Abstract
Previous work showed that calmodulin (CaM) and Ca2+-CaM–dependent protein kinase II (CaMKII) are somehow involved in cardiac hypertrophic signaling, that inositol 1,4,5-trisphosphate receptors (InsP3Rs) in ventricular myocytes are mainly in the nuclear envelope, where they associate with CaMKII, and that class II histone deacetylases (e.g., HDAC5) suppress hypertrophic gene transcription. Furthermore, HDAC phosphorylation in response to neurohumoral stimuli that induce hypertrophy, such as endothelin-1 (ET-1), activates HDAC nuclear export, thereby regulating cardiac myocyte transcription. Here we demonstrate a detailed mechanistic convergence of these 3 issues in adult ventricular myocytes. We show that ET-1, which activates plasmalemmal G protein–coupled receptors and InsP3 production, elicits local nuclear envelope Ca2+ release via InsP3R. This local Ca2+ release activates nuclear CaMKII, which triggers HDAC5 phosphorylation and nuclear export (derepressing transcription). Remarkably, this Ca2+-dependent pathway cannot be activated by the global Ca2+ transients that cause contraction at each heartbeat. This novel local Ca2+ signaling in excitation-transcription coupling is analogous to but separate (and insulated) from that involved in excitation-contraction coupling. Thus, myocytes can distinguish simultaneous local and global Ca2+ signals involved in contractile activation from those targeting gene expression.
Introduction
Ca2+, calmodulin (CaM), and Ca2+-CaM–dependent protein kinase II (CaMKII) signaling have been implicated in the regulation of gene expression in cardiac hypertrophy and heart failure (HF) (1–8). CaMKII expression is elevated in human HF (9) and animal models of HF (10), and overexpression of either CaM or CaMKII can induce hypertrophy and HF (4, 5, 7, 8). CaMKII inhibition protects against structural heart disease (11). However, the mechanisms by which CaMKII alters gene expression in heart and the way in which cardiac myocytes distinguish Ca2+ transients that occur at every heartbeat from those meant to regulate transcription are enigmas that are addressed here.
CaMK can phosphorylate type II histone deacetylases (HDACs) (12). These HDACs (HDAC4, 5, 7, and 9) normally repress transcriptional activation (e.g., activation driven by myocyte enhancer factor-2 [MEF2]) and favor condensed DNA. When HDAC is phosphorylated in response to neurohumoral stimuli, it is exported from the nucleus (in association with the chaperone protein 14-3-3), MEF2 is derepressed, and a hypertrophic program of cardiac gene expression is activated (13). Indeed, genetic knockout of these HDACs results in marked cardiac hypertrophy (14). Nonetheless, it is unknown how CaMKII is activated by neurohumoral stimuli that induce hypertrophy or whether this causes HDAC nuclear export in adult ventricular myocytes.
Inositol 1,4,5-trisphosphate receptors (InsP3Rs) are ubiquitous intracellular Ca2+ release channels. They are present in cardiac myocytes, albeit at lower levels than the related ryanodine receptor (RyR), which is the main source of Ca2+ in excitation-contraction coupling (ECC) (15). The type 2 InsP3R (InsP3R2) is the predominant subtype in cardiac myocytes (16), but its role in heart is poorly understood. In atrial myocytes, some InsP3R are located near RyRs at sarcoplasmic reticulum (SR) Ca2+ release sites in the cell periphery, and these may contribute to altered ECC and arrhythmogenesis in atria (17, 18). In ventricular myocytes, InsP3R2 are mainly in the nuclear envelope where they complex with CaMKII (19), and evidence for ventricular InsP3R in ECC or myocyte Ca2+ transients is mainly negative (17, 18). Thus, the role of InsP3R2 in ventricular myocytes and their functional relationship with CaMKII colocalized at the nucleus are unknown. Here we explore whether InsP3R, CaMKII, and HDAC signaling pathways converge.
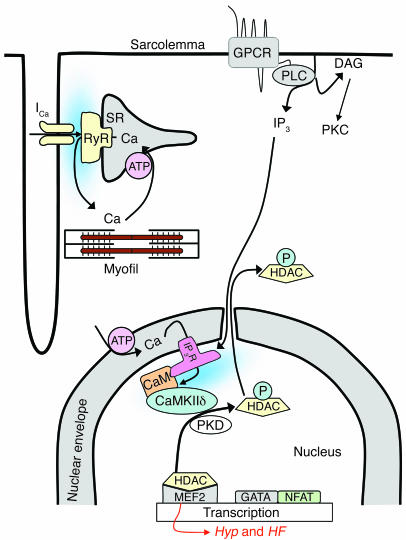
We tested the hypothesis (Figure 1) that stimulation of adult ventricular myocytes by the physiological hypertrophic agonist endothelin-1 (ET-1) activates HDAC5 nuclear export by a local Ca2+-dependent pathway at the nuclear envelope. To accomplish this, InsP3 produced by ET-1–induced activation of phospholipases causes very local Ca2+ release via InsP3R2 in the nuclear envelope, which in turn activates CaMKII, phosphorylates HDAC5, and causes HDAC5 nuclear export. A corollary hypothesis is that the Ca2+ involved in this activation is relatively insulated from the Ca2+ transients associated with each heartbeat (i.e., that responses to neurohumoral stimuli and cytosolic Ca2+ transients are distinct). This paradigm is analogous to local control involved in Ca2+-induced Ca2+ release during ECC and to Ca2+-dependent inactivation of Ca2+ current (20).
Figure 1.
Working hypothesis. ET-1 induces HDAC5 nuclear export via a pathway involving InsP3 (IP3) and InsP3R (IP3R) with associated CaM and CaMKII, leading to HDAC5 phosphorylation and nuclear export, thereby derepressing the transcription factor MEF2 (see text for additional details). Hyp, hypertrophy.
Results
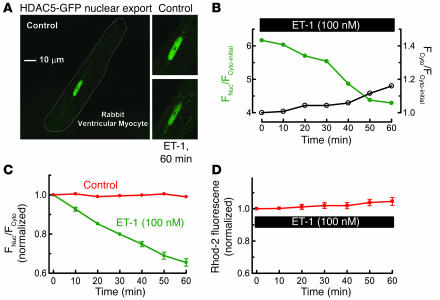
Figure 2A shows a resting adult rabbit ventricular myocyte in which HDAC5 tagged with GFP (HDAC5-GFP) was expressed by adenoviral infection. HDAC5 was initially largely confined to the nucleus. ET-1 exposure induced a time-dependent export of HDAC5 from the nucleus (Figure 2, A–C). Phenylephrine also caused HDAC5 nuclear export (data not shown). Notably, basal [Ca2+]i (assessed using Rhod-2) was not significantly altered (Figure 2D), nor was there any detectable local or global Ca2+ transients or [Ca2+]i elevation in or around the nucleus or elsewhere (see below and refs. 18, 21).
Figure 2.
ET-1 induces nuclear export of HDAC5. (A) ET-1 (100 nM) was applied for 60 minutes to quiescent rabbit ventricular myocyte expressing the fusion protein HDAC5-GFP. (B) Individual traces of nuclear and cytosolic fluorescence (FNuc/FCyto) for a single myocyte, where each trace is normalized to the Fcyto before ET-1 exposure (Fcyto-initial). (C) HDAC5 nuclear export was analyzed as decrease of FNuc/FCyto, normalized to the initial ratio (6.3 ± 0.4; n = 12). The control group was treated the same, except without ET-1 application (n = 10). (D) [Ca2+]i measured by Rhod-2 fluorescence upon exposure to 100 nM ET-1 (n = 7). Long-term exposure to ET-1 induced further HDAC5 export, such that after 24 hours the nucleus was virtually depleted of HDAC5 (not shown), analogous to effects in cultured neonatal rat myocytes (31).
Role of InsP3 and nuclear envelope Ca2+ stores in ET-1–induced HDAC5 export.
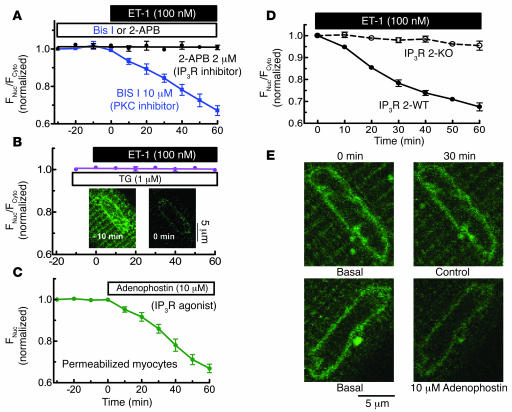
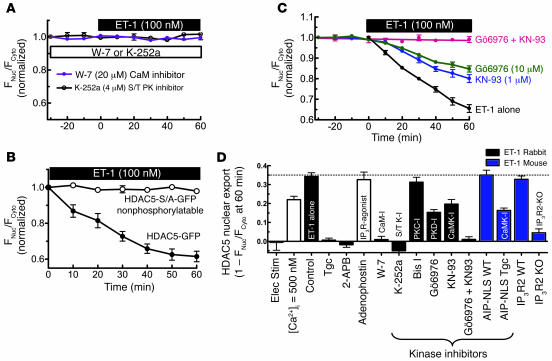
Phospholipase C produces both InsP3 and diacylglycerol (DAG), an activator of PKC. Either second messenger could mediate ET-1 effects on HDAC export. However, we found that ET-1–induced HDAC5 nuclear export was completely blocked by the InsP3R inhibitor 2-aminoethoxydiphenyl borate (2-APB) but unaffected by the PKC inhibitor bisindolylmaleimide I (Bis I) (Figure 3A). These data suggest that InsP3 rather than PKC is the critical second messenger in ET-1–induced HDAC5 nuclear export.
Figure 3.
ET-1–induced HDAC5 nuclear export is dependent on InsP3R and Ca2+ in InsP3-sensitive stores. (A) HDAC5-GFP–expressing myocytes were pretreated with 2 μM 2-APB (n = 6) or 10 μM Bis I (n = 5) for 30 minutes, followed by application of 100 nM ET-1. (B) SR and nuclear envelope Ca2+ stores were depleted by preincubation with SR/ER Ca2+-ATPase (SERCA) inhibitor TG 1 μM (n = 7) for 10 minutes. Insets are the Fluo-5N images, showing that this TG treatment depleted nuclear envelope Ca2+ stores. (C) In permeabilized HDAC5-GFP expressing myocytes, 10 μM adenophostin was applied after 30 minutes at 100 nM [Ca2+]i (n = 8). HDAC5 nuclear export was measured as the decrease of nuclear fluorescence. In the permeabilized cell, cytosolic concentration is irrelevant as HDAC5-GFP can readily diffuse to the bath. (D) ET-1–induced HDAC5 nuclear export assessed in mouse ventricular myocytes that lack InsP3R2 (InsP3R 2 KO) or WT littermates. (E) Fluo-5N–loaded myocytes were permeabilized and treated without or with 10 μM adenophostin for 30 minutes. Nuclear envelope Ca2+ release was indicated by decreased Fluo-5N fluorescence.
Complementary experiments indicate (Figure 3B) that the selective SR/ER Ca2+-ATPase (SERCA) inhibitor thapsigargin (TG) (which depletes both SR and ER Ca2+ stores) completely abolished ET-1–induced HDAC5 nuclear export. To confirm that TG depleted Ca2+ in the nuclear envelope, parallel studies were done using the low affinity Ca2+ sensor Fluo-5N, which can be trapped in the SR and nuclear envelope to sense free [Ca2+] inside Ca2+ stores (22). Because of the low Ca2+ affinity of Fluo-5N (Kd ∼ 400 μM), fluorescence is only bright in organelles like SR and nuclear envelope, where free [Ca2+] is approximately equal to 1 mM. The inset in Figure 3B shows that 1 μM TG preincubation for 10 minutes depleted the nuclear envelope Ca2+ stores.
In addition, direct InsP3R activation in permeabilized myocytes by the selective InsP3R agonist adenophostin could fully mimic the effect of ET-1 (Figure 3C). These data further demonstrate that InsP3-dependent Ca2+ stores can induce HDAC5 translocation.
To more directly test the involvement of InsP3R2 in ET-1–induced HDAC5 nuclear export, we used mouse ventricular myocytes in which the InsP3R2 gene had been knocked out (InsP3R2 KO) (23). In myocytes from WT mice, ET-1 induced HDAC5 export to an extent similar to that seen in rabbit myocytes (Figure 3D versus Figure 2C). However, in InsP3R 2 KO mice, ET-1–induced HDAC5 nuclear export was abolished (Figure 3D).
Using Fluo-5N to assess [Ca2+] in the nuclear envelope (as in Figure 3B), we found that adenophostin (or InsP3; not shown) mobilized Ca2+ from the nuclear envelope (Figure 3E), consistent with activation of InsP3Rs at that site (19). As in prior studies using Fluo-3 or Rhod-2 in adult ventricular myocytes (18), we did not detect ET-1– or adenophostin-induced Ca2+ mobilization near the nuclear envelope. This may reflect the small number of InsP3R2, the low unitary Ca2+ flux rate (versus RyRs), nonsynchronized openings (versus coactivation of RyRs in Ca2+ sparks), or diffusion and dilution of Ca2+. Measuring [Ca2+] inside the nuclear envelope obviated these limitations. Thus, Figure 3 shows that activation of InsP3R2 and Ca2+ release from nuclear envelope stores is necessary and sufficient to trigger HDAC5 nuclear export initiated by ET-1.
Local versus global Ca2+ signals in ET-1–induced HDAC5 export.
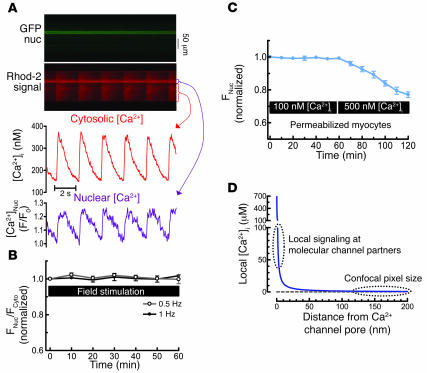
Next, we assessed the ability of the normal global Ca2+ transients that accompany each heartbeat to trigger HDAC5 nuclear export. Figure 4A shows simultaneous measurements of nuclear HDAC5 and Ca2+ transients in the cytosol and nucleus. Electrically stimulated Ca2+ transients (at either 0.5 or 1 Hz) failed to induce detectable nuclear HDAC5 export (Figure 4B). In permeabilized myocytes where [Ca2+]i is controlled, HDAC5 remained nuclear for at least 60 minutes at resting [Ca2+]i equal to 100 nM, and only when [Ca2+]i was chronically elevated to 500 nM (which does not happen in the living cell) was HDAC5 slowly exported (to half the extent induced by ET-1; Figure 4C). Activation of HDAC5 nuclear export was thus isolated from the physiological swings of bulk cytosolic and nuclear [Ca2+]. This is consistent with results of Schneider and his colleagues (24), who showed in skeletal muscle that pacing did not cause HDAC5 nuclear export or CaM nuclear import (24). However, they did show that pacing (10 Hz train stimulation) activated HDAC4 nuclear export and nuclear import of nuclear factor of activated T cells (NFAT) (24, 25). This may be due to the different local Ca2+ signal decoding in translocation of HDAC4 and NFAT versus HDAC5 and CaM (and skeletal muscle coding may differ from heart).
Figure 4.
HDAC5 nuclear export is Ca2+ dependent but not activated by global Ca2+ transients. (A) Adenoviral HDAC5-GFP–infected myocytes were also loaded with Rhod-2 to measure [Ca2+]i. Rhod-2 is more concentrated in the nucleus (nuc), complicating Ca2+ transient calibration there. (B) HDAC5 remained nuclear when myocytes were field stimulated at 0.5 Hz (n = 5) or 1 Hz (n = 7) for 60 minutes or even at 2 Hz (not shown). (C) In permeabilized myocytes, internal [Ca2+] was increased from 100 nM to 500 nM (at 60 minutes) (n = 7). 2,3 butanedione monoxime (5 mM) was used to prevent contraction. (D) Expected local [Ca2+] gradient around the mouth of an InsP3R Ca2+ channel: [Ca2+]i = [Ca2+]Init + q/(2πDr) × erfc{r/(2√Dr)}, where q = single channel current (0.1 pA), D = diffusion coefficient (600 μm2/s), [Ca2+]Init = 100 nM, erfc = complementary error function, and r = radial distance from the channel mouth for hemispheric diffusion. This is the steady state, achieved in approximately 10 μs without buffering and much less than 1 ms when local Ca2+ buffering is included (26).
This failure of HDAC5 export to respond to global pulsatile [Ca2+]i elevations is reminiscent of the well-accepted local control of SR Ca2+ release during cardiac ECC. There, local Ca2+ current activation is required at each junction where sarcolemma couples with SR, allowing each junction to function independently (15, 20). The local [Ca2+]i near the Ca2+ channel mouth (>20 μM) can activate local SR Ca2+ release, but [Ca2+]i declines as it diffuses from the source such that global [Ca2+]i (even at the Ca2+ transient peak, approximately 1 μM) is not at the threshold for triggering SR Ca2+ release at junctions where no Ca2+ channel opens (see Figure 4D) (20, 26). The relative insensitivity of HDAC5 export to global Ca2+ transients observed here would be expected if high local [Ca2+]i extremely close to the InsP3R is likewise needed to trigger the cascade of events leading to HDAC5 nuclear export.
Importantly, the volume sensed in a single confocal pixel (150 × 150 × 1000 nm = 2.3 × 107 nm3) is more than 10,000 times larger than a 20 nm hemisphere (2100 nm3) around the Ca2+ channels involved in either ECC or nuclear InsP3R2 (where the Ca2+-dependent proteins are). This means that saturation of Ca2+ indicator in this small volume can be impossible to see using optical methods (0.01% change in signal), although it physically must occur.
CaM and protein kinases are involved in ET-1–induced HDAC5 export.
We also explored downstream links between Ca2+ release from InsP3R and HDAC phosphorylation. CaM and CaMKII associate with the InsP3R (19, 27), and prior work has focused on how they regulate the InsP3R (19, 27–30) (e.g., CaMKII phosphorylates the InsP3R2, which feeds back on channel gating; ref. 19). We hypothesize that Ca2+ released by the InsP3R and consequent activation of CaM and CaMKII mediate local Ca2+ signaling to other downstream targets such as HDAC. In support of this possibility, pretreatment of myocytes with the CaM antagonist W-7 completely prevented ET-1–induced HDAC5 nuclear export (Figure 5A), implicating CaM as a local mediator.
Figure 5.
Role of CaM and protein kinase in ET-1–induced HDAC5 nuclear export. (A) Myocytes were preincubated for 30 minutes with 4 μM K-252a (n = 8) or 20 μM W-7 (n = 7) followed by ET-1 (100 nM) application. (B) ET-1–induced nuclear export of HDAC5-GFP versus mutant nonphosphorylatable HDAC5 (HDAC5-S/A-GFP; n = 4). (C) Adv-GFP-HDAC5–infected myocytes were pretreated with 1 μM KN-93 (n = 6) or 10 μM Gö6976 (n = 8), separately or simultaneously for 20–30 minutes followed by ET-1 application. (D) Summary of HDAC5 nuclear export under different conditions. Elec stim, electrical stimulation; S/T PK, serine/threonine protein kinase; Tgc, transgenic.
HDAC5 phosphorylation is required for this effect because the broad spectrum serine/threonine protein kinase inhibitor K-252a completely abolished HDAC5 export (Figure 5A) despite the fact that PKC inhibition had no effect (Figure 3A). Furthermore, the nonphosphorylatable mutant HDAC5-S/A-GFP (where both regulatory phosphorylation sites on HDAC5 were mutated to alanine) was not exported from the nucleus in response to ET-1 stimulation (Figure 5B).
CaMKII inhibition by KN-93 inhibited HDAC5 export, but only by approximately 50% (Figure 5, C and D). To further test the role of nuclear CaMKII, we used mouse myocytes isolated from transgenic mice in which a CaMKII autocamtide inhibitory peptide (AIP) was targeted to the nucleus via a nuclear localization signal (AIP-NLS; Figure 5D). In the WT littermates, ET-1–induced HDAC5 nuclear export was similar to that in the other WT mice and rabbits used here. However, in the AIP-NLS mice, ET-1–induced HDAC5 nuclear export was inhibited approximately 50% (Figure 5D). This is comparable in extent to the KN-93 effect in rabbit and is most consistent with the critical CaMKII action being inside the nucleus. Collectively, these data implicate CaM and nuclear CaMKII in HDAC export.
While CaMK is an HDAC kinase (12), it was recently reported that protein kinase D (PKD) was the primary mediator of HDAC5 phosphorylation in cultured neonatal rat ventricular myocytes stimulated with ET-1 (31). Figure 5C shows that inhibition of PKD with Gö6976 also inhibited HDAC5 by 50% (comparable to CaMKII inhibition), and when combined with KN-93, completely prevented HDAC5 nuclear export. This suggests that CaMKII and PKD both contribute to the ET-1–induced HDAC5 nuclear export in adult rabbit myocytes. Notably, PKD and CaMKII show similarities in terms of active sites and substrate specificity (32, 33); thus, these kinases may both contribute to HDAC5 phosphorylation in vivo.
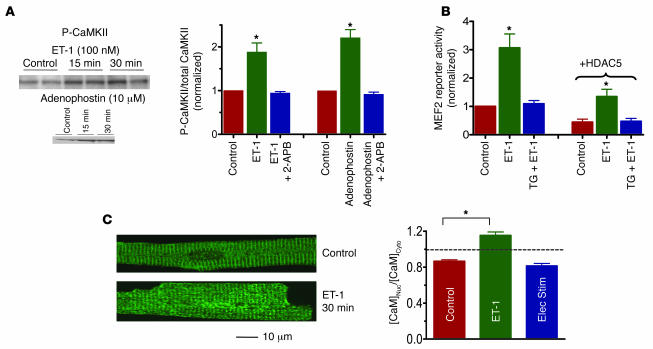
CaMKII activated directly by Ca2+-CaM can then be autophosphorylated, resulting in sustained CaMKII activation, even when [Ca2+]i declines (34). Exposure of resting myocytes to ET-1 (Figure 6A) induced CaMKII autophosphorylation, and adenophostin (the InsP3R agonist) had the same effect in permeabilized myocytes (again, without detectable global or local [Ca2+]i elevation). Both effects were completely blocked by the InsP3R inhibitor 2-APB. Thus, CaMKII activation, like HDAC export, appears to be mediated through Ca2+ release from InsP3-sensitive stores.
Figure 6.
CaMKII autophosphorylation, MEF2 activity, and CaM translocation. (A) Adult rabbit myocytes were treated with 100 nM ET-1 or 10 μM adenophostin as in Figures 2B and 3C, with cells quickly frozen for CaMKII autophosphorylation measurement by Western blot (n = 3 for each group). (B) Adenoviral MEF2-luciferase reporter was expressed in rabbit ventricular myocyte (± Adv-HDAC5-GFP) and challenged for 1 hour with 100 nM ET-1 (± pretreatment for 10 minutes with 1 μM TG). Luciferase activity was normalized to control (without HDAC5-GFP). (C) Rabbit myocytes ± ET-1, with CaM movement detected by immunocytochemistry during rest (control), exposure to 100 nM ET-1, or electrical stimulation at 1 Hz (n = 8 for each group). *P < 0.05.
Figure 5D summarizes HDAC5 export data, obtained at 1 hour of ET-1 exposure in the presence of various inhibitors (or activators alone) in rabbit and mouse myocytes. Most aspects of our working hypothesis (Figure 1) are supported.
Does ET-1 stimulate Ca2+ store–dependent activation of MEF2
? To test whether Ca2+-dependent HDAC5 nuclear export activates transcription, we used a MEF2-luciferase reporter adenovirus expressed in adult rabbit ventricular myocytes (Figure 6B). ET-1 increased luciferase expression after 1 hour of exposure (and 24 hours after incubation to allow accumulation of gene product). This effect was completely prevented by pretreatment with TG, demonstrating that intracellular Ca2+ stores are required. Coexpression with HDAC5-GFP reduced the MEF2 reporter expression overall, but the TG-sensitive stimulation by ET-1 to approximately 300% was unaltered. Thus, basal MEF2 activation is repressed when we express exogenous HDAC5-GFP. This is also a useful internal control experiment, that is, it means that the HDAC5-GFP we express is (a) functional (repressing MEF2) and (b) not excessive enough to shut down the control under study. These results again suggest that Ca2+ release from stores is required for potent ET-1–induced MEF2 transcriptional activation and that it is HDAC5 sensitive.
CaM also translocates to the nucleus upon ET-1 exposure.
In neurons, CaM can also be translocated to the nucleus, where it can participate in transcriptional regulation (35). In our case, CaM translocation to the nucleus could synergize with the ET-1–induced activation (e.g., by enhancing nuclear CaMKII activity). We measured CaM movements in adult rabbit ventricular myocytes after ET-1 treatment or electrical pacing (Figure 6C). After 30 minutes of ET-1 exposure in intact resting myocytes, there was a significant shift of CaM from cytosol to nucleus (measured by immunofluorescence), but pacing at 1 Hz for 30 minutes or even 60 minutes (not shown) did not cause significant CaM movement. Thus, CaM may also move to the nucleus in response to ET-1, and this could synergize with the Ca2+-, InsP3R-, and CaMKII-dependent signals involved in activating HDAC5 nuclear export.
Discussion
The present study provides novel compelling evidence for a working model of ET-1–induced transcriptional regulation in adult ventricular myocytes as illustrated in Figure 1. That is, ET-1 stimulates InsP3 production, which activates InsP3R2 on the nuclear envelope to release Ca2+, causing local Ca2+-dependent activation of nuclear CaMKII, HDAC5 phosphorylation, consequent HDAC5 nuclear export, and activation (derepression) of MEF2-dependent transcription. This provides a clear and unifying mechanistic explanation for how Ca2+, CaM, CaMKII, and InsP3R can regulate transcription in adult ventricular myocytes in response to neurohumoral signals and potentially be involved in altered gene expression in hypertrophy and HF.
InsP3 and InsP3R in ET-1–induced HDAC5 export.
Many G protein–coupled receptors are known to activate phospholipases and cause production of both InsP3 and DAG, the latter of which activates several PKC isoforms. While PKC activation causes many downstream effects, some of which alter transcription (36), the ET-1–induced HDAC5 nuclear export studied here was entirely attributable to InsP3-dependent signaling. This is especially salient because the functional role of InsP3 and InsP3R in ventricular myocytes has been a puzzle for some time. Indeed, while InsP3 in SR of atrial myocytes may modulate ECC and arrhythmogenesis, in ventricular myocytes, InsP3R are localized to the nuclear envelope (19) and do not normally alter ECC (17, 18). We have drawn the InsP3R2 facing the inside of the nucleus in Figure 1. While consistent with our data and working model and results in skeletal muscle (37), this still requires confirmation in cardiac myocytes.
One might question whether InsP3 can diffuse from the plasma membrane to the nucleus. However, it should be borne in mind that ventricular myocytes have an intense transverse tubule network such that no place in the cell is far from the sarcolemma. Additionally, Remus et al. (38), using a novel fluorescent InsP3 sensor, demonstrated that ET-1, angiotensin II, and phenylephrine all induce rapid increases in [InsP3] in adult ventricular myocytes. They also showed that InsP3 could diffuse from one end of the cell to the other (including the nucleus) and that [InsP3] in the nucleus lagged only slightly behind the rise in the cytosol. Thus, it makes sense that InsP3 produced at the sarcolemma can diffuse to nuclear InsP3R. Indeed, Allbritton et al. (39) showed that in cells, InsP3 (via diffusion) serves as a much better long-distance Ca2+-signal controller than Ca2+ itself.
CaMKII is a local partner of nuclear InsP3R.
In ventricular myocytes, the InsP3R2 associates with CaMKII (19), and CaM is also known to interact with InsP3R (27, 28). Moreover, activation of InsP3R-associated CaMKII can phosphorylate the InsP3R2 and decrease its open probability (19). This may be a negative feedback mechanism, such that Ca2+ released from the InsP3R activates local CaMKII, which feeds back to shut off further Ca2+ release from the nuclear envelope. The activated CaMKII could then go on to phosphorylate other targets. In the present study we have identified 1 downstream target of this local InsP3R-dependent Ca2+ release and CaMKII activation (HDAC5). We also show that InsP3R activation is necessary and sufficient to trigger ET-1–induced CaMKII autophosphorylation and HDAC5 nuclear export.
Signaling may differ between neonatal and adult ventricular myocytes.
The control of HDAC5 nuclear export reported here in adult rabbit and mouse ventricular myocytes differs somewhat from reports in Cos-1 cells or cultured neonatal rat ventricular myocytes (31). In those systems, PKD was dominant over CaMKII, and there was little evidence of Ca2+ dependence. These differences emphasize that there may be important developmental and/or cell type–specific changes in how this signaling system is controlled. This regulatory pathway might also be modified in pathophysiological states (e.g., in HF, where there is intense neurohumoral activation, and both CaMKII and InsP3R expression are greatly increased; refs. 9, 10, 40).
Our data show that ET-1–induced HDAC5 nuclear export is entirely Ca2+ and CaM dependent. This Ca2+-CaM dependence is normal for CaMKII, but there is no prior work that shows that PKD is activated by Ca2+. It is possible that Ca2+ (and/or CaM) is involved in this particular pathway for PKD activation or translocation, but the mechanism might still be indirect. The possible role of Ca2+ or CaM in the activation of PKD may require additional study (and in the appropriate cellular context).
Local versus global [Ca2+] is important in activating HDAC5 translocation.
It is remarkable that the activation of HDAC5 nuclear export here is entirely Ca2+ dependent but that global Ca2+ transients (rising to 0.5–1 μM), even in the nucleus, are unable to significantly activate HDAC5 translocation. However, this is entirely consistent with very local Ca2+ signaling to proteins that are physically associated with the InsP3R channel (e.g., CaM and CaMKII). Indeed, very high local [Ca2+] (>> 10 μM; Figure 4D) must exist around the InsP3R, and the associated CaM and CaMKII must be exposed to it (note that Ca2+ binds to CaM with Kd values in the 1–10 μM range). This scenario is expected for CaMKII, as in other systems where CaMKII is activated by high local [Ca2+] and the CaMKII activation state may sense local Ca2+ spikes in an integrating manner (41). This may also relate to the relatively modest affinity of Ca2+-CaM for CaMKII (∼200-fold lower than for calcineurin, which would be expected to sense [Ca2+] differently than CaMKII).
The data here reveal a novel local Ca2+ control system for excitation-transcription coupling (ETC) in adult ventricular myocytes. This pathway is expected to be important in cardiac transcriptional regulation since both the initiating stimulus (ET-1) and end points studied here (HDAC5 nuclear export and MEF2 activation) have accepted roles in this process. This pathway demonstrates a long sought for and important signaling role for InsP3R in ventricular myocyte which is independent of ECC and centered at the nuclear envelope. Indeed, the local Ca2+ control features of this ETC pathway resemble that in ECC. A remarkable feature of ETC here is that the local Ca2+ signaling is relatively insulated from the large swings of cytosolic and nuclear [Ca2+]i that occur at every heartbeat. This may be an extremely important way by which myocytes can use neurohumoral signaling via Ca2+-dependent mechanisms without being distracted by the “noise” of beat-to-beat Ca2+ transients. It may also complement other Ca2+- and CaM-dependent transcriptional factors, such as the calcineurin/NFAT pathway (42), which may respond to very different local or global Ca2+ signals than shown here for CaMKII/ HDAC5. Likewise, CaMKII may activate transcription via phosphorylating cAMP response element-binding protein (CREB), as seen in neurons, but this is not yet apparent in heart (35, 43, 44).
These experiments mechanistically explain how InsP3, InsP3R, CaM, and CaMKII work in concert to activate HDAC5 translocation and thus transcription in cardiac myocytes. They also provide a functional role for the robust physical association between InsP3R2 and CaMKII on the nuclear envelope (19). CaMKII in this complex can thus signal transcriptional activation but also feed back by phosphorylating the InsP3R2 to inhibit further Ca2+ release (19), creating an elegant fine tuning system. Since both CaMKII and InsP3R are upregulated in HF, this may also constitute a pathway that contributes to either the development or reinforcement of the hypertrophic or HF phenotype.
Methods
Myocyte isolation and adenoviral infection.
All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Loyola University Chicago Institutional Animal Care and Use Committee. Myocytes were isolated as previously described (45). Myocyte superfusate for experiments contained 140 mM NaCl; 4 mM KCl; 1 mM MgCl2; 2 mM CaCl2 (rabbit) or 1 mM CaCl2 (mouse); 10 mM HEPES; and 10 mM glucose; pH 7.4; 23°C.
Myocytes were seeded on laminin-coated culture inserts. Culture media for rabbit were supplemented with serum-free medium 199 (M199) or in mouse with minimum essential medium containing 1% FBS. Some mouse experiments were done without 1% FBS, and results were similar. Myocytes were infected for 2 hours at MOI equal to 10 with recombinant replication-deficient adenovirus expressing HDAC5-GFP (or HDAC5-S/A-GFP, a mutant in which the critical phosphorylation sites (12) at serine 259 and 498 are mutated to alanine) under the cytomegalovirus promoter, with subsequent culture for 24–36 hours. GFP fluorescence indicated infection and localization. For MEF2-luciferase reporter experiments, cultured rabbit ventricular myocytes were infected with adenovirus encoding MEF2-luciferase gene for 48 hours total (with or without coexpression with GFP-HDAC5). After the first 24 hours, myocytes were exposed (or not) for 1 hour to 100 nM ET-1 with or without TG 1 μM pretreatment for 10 minutes before ET-1 exposure. Then, ET-1 and TG were removed and cells were incubated for another 24 hours to allow the effects of ET-1 on MEF2-luciferase expression to occur.
Genetically modified mice.
The InsP3R2 gene was ablated as described to generate InsP3R2 KO mice (23). Transgenic mice with nuclear-targeted AIP (a specific and potent CaMKII inhibitor) were generated similarly to those in Ji et al. (46). A synthetic gene encoding 4 repeats of AIP, KKALRRQEAVDAL, was placed between a FLAG epitope, DYKDDDDK (3′), and an SV40 large T antigen nuclear location signal, PKKRKV (5′). This expression unit was cloned into a pBluescript vector between the 5.5-kb murine α-myosin heavy chain promoter (from J. Robbins, Cincinnati Children’s Hospital, Cincinnati, Ohio, USA) and an SV40 polyadenylation signal. Purified linearized transgene was injected into pronuclei of fertilized mouse oocytes by the University of Cincinnati Transgenic Core Facility. Founders were bred with FVB/N WT mice for 4 generations.
Reagents.
Rhod-2 and Fluo-5N were from Invitrogen Corp., ET-1 and TG from Sigma-Aldrich. W-7, 2-APB, adenophostin A, and K-252a were from Calbiochem. KN-93 was from Seikagaku Corp. and Bis I and Gö6976 from A.G. Scientific Inc. CaMKII phosphorylation–specific antibody was from Affinity BioReagents, and Western blots were as described previously (6). CaM monoclonal antibody was from Upstate USA Inc., with immunocytochemistry as described (35).
MEF2-luciferase assay.
The cultured myocytes were lysed in lysis buffer (Promega). Luciferase activity was determined with the luciferase assay kit (Promega).
Ca2+ indicators loading and confocal imaging.
Myocytes were loaded with 10 μM Rhod-2 AM for 30 minutes (15 minutes for deesterification) or with 10 μM Fluo-5N AM for 90 minutes (60 minutes for deesterification). GFP-HDAC5 signals were measured by confocal microscopy with argon laser excitation at 488 nm and emitted fluorescence (F) at 500–530 nm. Rhod-2 was excited with a HeNe laser at 543 nm with emission at greater than 570 nm, and cytosolic [Ca2+]i was calibrated as follows: [Ca2+]i= Kd(F/F0)/(Kd/[Ca2+]dias + 1 – F/F0), assuming Kd=710 nM, [Ca2+]dias=150 nM, and F0=diastolic F. Fluo-5N excited at 488 nm, with emission greater than 500 nm (22).
Image-J software (http://rsb.info.nih.gov/ij/) was used for image analysis, with the intensity of regions of interest normalized to area. Confocal line scan (1-dimensional x-t) and 2-dimensional imaging were performed with a ×40 oil immersion objective with temporal resolution of 166 lines s–1.
Myocyte permeabilization.
Myocytes, in relaxation solution for 2 minutes, were permeabilized by adding saponin (50 μg/ml) for 20 seconds and then exposed to internal solution containing 0.5 mM EGTA; 10 mM HEPES; 120 mM K-aspartate, 1 mM free Mg; 100 nM free Ca2+; 5 mM ATP; 10 mM reduced glutathione; 5 mM phosphocreatine di-tris; 5U/ml creatine phosphokinase; 8% dextran; pH 7.2. Relaxing solution was the same except EGTA was 0.1 mM and Ca2+ was omitted. When required, 10 μM adenophostin was added to the internal solution or free [Ca2+] was changed. Low laser power (4%) was used to minimize Fluo-5N photobleaching.
Statistics.
Results are mean ± SEM with significance (P < 0.05) determined using unpaired 2-tailed Student’s t test.
Acknowledgments
We thank Jayme O’Brien for technical assistance. We thank J.D. Molkentin for adenovirus-encoding MEF2-luciferase reporter. This work was supported by NIH grants HL-30077, HL-46345, and HL80101 (to D.M. Bers and J. Heller Brown) and an American Heart Association predoctoral fellowship (X. Wu) and scientific development grant (T. Zhang).
Footnotes
See the related beginning on page .
Nonstandard abbreviations used: AIP, autocamtide inhibitory peptide; 2-APB, 2-aminoethoxydiphenyl borate; Bis I, bisindolylmaleimide I; CaM, calmodulin; CaMKII, Ca2+-CaM–dependent protein kinase II; DAG, diacylglycerol; ECC, excitation-contraction coupling; ET-1, endothelin-1; ETC, excitation-transcription coupling; HDAC, histone deacetylase; HF, heart failure; InsP3, inositol 1,4,5-trisphosphate; InsP3R, InsP3 receptor; MEF2, myocyte enhancer factor-2; NFAT, nuclear factor of activated T cells; PKD, protein kinase D; RyR, ryanodine receptor; SR, sarcoplasmic reticulum; TG, thapsigargin.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Frey N, McKinsey TA, Olson EN. Decoding calcium signals involved in cardiac growth and function. Nat. Med. 2000;6:1221–1227. doi: 10.1038/81321. [DOI] [PubMed] [Google Scholar]
- 2.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 3.Zhu W, et al. Ca2+/calmodulin-dependent kinase II and calcineurin play critical roles in endothelin-1-induced cardiomyocyte hypertrophy. J. Biol. Chem. 2000;275:15239–15245. doi: 10.1074/jbc.275.20.15239. [DOI] [PubMed] [Google Scholar]
- 4.Gruver CL, DeMayo F, Goldstein MA, Means AR. Targeted developmental overexpression of calmodulin induces proliferative and hypertrophic growth of cardiomyocytes in transgenic mice. Endocrinology. 1993;133:376–388. doi: 10.1210/endo.133.1.8319584. [DOI] [PubMed] [Google Scholar]
- 5.Colomer JM, Means AR. Chronic elevation of calmodulin in the ventricles of transgenic mice increases the autonomous activity of calmodulin-dependent protein kinase II, which regulates atrial natriuretic factor gene expression. Mol. Endocrinol. 2000;14:1125–1136. doi: 10.1210/mend.14.8.0496. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez MT, Zhao XL, Schulman H, Brown JH. The nuclear deltaB isoform of Ca2+/calmodulin-dependent protein kinase II regulates atrial natriuretic factor gene expression in ventricular myocytes. J. Biol. Chem. 1997;272:31203–31208. doi: 10.1074/jbc.272.49.31203. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, et al. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ. Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 8.Zhang T, et al. The cardiac-specific nuclear delta(B) isoform of Ca2+/calmodulin-dependent protein kinase II induces hypertrophy and dilated cardiomyopathy associated with increased protein phosphatase 2A activity. J. Biol. Chem. 2002;277:1261–1267. doi: 10.1074/jbc.M108525200. [DOI] [PubMed] [Google Scholar]
- 9.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ. Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 10.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat. Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 12.McKinsey TA, Zhang CL, Lu J, Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000;408:106–111. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003;17:1937–1956. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- 14.Zhang CL, et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–488. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 16.Perez PJ, Ramos-Franco J, Fill M, Mignery GA. Identification and functional reconstitution of the type 2 inositol 1,4,5-trisphosphate receptor from ventricular cardiac myocytes. J. Biol. Chem. 1997;272:23961–23969. doi: 10.1074/jbc.272.38.23961. [DOI] [PubMed] [Google Scholar]
- 17.Mackenzie L, et al. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J. Physiol. 2002;541:395–409. doi: 10.1113/jphysiol.2001.013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J. Physiol. 2004;555:607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 2005;280:15912–15920. doi: 10.1074/jbc.M414212200. [DOI] [PubMed] [Google Scholar]
- 20.Bers, D.M. 2001. Excitation-contraction coupling and cardiac contractile force. 2nd edition. Kluwer Academic Publishers. Dordrecht, Holland. 427 pp.
- 21.Lipp P, et al. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Curr. Biol. 2000;10:939–942. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- 22.Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ. Res. 2003;93:40–45. doi: 10.1161/01.RES.0000079967.11815.19. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate(IP3)-receptor type 2-deficient mice. Circ. Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Randall WR, Schneider MF. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J. Cell Biol. 2005;168:887–897. doi: 10.1083/jcb.200408128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Cseresnyes Z, Randall WR, Schneider MF. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J. Cell Biol. 2001;155:27–39. doi: 10.1083/jcb.200103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bers DM, Peskoff A. Diffusion around a cardiac calcium channel and the role of surface bound calcium. Biophys. J. 1991;59:703–721. doi: 10.1016/S0006-3495(91)82284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada M, et al. The calmodulin-binding domain in the mouse type 1 inositol 1,4,5-trisphosphate receptor. Biochem. J. 1995;308:83–88. doi: 10.1042/bj3080083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardy TJ, Taylor CW. A novel role for calmodulin: Ca2+-independent inhibition of type-1 inositol trisphosphate receptors. Biochem. J. 1998;334:447–455. doi: 10.1042/bj3340447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michikawa T, et al. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,4,5-trisphosphate receptor. Neuron. 1999;23:799–808. doi: 10.1016/s0896-6273(01)80037-4. [DOI] [PubMed] [Google Scholar]
- 30.Missiaen L, et al. The bell-shaped Ca2+ dependence of the inositol 1,4, 5-trisphosphate-induced Ca2+ release is modulated by Ca2+/calmodulin. J. Biol. Chem. 1999;274:13748–13751. doi: 10.1074/jbc.274.20.13748. [DOI] [PubMed] [Google Scholar]
- 31.Vega RB, et al. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol. Cell. Biol. 2004;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valverde AM, Sinnett-Smith J, Van Lint J, Rozengurt E. Molecular cloning and characterization of protein kinase D: a target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc. Natl. Acad. Sci. U. S. A. 1994;91:8572–8576. doi: 10.1073/pnas.91.18.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doppler H, Storz P, Li J, Comb MJ, Toker A. A phosphorylation state-specific antibody recognizes Hsp27, a novel substrate of protein kinase D. J. Biol. Chem. 2005;280:15013–15019. doi: 10.1074/jbc.C400575200. [DOI] [PubMed] [Google Scholar]
- 34.MacNicol M, Jefferson AB, Schulman H. Ca2+/calmodulin kinase is activated by the phosphatidylinositol signaling pathway and becomes Ca2+-independent in PC12 cells. J. Biol. Chem. 1990;265:18055–18058. [PubMed] [Google Scholar]
- 35.Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- 36.Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J. Clin. Invest. 2005;115:527–537. doi:10.1172/JCI200524178. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardenas C, et al. Nuclear inositol 1,4,5-trisphosphate receptors regulate local Ca2+ transients and modulate cAMP response element binding protein phosphorylation. J. Cell Sci. 2005;118:3131–3140. doi: 10.1242/jcs.02446. [DOI] [PubMed] [Google Scholar]
- 38.Remus TP, et al. Biosensors to measure InsP3 concentration in living cells with spatio-temporal resolution. J. Biol. Chem. 2006;281:608–616. doi: 10.1074/jbc.M509645200. [DOI] [PubMed] [Google Scholar]
- 39.Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- 40.Go LO, et al. Differential regulation of 2 types of intracellular calcium release channels during end-stage heart failure. J. Clin. Invest. 1995;95:888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 42.Molkentin JD, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc. Res. 2004;63:476–486. doi: 10.1016/j.cardiores.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 44.Passier R, et al. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J. Clin. Invest. 2000;105:1395–1406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bassani JW, Bassani RA, Bers DM. Calibration of indo-1 and resting intracellular [Ca2+]i in intact rabbit cardiac myocytes. Biophys. J. 1995;68:1453–1460. doi: 10.1016/S0006-3495(95)80318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji Y, et al. Targeted inhibition of Ca2+/calmodulin-dependent protein kinase II in cardiac longitudinal sarcoplasmic reticulum results in decreased phospholamban phosphorylation at threonine 17. J. Biol. Chem. 2003;278:25063–25071. doi: 10.1074/jbc.M302193200. [DOI] [PubMed] [Google Scholar]