Abstract
Homozygous lines of barley overexpressing a wheat thioredoxin h transgene (up to 30-fold) were generated earlier by using a B1-hordein promoter with a signal peptide sequence for targeting to the protein body and found to be enriched in starch debranching enzyme (pullulanase). Here, we describe the effect of biochemically active, overexpressed thioredoxin h on germination and the onset of α-amylase activity. Relative to null segregant controls lacking the transgene, homozygotes overexpressing thioredoxin h effected (i) an acceleration in the rate of germination and appearance of α-amylase activity with a 1.6- to 2.8-fold increase in gibberellin A1 (GA1) content; (ii) a similar acceleration in the appearance of the α-amylase activity in deembryonated transgenic grain incubated with gibberellic acid; (iii) a 35% increase in the ratio of relative reduction (abundance of SH) of the propanol soluble proteins (hordein I fraction); and (iv) an increase in extractable and soluble protein of 5–12% and 11–35%, respectively. Thioredoxin h, which was highly reduced in the dry grain, was degraded in both the null segregant and homozygote after imbibition. The increase in α-amylase activity and protein reduction status was accompanied by a shift in the distribution of protein from the insoluble to the soluble fraction. The results provide evidence that thioredoxin h of the starchy endosperm communicates with adjoining tissues, thereby regulating their activities, notably by accelerating germination of the embryo and the appearance of α-amylase released by the aleurone.
Thioredoxin h, a 12-kDa extraplastidic protein containing a redox-active disulfide group, plays a regulatory role in mobilizing nitrogen and carbon resources in the starchy endosperm of wheat during germination and early seedling development (1, 2). Similar mobilization events were recently shown to take place in barley and rice starchy endosperm (3, 4). In an earlier study, grain from homozygous lines of transgenic barley overexpressing wheat thioredoxin h (up to 30-fold) were generated by using a barley starchy endosperm-specific B1-hordein promoter with a signal peptide sequence for targeting to the protein body (5). The finding that the transgenic grain overproduced starch debranching enzyme raised the question as to whether other physiological changes occur during germination and early seedling development. We have pursued this question and now report that thioredoxin h alters fundamental properties, including the rate of germination, production of α–amylase that is regulated by gibberellin (GA), and the amount of soluble protein. The results provide evidence that thioredoxin h of the starchy endosperm communicates with the embryo and the aleurone, thereby influencing key activities.
Materials and Methods
Biological Materials.
Dry mature grain from the transgenic lines of greenhouse-grown or, as indicated, field-grown barley (Hordeum vulgare cv. Golden Promise) overexpressing wheat thioredoxin h were generated and cultivated by using either a D-hordein promoter (Dh) targeted to the cytosol or a B1-hordein promoter with signal sequence (Bhss) targeted to the protein body (5, 6). The homozygous lines with the signal sequence were designated GPdBhssBarWtrx-29-3-2 and GPdBhssBarWtrx-2-1-15. The corresponding line without signal sequence was GPDhBarWtrx-9-1-4. The null segregant controls for the above transgenic lines were designated GPdBhssBarWtrx-29-11-10, GPdBhswsBarWtrx-2-5-17 and GPDhBarWtrx-9-13-5, respectively. Thioredoxin h and NADP-thioredoxin reductase (NTR) from bread wheat (Triticum aestivum) were purified as described (7). The purified anti-wheat thioredoxin h and anti-spinach NTR sera were generated as described earlier (8).
Unless otherwise indicated, the data shown below were obtained with the homozygous line overexpressing the thioredoxin h gene with B1-hordein promoter and signal sequence (GPdBhssBarWtrx-29-3-2) and the corresponding null segregant (GPdBhssBarWtrx-29-11-10). As indicated, similar data were obtained with an independent transformant (GPdBhssBar- Wtrx-2-1-15) and its null segregant (GPdBhssBarWtrx-2-5-17). One experiment (see Fig. 2) was also conducted with a homozygous line transformed with the thioredoxin h gene coupled to a D-hordein promoter without signal sequence (GPDhBarWtrx-9-1-4) and its null segregant (GPDhBarWtrx-9-13-5).
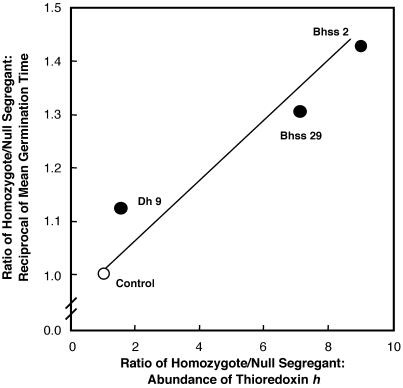
Fig 2.
Germination velocity of barley grain vs. abundance of thioredoxin h. Dh 9 is a transgenic barley line overexpressing thioredoxin h targeted to the cytosol; Bhss 2 and 29 are barley lines overexpressing thioredoxin h targeted to the protein body. The control (open circle) is a theoretical point.
Chemical Reagents.
Standard chemicals were purchased commercially. PEG-MAL (maleimide-polyethylene glycol) was purchased from Shearwater Polymers (Huntsville, AL).
Protein Determination.
Protein was estimated according to Bradford (9) as described (1).
Germination and Extraction of Grain.
Grain was germinated as described earlier (1, 2, 5). The mean germination time (reciprocal of Kotowski coefficient) was calculated as described by McCafferty and Bryce (10). In these experiments, the same plates of grain were monitored daily. In this study, germination refers to emergence of the radicle through the seed coat.
Extraction and Separation of Proteins for Determining Redox Status.
One gram of grain was ground in a mortar and pestle and sequentially extracted at 25°C for albumin (buffer-soluble), globulin (salt-soluble), hordein (propanol-soluble), and glutelin (acid-soluble) fractions by using a modification (3) of the procedure of Shewry et al. (11). Monobromobimane (mBBr)-labeled protein samples were separated according to Laemmli (12) as before (1).
α-Amylase Activity, Isoelectric Focusing, and Western Blots.
Grain was extracted as above with 30 mM Tris⋅HCl buffer (pH 7.9), supplemented with 1 mM EDTA and 0.5 mM PMSF, by using a ratio of 1:3 to 1:6 wt/vol of tissue to buffer depending on developmental stage. The supernatant fraction was stored in 0.1-ml aliquots at −80°C or as lyophilized powder (for enzymatic analysis). Total amylase activity was analyzed either by enzyme assay (13) or by native activity gel. Electrophoresis was performed on 6% acrylamide gels (140 × 160 × 1.5 mm) according to Laemmli (12) except that SDS was omitted from all solutions. The resolving gel was supplemented with 0.5% Lintner soluble potato starch (Sigma). Lyophilized samples were dissolved in deionized water and mixed 1:1 with a sample buffer (0.25 M Tris⋅HCl, pH 6.8 containing 50% glycerol, 0.04% bromophenol blue, and 3 mM CaCl2). Fifty micrograms of protein of each sample were loaded in each lane. Electrophoresis was carried out at 80 mA per gel at 4°C until the dye front moved to the bottom edge of the gel (typically 4–5 h). After electrophoresis, gels were incubated in 100 ml of 0.1 M succinate buffer (pH 5.0) for 1–2 h at 37°C, then stained for 5 min in a solution containing 2.5 mM I2 and 0.5 M KI and finally washed in distilled water. The gel stained dark blue except for the regions containing amylase activity, which remained clear. Bands were captured by using a Bio-Rad Gel Doc 1000.
Quantitative Analysis of GAs.
Whole grain samples for GA analysis were frozen in liquid N2, freeze-dried, ground to a fine powder in a ball mill, and stored at −20°C. Aliquots (1 g) were analyzed for GA content by combined gas chromatography-mass spectrometry as described (14), except that quantitation was based on mass chromatograms generated from full scans rather than on selected reaction monitoring.
α-Amylase Isozyme Patterns.
α-Amylase isozymes were analyzed by Western blots obtained with a Mini TransBlot Electrophoretic Transfer Cell (Bio-Rad). Protein (15–30 μg per lane) was separated on the pH 3–10 isoelectric focusing gels (Invitrogen) according to the manufacturer's instructions and was transferred to nitrocellulose at a constant voltage of 100 V for 1 h at 4°C by using 0.75% acetic acid as transfer buffer. Nitrocellulose was blocked with 5% powdered milk in 20 mM Tris⋅HCl, pH 7.5 and 0.15 M NaCl (TBS) for 1 h, incubated for 4 h in a 1:1,000 dilution of primary antibody, rabbit anti-barley high pI α-amylase, a gift from R. Jones (University of California, Berkeley). Blots were developed in p-nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) alkaline phosphatase color reagent. Blots were then reprobed with a second primary antibody, rabbit low pI anti-barley α-amylase, also a gift from R. Jones. Blots were developed in Naphthol Phosphate/Fast Red alkaline phosphatase color reagent (both probes according to Bio-Rad instructions). In each case, the secondary antibody was goat anti-rabbit alkaline phosphatase diluted 1:3,000. After the transfer, gels were stained with Coomassie blue to assure complete transfer. Western blot analysis after SDS/PAGE was also conducted as described (2).
Determination of Thioredoxin h Redox Status.
Ten deembryonated grain from the null segregant or homozygote were ground in liquid nitrogen as above for protein separation. Frozen powder equivalent to 0.1 ml was suspended in 1 ml of 50 mM Tris⋅HCl, 125 mM glycine, and 0.1% SDS at pH 8.8. PEG-MAL, 5 mg, was added after thawing and the mixture was rehomogenized. The homogenate was incubated on ice for 20–30 min for PEG-MAL labeling of the sulfhydryl groups and then centrifuged (20 min at 16,000 × g at 4°C). The recovered supernatant fractions were mixed with 0.1 ml 100% (wt/vol) trichloroacetic acid, incubated an additional hour on ice, and then centrifuged as before. The precipitated proteins were dissolved in extraction buffer and adjusted to contain equal amounts of protein (≈5 mg/ml). Aliquots of the PEG-MAL samples (15 and 40 μg for homozygote and null segregant, respectively) were subjected to SDS/PAGE (10–20% polyacrylamide Criterion precast gels; Bio-Rad). A partially purified wheat thioredoxin h preparation was used as the oxidized and reduced (with 0.05 mM DTT) standards. After electrophoresis (150 V, 90 min), the proteins were transferred to nitrocellulose by using a Hoefer blotting system (Amersham Pharmacia; 90 mA, 80 min, 4°C). Blots were probed first with rabbit anti-wheat thioredoxin h (6) and then with goat anti-rabbit peroxidase as above. The relative abundance and redox state of the thioredoxin h were determined by scanning the blots.
Results and Discussion
Evidence for Communication of Starchy Endosperm with Embryo: Germination Studies.
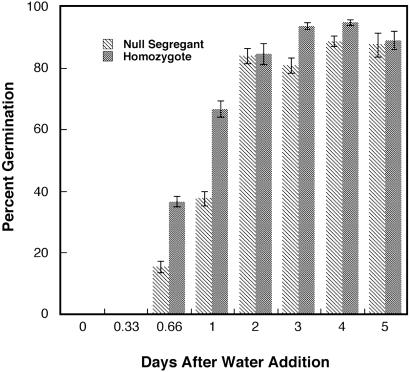
Fig. 1 demonstrates that homozygous barley grain overexpressing wheat thioredoxin h targeted to the protein body germinated faster than the null segregant counterpart. Thus, about twice as many grain germinated in 16 and 24 h in the transgenic vs. null segregant lines after H2O imbibition. However, after 2 days of germination, the percent germination of the transgenic and null lines was similar. That the observed acceleration was a direct result of overexpressed thioredoxin h was supported by the finding that null segregant grain incubated with 0.1 mM DTT showed a 60% enhancement in the rate of germination after 18 h whereas the homozygote did not (data not shown). The capability for accelerated germination seemed to be stable and was observed in seeds stored for up to 2 yr at 25°C. Further, accelerated germination was observed from seed of the above lines grown in the field (data not shown).
Fig 1.
Germination of transgenic barley grain overexpressing wheat thioredoxin h vs. null segregant (n = 3).
The question arises as to whether the faster rate of germination in the transgenic barley correlates with the amount of overexpressed thioredoxin h. A series of experiments was conducted to answer this question by using three independent transgenic lines of barley overexpressing different levels of thioredoxin h: one confined to cytosol (Dh 9) and two with protein body-targeted thioredoxin h (Bhss 2, Bhss 29). By using the three transgenic lines together with the corresponding null segregants, we observed a positive correlation between germination velocity (calculated as the coefficient of germination velocity or Kotowski coefficient) and the amount of thioredoxin h estimated from Western blots (Fig. 2). The finding with thioredoxin that targeting to the protein body markedly enhances expression of a transgene in barley (5) was subsequently confirmed and extended to other proteins (15).
Evidence for Communication of Starchy Endosperm with Aleurone: Studies with α-Amylase.
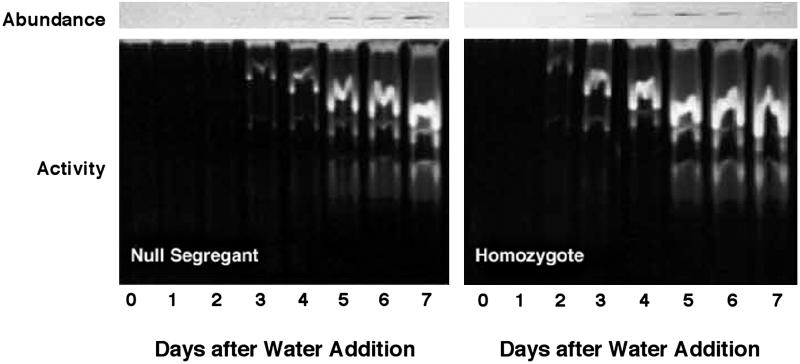
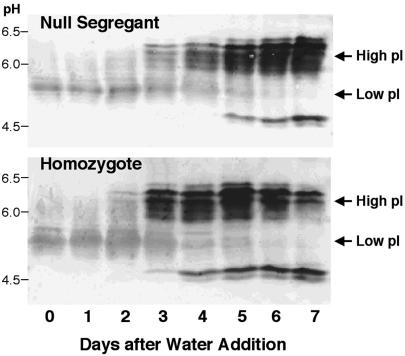
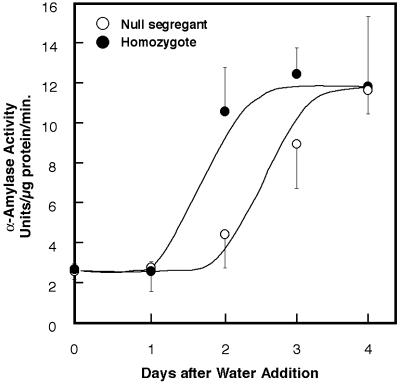
Our previous finding of increased pullulanase (starch debranching enzyme) activity in transgenic grain (5) raised the possibility that overexpressed thioredoxin h might enhance the activity of other hydrolytic enzymes associated with germination. Earlier work showed that α-amylase activity is increased in vitro by the thioredoxin-linked reductive inactivation of its disulfide inhibitor protein (16). In the present study with isolated starchy endosperm, we observed that thioredoxin h accelerated the appearance of α-amylase by about one day with respect to both activity (Fig. 3, “activity” panels) and amount of enzyme (Fig. 3, “abundance” panels). The isoelectric focusing blots in Fig. 4 show that this enhancement was due mainly to an increase in the abundance of the high pI rather than the low pI form enzyme (17, 18), which, as found by others (19), was a minor component. Such an increase in abundance of the high pI form speaks against a major role of the thioredoxin-linked α-amylase/subtilisin inhibitor (16) in contributing to the accelerated onset of α-amylase activity.
Fig 3.
Change in the abundance of α-amylases (Upper) and activity of total amylases (Lower) of barley grain during germination and seedling growth: homozygote overexpressing thioredoxin h vs. null segregant.
Fig 4.
Western blot of isoelectric focusing gels showing change in abundance of isozymes of α-amylases, low pI and high pI, during germination and seedling growth of barley grain: homozygote overexpressing thioredoxin h vs. null segregant.
An additional feature in Fig. 4 warrants comment. The antibody used to detect the high pI form of α-amylase revealed an atypical minor component with a pI of 5 in addition to the typical component with a pI of 5.9–6.4. It seems likely that the minor component, which was also observed in a commercial barley α-amylase preparation from Sigma, represents a proteolytic product derived from the typical high pI form. Analyses revealed that the accelerated appearance of α-amylase seen with GPdBhssBarWtrx-29-3-2 also occurred with a second event, GPdBhssBarWtrx-2-1-15; thus, after 2 days, there were respective increases in α-amylase activity of 2- and 3-fold on both a protein and per grain basis. Parallel enzyme assays showed that the aleurone of the homozygous lines accumulated α-amylase more rapidly (by 1 day) and at higher levels (up to four times) than the null segregant counterparts (data not shown). Similarly, α-amylase appeared and peaked in the starchy endosperm 1 day earlier in the homozygote relative to the null segregant.
The above results prompt the question whether thioredoxin h of the starchy endosperm regulates the synthesis of GA by the embryo, which is required for synthesis of α-amylase. Accordingly, homozygote and null segregant grain were analyzed for GA. The analyses were carried out with both field- and greenhouse-grown samples. The results with field-grown seeds revealed that the homozygote produced ≈60% more of the active GA component, GA1, than the corresponding null segregant in the first 48 h after imbibition (0.8 vs. 0.5 ng per g of dry weight). During this period, the homozygote showed, relative to the null segregant, a 50% increase in α-amylase activity on a per grain basis. Parallel experiments with greenhouse-grown grain revealed a corresponding 3-fold increase in GA1 (9.2 vs. 3.3 ng per g of dry weight). This change was accompanied by a 4-fold increase of α-amylase activity. In view of these analyses, it is possible that the thioredoxin-linked acceleration of the appearance of α-amylase was due in part to an increase in GA. However, as seen below, thioredoxin also seems to act independently of GA in eliciting this response.
The data presented above pose the question whether the embryo is essential for the accelerated appearance of the α-amylase activity associated with the homozygous grain overexpressing thioredoxin h. The question was approached by replacing the embryo of the grain with GA, the hormone long known to be synthesized in the embryo and transported to the aleurone where it promotes the synthesis of this enzyme (20, 21). The results of Fig. 5 speak to this point and show that, when incubated with gibberellic acid, the deembryonated homozygous grain showed an acceleration in the appearance of the α-amylase activity. Thus, 2 days after GA addition, grain from the homozygous line overexpressing thioredoxin h showed on a protein basis a 2-fold increase in α-amylase activity (Fig. 5) relative to grain from the null segregant. The corresponding value on a per grain basis was 2-fold. Similar results were obtained with GPdBhssBarWtrx-2-1-15 (see legend to Fig. 5). On further incubation, the grain from the null segregant caught up in each case and reached the level of activity seen with the homozygote. The results demonstrate that, in the presence of GA, thioredoxin h of the starchy endosperm interacts directly with the aleurone to accelerate the formation of hydrolytic enzymes to provide nutrients for the developing seedling.
Fig 5.
Formation of α-amylase by deembryonated grain overexpressing thioredoxin h vs. null segregant. Grain was incubated as indicated with 1 μM gibberellic acid (n = 3 for both experiments). With GPdBhssBarWtrx-2-1-15, a second event, we observed increases on day 2 of 47% and 80% in α-amylase activity on a protein and per grain basis, respectively.
The question arises as to whether the enhancement effects described above are due not to the thioredoxin h targeted to the starchy endosperm but to the inadvertent production of this protein in the embryo and aleurone by a “leaky” promoter. Available evidence speaks against this possibility. First, in experiments with β-glucuronidase gene (gus; uidA), GUS expression driven by the B1-hordein promoter was not visible in the embryo (6, 22) or in the aleurone (22). Second, based on this finding, the D-hordein promoter is also largely specific; otherwise the point representing this promoter in Fig. 2 would deviate from the line. Third, earlier biochemical experiments showed hordeins were restricted to the starchy endosperm and were not present in the aleurone (23). Finally, experiments to be published elsewhere have revealed that the formation of α-amylase by isolated aleurone is enhanced by thioredoxin added in the presence of GA (unpublished results).
Change in Thioredoxin h.
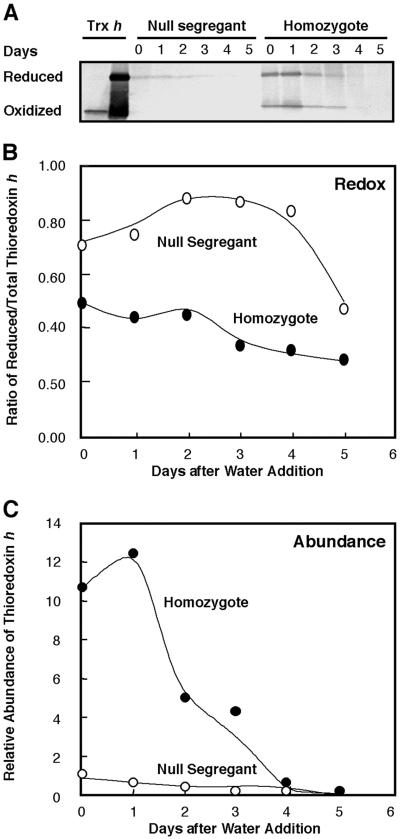
Earlier germination experiments with wheat revealed that thioredoxin h first undergoes reduction (from an initial 20% to a maximum of 80%), and then rapid proteolysis after imbibition (2). In the present study with barley, thioredoxin was more highly reduced in the dry grain of both the null segregant (70%) and homozygote (50%) (Fig. 6 A and B). Further reduction after imbibition was either sluggish (null segregant) or did not occur (homozygote). In both cases, the total amount of thioredoxin h decreased after imbibition (Fig. 6C) as observed previously for wheat (2). Changes similar to those in Fig. 6 were observed with deembryonated grain incubated with gibberellic acid (data not shown). It remains to be seen whether the absolute amount of thioredoxin or the ratio of the reduced and oxidized forms is important in effecting the changes described above. It should be noted that the absolute amount of reduced thioredoxin h was ≈5-fold higher in the homozygote than the null segregant.
Fig 6.
Abundance and redox status of thioredoxin h during germination and early seedling development: homozygote overexpressing thioredoxin h vs. null segregant. (A) Western blot showing the oxidized and reduced forms of thioredoxin h. (B) Change in redox state of thioredoxin h. (C) Relative abundance of thioredoxin h.
Studies on Storage Proteins.
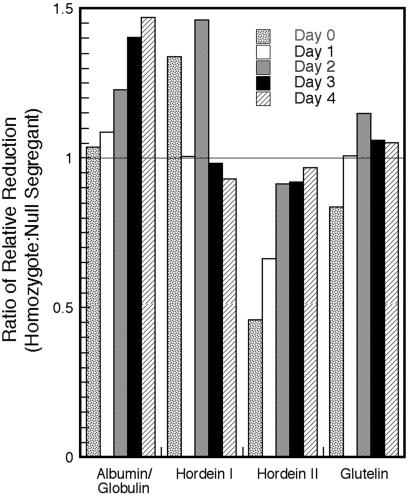
Redox status: The above results raise the question whether the high level of reduced thioredoxin in the homozygous dry grain effects change in the redox state of storage proteins of the starchy endosperm. We therefore assessed the sulfhydryl/disulfide (SH/SS) redox status of the soluble and insoluble protein fractions of transgenic barley in dry (day 0) and germinating grain (Fig. 7). The results suggest that the thioredoxin h in the transgenic “dry” seed led to a 35% increase in the ratio of relative reduction of the “free” (propanol-extractable) hordeins and to respective 50% and 15% decreases in the “bound” hordeins (hordein II) and glutelins, i.e., those requiring a sulfhydryl reagent (2-mercaptoethanol) for extraction. In brief, the increased level of thioredoxin h in dry grain caused an increase in the relative abundance of reduced (free) hordeins extractable by propanol at the expense of the oxidized (bound) proteins extracted only with a sulfhydryl reagent. This finding is in accord with the greater solubility of reduced sulfhydryl (-SH) as compared with the oxidized (S-S) proteins (11). There was little change in the aqueous (albumin/globulin) fractions of the dry grain. However, as the seedlings developed, there was, relative to the null segregant, an increase in the sulfhydryl groups in the albumin/globulin, the bound hordein (hordein II) and glutelin fractions of the homozygote. The change in the propanol-extractable hordein fraction (hordein I) was less consistent. It is noted that the water-soluble fraction used in this study contained proteins of both the aleurone and starchy endosperm. Recent findings have shown that starchy endosperm proteins of nontransformed barley undergo reduction during germination although the changes are less marked than with wheat (3).
Fig 7.
Change in redox status of protein fractions of transgenic barley grain overexpressing wheat thioredoxin h vs. null segregant during germination and early seedling development.
The increase in the ratio of relative reduction (SH) of proteins of dry homozygous grain enriched in thioredoxin h suggests that the overexpression effected a shift in distribution between the soluble and insoluble fractions. Analytical results indicated this to be the case. Relative to the null segregant, greenhouse-grown transgenic barley grain showed on a percentage basis an increase in the relative amount of protein recovered in the soluble (albumin/globulin) fraction by 2.7% (51.7% vs. 49.0%) and 10.3% (60.2% vs. 49.9%) in separate experiments. This increase, which was possibly due to thioredoxin-mediated structural changes in the hordein, was maintained during the first 2 days of germination, after which the null segregant showed a similar value (data not shown).
Concluding Remarks.
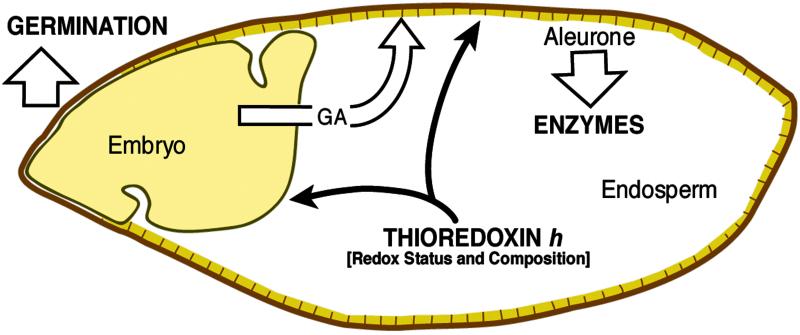
The present results confirm and extend earlier findings in underscoring the role of thioredoxin h in seed germination. Thioredoxin h overexpressed in barley starchy endosperm seemed to communicate directly with the embryo and the aleurone, respectively, to accelerate germination and the appearance of α-amylase (Fig. 8). In addition, based on current data, it is possible that thioredoxin h also enhances the synthesis of GA by the embryo, concordant with the more direct effects. The changes responsible for these accelerations could take place during either seed development or germination. In either case, thioredoxin h provides a redox-linked mechanism whereby the embryo and aleurone can sense the biochemical status of the adjoining starchy endosperm and adjust their activities accordingly. It will be of interest to learn to what extent changes in transcription, translation, and transport enable thioredoxin h in the starchy endosperm to communicate with adjoining tissues to accelerate these key events. An additional timely question is the relation of thioredoxin h to the endogenous factor concluded to be responsible for differences in the rate of α-amylase formation by two different barley cultivars (19).
Fig 8.
Role of thioredoxin h in communication between the starchy endosperm and the embryo and aleurone to enhance germination and enzyme formation. The segmented line represents the aleurone.
Acknowledgments
We thank S. Croker, P. Gaskin, and M. Torbett for the GA analyses. This work was supported by funds from the BioSTAR program of the University of California and Ventria Bioscience (Sacramento, CA). P.G.L. and B.B.B. were supported, respectively, by Cooperative Extension and the Agricultural Experiment Station of the University of California.
Abbreviations
PEG-MAL, maleimide-polyethylene glycol
GA, gibberellin
Dh, D-hordein promoter
Bhss, B1-hordein promoter with signal sequence
References
- 1.Kobrehel K., Wong, J. H., Balogh, A., Kiss, F., Yee, B. C. & Buchanan, B. B. (1992) Plant Physiol. 99, 919-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lozano R. M., Wong, J. H., Yee, B. C., Peters, A., Kobrehel, K. & Buchanan, B. B. (1996) Planta 200, 100-106. [Google Scholar]
- 3.Marx, C., Wong, J. H. & Buchanan, B. B. (2002) Planta, in press. [DOI] [PubMed]
- 4.Yano H., Wong, J. H., Cho, M.-J. & Buchanan, B. B. (2001) Plant Cell Physiol. 42, 879-883. [DOI] [PubMed] [Google Scholar]
- 5.Cho M.-J., Wong, J. H., Marx, C., Jiang, W., Lemaux, P. G. & Buchanan, B. B. (1999) Proc. Natl. Acad. Sci. USA 96, 14641-14646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho M.-J., Choi, H. W., Buchanan, B. B. & Lemaux, P. G. (1999) Theor. Appl. Genet. 98, 1253-1262. [Google Scholar]
- 7.Johnson T. C., Wada, K., Buchanan, B. B. & Holmgren, A. (1987) Plant Physiol. 85, 446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford N. A., Droux, M., Kosower, N. S. & Buchanan, B. B. (1989) Arch. Biochem. Biophys. 271, 223-239. [DOI] [PubMed] [Google Scholar]
- 9.Bradford M. (1976) Anal. Biochem. 72, 248-254. [DOI] [PubMed] [Google Scholar]
- 10.McCafferty K. A. & Bryce, J. H. (1999) Seed Sci. Technol. 27, 633-644. [Google Scholar]
- 11.Shewry P. R., Field, J. M., Kirkman, J. A., Fauks, A. J. & Miflin, B. J. (1980) J. Exp. Bot. 31, 393-407. [Google Scholar]
- 12.Laemmli U. K. (1970) Nature 227, 680-685. [DOI] [PubMed] [Google Scholar]
- 13.Jones R. L. & Varner, J. E. (1967) Planta 72, 155-161. [DOI] [PubMed] [Google Scholar]
- 14.Cole J. P., Phillips, A. L., Croker, S. J., Garcia-Lepe, R., Lewis, M. J. & Hedden, P. (1999) Plant J. 17, 547-556. [DOI] [PubMed] [Google Scholar]
- 15.Horvath H., Huang, J., Wong, O., Kohl, E., Okita, T., Kannangaru, C. G. & von Wettstein, D. (2000) Proc. Natl. Acad. Sci. USA 97, 1914-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao J., Yee, B. C., Wong, J. H., Kobrehel, K. & Buchanan, B. B. (1993) Plant Physiol. Biochem. 31, 799-804. [Google Scholar]
- 17.Jacobsen J. V. & Higgins, T. J. V. (1982) Plant Physiol. 70, 1647-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callis J. & Ho, T.-H. D. (1983) Arch. Biochem. Biophys. 224, 224-234. [DOI] [PubMed] [Google Scholar]
- 19.Skadsen R. W. (1993) Plant Physiol. 102, 195-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chrispeels M. J. & Varner, J. E. (1967) Plant Physiol. 42, 398-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobsen J. V. & Varner, J. E. (1967) Plant Physiol. 42, 1596-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knudsen S. & Müller, M. (1991) Planta 185, 330-336. [DOI] [PubMed] [Google Scholar]
- 23.Yupsanis T., Burgess, S. R., Jackson, P. J. & Shewry, P. R. (1999) J. Exp. Bot. 41, 385-392. [Google Scholar]