974. [DOI] [PubMed] [Google Scholar]
- 18.Vidal S., Doco, T., Williams, P., Pellerin, P., York, W. S., O'Neill, M. A., Glushka, J., Darvill, A. G. & Albersheim, P. (2000) Carbohydr. Res. 326, 277-294. [DOI] [PubMed] [Google Scholar]
- 19.Lukowitz W., Nickle, T. C., Meinke, D. W., Last, R. L., Conklin, P. L. & Somerville, C. R. (2001) Proc. Natl. Acad. Sci. USA 98, 2262-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillmor C. S., Poindexter, P., Lorieau, J., Palcic, M. M. & Somerville, C. R. (2002) J. Cell Biol. 156, 1003-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arioli T., Peng, L., Betzner, A. S., Burn, J., Wittke, W., Herth, W., Camilleri, C., Hofte, H., Plazinski, J., Birch, R., et al. (1998) Science 279, 717-720. [DOI] [PubMed] [Google Scholar]
- 22.An J., O'Neill, M. A., Albersheim, P. & Darvill, A. G. (1994) Carbohydr. Res. 252, 235-243. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi A., Edashige, Y., Ishii, T., Fujii, T. & Satoh, S. (1996) Planta 198, 634-639. [DOI] [PubMed] [Google Scholar]
- 24.Satoh S. (1998) Plant Cell Physiol. 39, 361-368. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M., Nakagawa, H., Asaka, T. & Matoh, T. (1999) Plant Physiol. 119, 199-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mollet J., Park, S., Nothnagel, E. A. & Lord, E. M. (2000) Plant Cell 12, 1737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishii T., Matsunaga, T., Pellerin, P., O'Neill, M. A., Darvill, A. & Albersheim, P. (1999) J. Biol. Chem. 274, 13098-13104. [DOI] [PubMed] [Google Scholar]
- 28.Lucas W. J., Bouche-Pillon, S., Jackson, D. P., Nguyen, L., Baker, L., Ding, B. & Hake, S. (1995) Science 270, 1980-1983. [DOI] [PubMed] [Google Scholar]
- 29.Haecker A. & Laux, T. (2001) Curr. Opin. Plant Biol. 4, 441-446. [DOI] [PubMed] [Google Scholar]
- 30.Blevins D. G. & Lukaszewski, K. M. (1998) Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 481-500. [DOI] [PubMed] [Google Scholar]