Abstract
The liver X receptors (LXRs) are nuclear receptors that play central roles in the transcriptional control of lipid metabolism. LXRs function as nuclear cholesterol sensors that are activated in response to elevated intracellular cholesterol levels in multiple cell types. Once activated, LXRs induce the expression of an array of genes involved in cholesterol absorption, efflux, transport, and excretion. In addition to their function in lipid metabolism, LXRs have also been found to modulate immune and inflammatory responses in macrophages. Synthetic LXR agonists promote cholesterol efflux and inhibit inflammation in vivo and inhibit the development of atherosclerosis in animal models. The ability of LXRs to integrate metabolic and inflammatory signaling makes them particularly attractive targets for intervention in human metabolic disease.
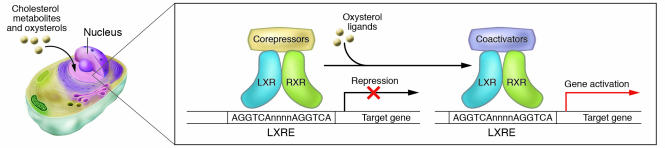
Liver X receptor-α (LXRα) and LXRβ (also known as NR1H3 and NR1H2, respectively) were cloned more than a decade ago based on sequence homology with other receptors. LXRs were originally considered “orphan” nuclear receptors, because their natural ligands were unknown (1, 2); however, these receptors were “adopted” following the discovery that metabolites of cholesterol — oxysterols — bind to and activate these receptors at physiological concentrations (2, 3). LXRα is highly expressed in the liver and at lower levels in the adrenal glands, intestine, adipose, macrophages, lung, and kidney, whereas LXRβ is ubiquitously expressed (4). The LXRs are ligand-dependent transcription factors that form permissive heterodimers with the retinoid X receptor (RXR); i.e., the complex can be activated by ligands of either partner (Figure 1). LXR/RXR heterodimers bind to LXR-responsive elements (LXREs) in DNA consisting of direct repeats (DRs) of the core sequence AGGTCA separated by 4 nucleotides (DR-4) (5). Like most other nuclear receptors that form heterodimers with RXR, LXRs reside within the nucleus, bound to cognate LXREs and in complex with corepressors such as silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) (6) and nuclear receptor corepressor (N-CoR) (7). In the absence of ligand, these corepressor interactions are maintained and the transcriptional activity of target genes is repressed. Binding of ligand to LXR results in a conformational change that facilitates coactivator-for-corepressor-complex exchange and transcription of target genes (8). Ligand activation of LXRs also inhibits transcription from promoters of certain genes (e.g., proinflammatory cytokines) that do not contain LXREs, a phenomenon referred to as trans-repression.
Figure 1.
LXRs are cholesterol-sensing transcription factors. Within the nucleus, LXR/RXR heterodimers are bound to LXREs in the promoters of target genes and in complex with corepressors (e.g., SMRT, N-CoR). In response to the binding of oxysterol ligands, the corepressor complexes are exchanged for coactivator complexes, and target gene expression is induced.
Studies over the last 5 years have established LXRs as key modulators of both lipid metabolism and inflammatory signaling (9). This Review will focus on recent advances in our understanding of the roles of LXRs in physiology and homeostasis as well as the links between LXR action and metabolic diseases such as atherosclerosis.
LXRs are master regulators of whole-body cholesterol homeostasis
The initial identification of oxysterols as physiological ligands of LXRs pointed to a possible role for these receptors in cholesterol metabolism (3, 10). Conclusive evidence for this notion came from studies of Lxra–/– mice, which display marked cholesteryl ester accumulation in their livers when challenged with a cholesterol-rich diet (11). This phenotype led to the identification of Cyp7a1, a member of the cytochrome p450 family of enzymes and the rate-limiting enzyme in the classical pathway of bile acid synthesis, as the first direct target of LXRs. The inability of Lxra–/– mice to induce hepatic Cyp7a1 expression results in a diminished ability to metabolize cholesterol to bile acids, and the accumulation of cholesteryl esters. Interestingly, the LXRE found in the promoter of rodent Cyp7a1 is not conserved in humans (12). LXRβ is also expressed in the liver, but Lxrb–/– mice do not display an obvious hepatic phenotype even when challenged with a high-cholesterol diet (13), indicating that LXRα is likely to be the dominant isoform in this tissue.
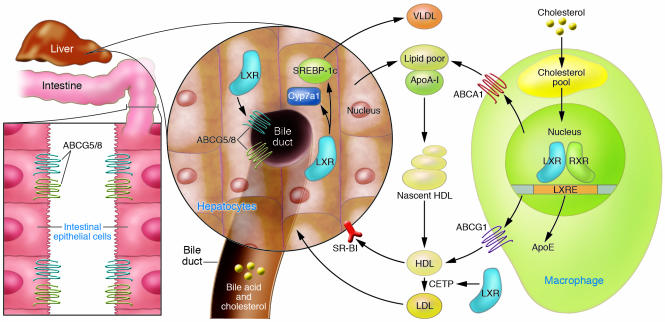
Subsequent studies demonstrated that LXRs also regulate a set of genes that participate in the process of reverse cholesterol transport — the transport of excess cholesterol in the form of HDL from peripheral tissue to the liver (14). In vivo activation of LXRs with a synthetic, high-affinity ligand increases HDL levels and net cholesterol secretion (15). To a large extent these activities are dependent on the ability of LXRs to control the expression of members of the ABC superfamily of membrane transporters (16), including ABCA1 (15, 17), ABCG5, ABCG8 (18, 19), and ABCG1 (20–22). Mutations in the ABCA1 gene are the cause of Tangier disease, a rare disorder that is characterized by the virtual absence of HDL in plasma of afflicted patients, the accumulation of cholesterol in tissue macrophages, and an increased incidence of cardiovascular disease (23–25). It is now well established that ABCA1 facilitates the efflux of cholesterol and phospholipids to lipid-poor lipoproteins (e.g., apoA-I), and its induction may contribute to the increase in plasma HDL levels seen with LXR ligand treatment (26, 27). The ability of LXR ligands to decrease intestinal absorption of cholesterol (15) appears to be mediated by induction of 2 other ABC transporters, ABCG5 and ABCG8 (18, 19). These apically localized transporters form a functional heterodimer that acts to limit cholesterol and plant-sterol absorption in the gut, and to mediate cholesterol efflux from hepatocytes into bile (28, 29). Mutations in either of the heterodimer partners cause the rare genetic disease sitosterolemia, which is characterized by increased absorption of plant sterols and premature atherosclerosis (30, 31). The role of LXRs in whole-body reverse cholesterol transport is summarized in Figure 2.
Figure 2.
Role of LXRs in reverse cholesterol transport from macrophages. The uptake of modified lipoproteins by macrophages results in increased LXR transcriptional activity and efflux of cholesterol to lipid-poor apoA-I by ABCA1 and to HDL by ABCG1. In humans, but not mice, induction of CETP expression transfers lipid from HDL to LDL. Once HDL/LDL is taken up by the liver, LXR promotes net cholesterol excretion. In rodents, but not humans, LXR induces expression of Cyp7a1, which initiates the conversion of cholesterol into bile acids. LXRs also induce cholesterol secretion into bile through the transporters ABCG5 and ABCG8. In the intestine, apical ABCG5 and ABCG8 also act to limit dietary cholesterol uptake.
LXR signaling in intermediary metabolism and energy balance
In addition to their ability to modulate cholesterol metabolism, LXRs are also key regulators of hepatic lipogenesis. Treatment of mice with synthetic LXR agonists elevates triglyceride levels in the liver as well as transiently in the plasma, an effect that poses a significant obstacle to the development of these compounds as human therapeutics (32, 33). The lipogenic activity of LXRs results from the upregulation of the master regulator of hepatic lipogenesis SREBP-c (34, 35), as well as induction of fatty acid synthase (33), acyl-CoA carboxylase, and stearoyl-CoA desaturase 1 (reviewed in ref. 36). An attempt to limit increases of hepatic and plasma triglycerides in response to LXR-agonist treatment by increasing hepatic fatty acid oxidation with a PPARα ligand was unsuccessful (37). Although the PPARα ligand strongly increased hepatic fatty acid oxidation, it was unable to counter the hepatic triglyceride accumulation in response to the LXR agonist. LXRs also positively regulate several enzymes involved in lipoprotein remodeling, including lipoprotein lipase, human cholesteryl ester transport protein (CETP), and the phospholipid transfer protein (PLTP). It is likely that some of the effects of activated LXR on plasma lipoproteins are mediated through action of these enzymes (36).
Glucose metabolism is also impacted by LXR activity. Two different synthetic agonists of LXRs have been reported to improve glucose tolerance in diabetic mouse (38) and rat models (39). This effect is associated with repression of the hepatic gluconeogenic genes phosphoenolpyruvate carboxykinase and glucose-6-phosphatase, and with the induction of glucokinase. Such changes would be expected to result in decreased hepatic glucose output and increased hepatic glucose utilization. The insulin-dependent glucose transporter 4 (GLUT4) has been shown to be a direct target of LXRs in white adipose fat, and its induction would be predicted to result in increased uptake and utilization of glucose by this tissue (38, 40). Recent studies have also demonstrated a role for LXRβ in pancreatic islet cells. Activation of LXRβ in these cells enhances glucose-dependent insulin secretion (41), a process that is impaired in cells from Lxrb–/– mice (42).
As outlined above, LXRs are intrinsically involved in key metabolic pathways, but the integrated action of LXRs on whole-body energy balance has been only recently addressed. Kalaany et al. (43) found that LXR-null mice are resistant to obesity when challenged with a diet high in both fat and cholesterol. Remarkably, this phenotype was dependent on the presence of cholesterol in the diet and was largely attributed to increased peripheral utilization of dietary fat as manifested by a marked enhanced metabolic rate. Moreover, an increase in expression and activity of deiodinase 2 (Dio2), an enzyme that generates active thyroid hormone (T3) from its inactive form (T4), was seen in livers of LXR-null mice fed the cholesterol-containing diet and would be predicted to increase hepatic utilization of dietary fat as well. The mechanism by which dietary cholesterol signals change in systemic metabolism is not yet known. Regardless, this study emphasizes the central role of LXRs as regulators of fat storage and utilization. New roles for LXRs in other tissues (e.g., brain, skin, fat, etc.) and metabolic pathways are also rapidly emerging (44).
LXRs as regulators of macrophage cholesterol metabolism
In addition to their essential role in innate immunity, macrophages are central to the development of the atherosclerotic lesion because of their ability to take up modified lipoproteins and to release inflammatory mediators (45, 46). Within the lesion, macrophages are postulated to accumulate ligands of LXRs by several distinct pathways. Uptake of modified lipoproteins may provide the cell with preformed oxysterol activators of LXR. Ligands may also be generated intracellularly from accumulated cholesterol by action of the mitochondrial Cyp27 (47). Although Cyp27 is not a direct target of LXRs, its enzymatic product 27-hydroxycholesterol is an LXR ligand (48, 49), albeit a relatively weak one (50). Additionally, the intracellular production of 24-(S),25-epoxycholesterol, a potent naturally occurring LXR ligand, in rodent and human macrophages was reported recently (51). Increasing the levels of this metabolite by partially inhibiting the enzyme 2,3-oxidosqualene:lanosterol cyclase results in increased LXR transcriptional activity (52).
A primary function of LXRs in macrophages is to maintain cellular cholesterol homeostasis. Activation of LXRs in lipid-loaded macrophages leads to induction of genes involved in the cholesterol efflux pathway in an attempt to reduce the intracellular cholesterol burden. The ABC transporters discussed above are critical for the ability LXRs to enhance efflux to cholesterol acceptors. Expression of ABCA1 is strongly induced by natural and synthetic LXR ligands as well as by loading of cells with modified lipoproteins. This induction has been attributed to the presence of LXREs in the proximal promoter of the ABCA1 gene (15, 17, 26, 27). LXRs are in fact essential for lipid-inducible ABCA1 expression, as induction is lost in macrophages from Lxrab double-knockout mice (Lxrab–/– mice). Conversely, LXRs are unable to stimulate cholesterol efflux to lipid-poor lipoproteins in fibroblasts from Tangier disease patients, demonstrating that ABCA1 is essential for the LXR-mediated efflux pathway (27). The importance of ABCA1 for atherogenesis is underscored by the fact that macrophage-specific loss of this gene results in increased lesion formation in murine models (53, 54).
ABCG1, another member of the ABC transporter family, is also strongly induced by cholesterol loading of macrophages (22, 55) and was recently identified as a direct target of LXRs in mouse and human cells (20, 21). Induction of ABCG1 may provide an additional pathway for cholesterol efflux from macrophages or may act in concert with ABCA1. ABCG1 is thought to function as a homodimer (56), although a functional partnership with ABCG4 has been also suggested (57). In in vitro assays, ABCG1 has been demonstrated to facilitate cholesterol efflux to HDL-2 and -3 particles, but not to apoA-I, thus distinguishing it mechanistically from ABCA1 (55, 56, 58). At present, however, the cellular localization of ABCG1 is not defined, and it is therefore unclear whether ABCG1 directly mediates efflux to HDL particles or facilitates this process by influencing intracellular cholesterol trafficking. In line with the latter possibility is a recent study demonstrating that activation of LXRs in human macrophages boosts cholesterol trafficking to the plasma membrane at the expense of esterification (59).
The generation and initial characterization of Abcg1–/– mice has revealed striking phenotypes that point to a critical function for this transporter in whole-body lipid homeostasis (58). In support of in vitro experiments, macrophages lacking ABCG1 showed a diminished cholesterol efflux capacity to HDL. Cholesterol efflux to apoA-I, which is mainly mediated by ABCA1, was unchanged, however. In accordance with these findings, lipid-laden macrophages were detected in the lungs and liver of Abcg1-null mice after 9 weeks of a high-fat and -cholesterol diet. Remarkably, this phenotype was not accompanied by changes in the profile of plasma lipoproteins. It is tempting to speculate that ABCG1 activity, like ABCA1 activity, would be antiatherogenic, but this remains to be tested directly. The closely related protein ABCG4 is also modestly induced in macrophages by cholesterol loading and by LXR ligands and has been reported to promote cholesterol efflux to HDL particles when overexpressed in HEK293 cells (56, 60). Studies of the physiological roles of this transporter are eagerly awaited.
An additional mechanism that may contribute to the LXR-driven reverse cholesterol transport is the induction of a subset of apolipoproteins that may serve as cholesterol acceptors. Specifically, LXRs induce Apoe gene expression in macrophages and adipose tissue, but not in the liver (61). Additionally, the Apoc gene cluster (ApocI, ApocII, and ApocIV) is also induced by LXRs in macrophages (62), and Apod is a target for LXR in adipose tissue (63). The significance of the induction of the Apoc cluster and of Apod by LXR for lipoprotein metabolism is at present unknown. In contrast, the protective role of Apoe in atherogenesis is well established. Loss of macrophage apoE leads to increased lesions, whereas overexpression of apoE in these cells is protective (reviewed in ref. 64). More recently, LXR was shown to directly regulate hepatic, but not intestinal, Apoa4 in mouse liver, and APOA4 in the human HepG2 cell line (65). In humans, plasma levels of APOA4 are inversely correlated with cardiovascular disease. Whether APOA4 is regulated in macrophages by LXR is at present unknown. APOA5 is the only apolipoprotein known to be repressed by LXRs (66). Repression of hepatic APOA5 by LXR is not direct but appears to be secondary to induction of SREBP-1c. As increased levels of APOA5 are strongly correlated with reduced plasma triglycerides (67), repression by LXR may contribute to the hypertriglyceridemic effects of synthetic LXR agonists (32).
An additional level of LXR regulation, beyond ligand availability, is the level of LXRα receptor expression. In human and rodent macrophages, PPARγ agonists induce the expression of LXRα, suggesting a functional link between the uptake of oxidized LDL (oxLDL) and cholesterol removal (68). Furthermore, in human macrophages LXRα is able to induce its own transcript via an autoregulatory loop (69, 70). This autoregulation does not occur in rodent macrophages, however.
Collectively, the studies highlighted above point to the central role of LXRs in governing cholesterol efflux from macrophages. Under conditions of increased intracellular cholesterol levels, as is the case in lesion macrophages, these pathways would be expected to impact disease development. However, the presence of lipid-laden macrophages in various tissues of Lxrab–/– mice demonstrates that these same pathways are important for normal cholesterol homeostasis as well (71, 72).
LXRs as regulators of macrophage inflammatory signaling
Activation of inflammatory signaling pathways and release of inflammatory mediators are fundamental to the diverse immune functions of macrophages. The microenvironment present within the atherosclerotic lesion is proinflammatory and results in activation of these same pathways. A substantial number of studies demonstrate that excessive inflammation within the arterial wall is a risk factor for cardiovascular disease and promotes atherogenesis (45, 46). Therefore, factors that act to limit inflammation in this setting may prove to be beneficial in reducing disease progression.
Considerable evidence has emerged to indicate that, in addition to inducing genes involved in reverse cholesterol transport, LXRs reciprocally repress a set of inflammatory genes after bacterial, LPS, TNF-α, or IL-1β stimulation (73). Examples of such genes include those involved in generation of bioactive molecules such as iNOS and COX2, IL-6 and IL-1β, the chemokines monocyte chemoattractant protein-1 (MCP-1) and MCP-3, and MMP9 (73, 74). LXR ligands repress these genes in macrophages derived from WT, Lxra–/–, and Lxrb–/– mice but are unable to do so in macrophages from Lxrab–/– mice, indicating that both LXR isoforms possess antiinflammatory activity. Subsequent work has suggested that tissue factor and osteopontin, both inflammatory genes associated with an increased risk for developing atherosclerosis, are subject to similar repression by LXR ligands in macrophages (75, 76).
Inhibition of inflammatory signaling by LXR is not limited to isolated macrophages but also manifests itself in vivo. Experiments in several different models have confirmed the antiinflammatory effects of LXRs. When challenged i.p. with LPS, Lxrab–/– mice exhibit an exacerbated systemic inflammatory response and increased hepatic expression of iNOS, TNF-α, and IL-1β (73). Synthetic LXR agonists also reduce inflammation in a model of irritant contact dermatitis (73). A similar result was reported by Fowler et al., who found that LXR ligands showed activity comparable to that of a steroid-based drug in an oxazolone-induced allergic dermatitis model (77). Furthermore, administration of LXR ligands to mice inhibits tissue factor expression in the kidney and lung after an LPS challenge (75). Finally, in 2 mouse models of chronic atherogenic inflammation, Apoe–/– and Ldlr–/– mice, administration of LXR ligands repressed the aortic expression of MMP9 and tissue factor while inducing expression of ABCA1 (73, 75).
The mechanism underlying the repression of inflammatory genes by LXRs is poorly understood. LXREs have not been identified in the proximal promoters of the repressed genes; this points to an indirect mechanism. In addition to possible competition for transcriptional coactivators (78, 79), the body of evidence suggests that inhibition of the NF-κB pathway is involved (Figure 3). Inhibition of this pathway does not entail inhibition of NF-κB translocation to the nucleus, binding to DNA, or degradation of the NF-κB inhibitor IκB (73–75). Most likely, trans-repression of NF-κB by LXR involves a nuclear event. In a recent study of trans-repression of the iNOS promoter by PPARγ, sumoylation of PPARγ was identified as a possible mechanism involved in this process (80). Sumoylated PPARγ was suggested to prevent the LPS-dependent exchange of corepressors for coactivators, thus maintaining the iNOS promoter in a repressed state. Whether this is the case for LXR and other nuclear receptors remains to be tested.
Figure 3.
Integration of lipid metabolic and inflammatory signaling in macrophages by LXRs. Recognition of cytokines, bacterial components, or intact pathogens by their corresponding receptors initiates expression of proinflammatory genes (e.g., iNOS). Activation of the TLR3/4 receptors by these signals blocks LXR-dependent gene transcription and cholesterol efflux from macrophages via an IFN regulatory factor 3–dependent (IRF3-dependent) pathway. On the other hand, ligand activation of LXRs inhibits NF-κB–dependent induction of inflammatory gene expression. Intracellular bacteria induce LXRα expression, possibly through a NOD2-dependent pathway, and promote macrophage survival, through induction of Api6 (also known as AIM and SPα) and other targets.
Participation in both metabolic and inflammatory control is a common feature of a number of different nuclear receptor signaling pathways. For example, Ogawa et al. (81) demonstrated recently that LXR, PPARγ, and the glucocorticoid receptor repress an overlapping yet distinct set of inflammatory genes in a stimulus-dependent manner. In addition to pointing out the possibility of treating inflammatory-related diseases in a combinatorial approach that targets 2 or more of these receptors, this study also underscores the complexity of inflammatory gene regulation and the likelihood that these nuclear receptors have unique functions within the immune system.
LXR and atherosclerosis
Atherosclerosis is characterized by both alterations in lipid metabolism and the development of a chronic inflammatory state within the arterial wall. The ability of LXRs to increase reverse cholesterol transport and attenuate inflammation in macrophages, as outlined above, would therefore be predicted to be beneficial in this setting. Both gain-of-function and loss-of-function studies indicate that activation of the LXR pathway is antiatherogenic. Treatment of atherosclerosis-prone Apoe–/– and Ldlr–/– mice with a synthetic LXR ligand led to an approximately 50% decrease in lesion size (82). Expression of ABCA1 and ABCG1 was induced, whereas that of inflammatory genes was repressed, in aortic samples from ligand-treated mice, suggesting a plausible mechanism for the beneficial outcome. In contrast, macrophage-specific loss of LXRs achieved by transplantation of bone marrow from Lxrab–/– mice into either Apoe–/– or Ldlr–/– mice resulted in a marked increase in lesion size (71). A recent study further established that treatment of Ldlr–/– mice with an LXR agonist reduced the size of preexisting lesions and that this reduction was dependent on LXR activity in macrophages (83). The significance of this finding lies in the fact that most humans presenting with signs of cardiovascular disease already have substantial lesion development. The relative contribution of enhanced cholesterol efflux and repression of inflammation to the beneficial activity of LXRs in these experimental settings remains to be determined. Regardless, these studies strongly support the hypothesis that the macrophage LXR pathway is an important homeostatic mechanism that helps to protect against cholesterol overload, and they point to the LXR pathway as an attractive target for intervention in cardiovascular disease.
LXRs and innate immunity
Bacterial and viral pathogens have long been suspected to contribute to cardiovascular disease risk based on epidemiological studies and on experimental models of infection in atherosclerosis-prone mice (84–86). The molecular mechanisms underlying this effect are poorly understood, as is the link between cholesterol metabolism and innate immunity. Recent studies have uncovered a common mechanism by which different microbial pathogens might contribute to foam cell formation and accelerate lesion development: interference with LXR-dependent cholesterol metabolism (78). The innate immune system recognizes conserved motifs found in microbes through so-called pattern recognition receptors that include the TLR family of proteins (87). Activation of TLR3 or TLR4 during bacterial or viral infection of macrophages severely compromises the expression of Abca1, Abcg1, Apoe, and other LXR target genes both in vitro and in vivo (Figure 3). Consistent with these effects on LXR-dependent gene expression, activation of TLR3 or TLR4 potently inhibits cholesterol efflux from macrophages. TLR3/4–dependent inhibition of LXR is accomplished through activation of the viral response transcription factor IFN regulatory factor 3; however, the mechanism by which this factor blocks LXR action remains to be determined. LXR-TLR cross-talk provides a potential mechanism to explain how microbial infections might interfere with cholesterol metabolism and contribute to cardiovascular disease. Moreover, these studies emphasize the ability of LXRs to integrate inflammatory and metabolic signaling.
Recent studies also point to an unexpected function of LXR signaling in the innate immune response. Mice lacking LXRs were found to be highly susceptible to infection with the intracellular pathogen Listeria monocytogenes (88). This phenotype was recapitulated by transplantation of bone marrow from Lxrab–/– mice into WT mice, suggesting that altered macrophage function was a major contributor to susceptibility. Furthermore, the inability of LXR-null mice to mount an appropriate response to L. monocytogenes infection correlated with accelerated rates of macrophage apoptosis. The increased susceptibility of LXR-null macrophages to pathogen-induced apoptosis results, at least in part, from the loss of regulation of the antiapoptotic gene Api6 (also known as Aim and Spα) by LXRα. Similarly, Valledor et al. (89) showed that activation of LXR/RXR heterodimers by synthetic and natural ligands inhibited macrophage apoptosis in response to apoptotic stimuli (e.g., cycloheximide), and infection with Bacillus anthracis, E. coli, and Salmonella typhimurium. This activity was attributed to induction of Spα and other antiapoptotic factors, as well as to inhibition of a set of proapoptotic genes. Remarkably, Spα is the first LXR isoform–selective target gene to be described. The selective regulation of Spα by LXRα and the induction of LXRα mRNA during infection, possibly through a NOD2-dependent pathway, suggest that this isoform may have unique functions in innate immunity.
The ability of the LXR pathway to enhance macrophage survival through induction of the antiapoptotic Spα gene also highlights a common pathway used for both metabolic and immune control. In addition to being induced in the setting of bacterial infection, Spα is also upregulated during macrophage lipid loading. The importance of this macrophage survival pathway in atherogenesis was recently elucidated (90). Macrophages from Spα–/– mice are highly susceptible to oxLDL loading–induced apoptosis in vitro and undergo massive apoptosis within atherosclerotic lesions in vivo. As a result, early atherosclerotic lesions in Spα–/–Ldlr–/– mice are reduced compared with those in Spα+/+Ldlr–/– mice. The study of LXR function in macrophages and other immune cells is unraveling previously unrecognized links between immunity and metabolism.
LXRs as potential drug targets
The ability of LXRs to promote reverse cholesterol transport, to limit inflammation, and to improve glucose tolerance makes them attractive targets for drugs developed for the treatment of cardiovascular, metabolic, and/or inflammatory diseases. However, the finding that the first-generation synthetic ligands of LXR markedly increase hepatic lipogenesis and plasma triglyceride levels (32, 36) is an obstacle that needs to be cleared. The increase in hepatic lipogenesis has been attributed in large part to direct induction of SREBP-1c expression by LXRs (35). This suggests that a more nuanced agonist designed to increase reverse cholesterol transport, but not to induce hepatic SREBP-1c expression, would be a better therapeutic. The idea of designing partial, or gene-specific, agonists of nuclear receptors has precedents (e.g., estrogen receptor [ref. 91]) and, in the case of LXRs, may even have a good rationale. LXRs regulate ABCA1 and SREBP-1c in a distinct fashion (92). Whereas loss of LXRs results in increased expression of ABCA1 due to loss of derepression, SREBP-1c levels are substantially reduced, indicating that LXRs interact differently with the basal transcription machinery present in these promoters. A newly developed LXR ligand has recently been reported to have such selective activity (93); however, this compound has not yet been widely studied. In a similar vein one could envision the design of LXR ligands that possess only antiinflammatory properties. These could be potentially used as substitutes for, or in combination with, glucocorticoids.
An alternative approach to the undesirable effects of LXR agonists on hepatic lipogenesis is to develop isoform-specific LXR ligands. The rationale here is that LXRβ-specific ligands may induce the desired reverse cholesterol pathway but circumvent the hepatic complications that are attributed to LXRα (13). On its face this seems like an excellent option; however, the crystal structures of the ligand-binding domains of LXRα and LXRβ indicate that binding pockets are virtually identical, making it very difficult to achieve highly selective agonists (94). Despite this hurdle, Lund et al. (95) recently provided data to support the viability of this approach. Treating Lxra-null mice with a nonselective LXR ligand was found to result in increased HDL levels, but no hepatic accumulation of triglycerides. Whether activation of LXRβ is sufficient to increase whole-body cholesterol secretion remains to be demonstrated, however. Alternatively, specific allosteric modulators designed to interact with divergent regions of these 2 receptors may offer a better solution.
Finally, species-dependent metabolic differences cannot be overlooked. Most studies proving the beneficial outcome of LXR activation for cardiovascular disease were performed in mice. In addition to differences in gene regulation by mouse and human LXRs (e.g., in the case of Cyp7a1), mice also lack plasma CETP activity that is present in humans. A recent study demonstrated that in 2 CETP-containing animal models, Syrian hamsters and cynomolgus monkeys, activation of LXRs induced a significant increase in LDL cholesterol levels that was not previously observed in mice (96). Direct induction of CETP by LXRs, which has been demonstrated in human cell lines, may, in part, contribute to this increase (97). These findings emphasize that any LXR-based therapy needs to be tested in animals that are more similar in their metabolic pathways to humans.
Summary
Since losing their orphan status, LXRs have rapidly adopted new physiological roles. These receptors are now known to be involved in cholesterol, fat, and glucose metabolism. Moreover, LXRs have emerged as integrators of metabolism and inflammatory signaling. LXR agonists show promise as potential therapeutics, given their antiatherogenic and antiinflammatory properties. Future work will continue to define the roles of LXRs in immunity and metabolism and will further explore the LXR signaling pathway as a target in human cardiovascular and inflammatory disease.
Acknowledgments
Noam Zelcer is supported by a long-term postdoctoral fellowship from the International Human Frontier Science Program Organization. Peter Tontonoz is an Investigator of the Howard Hughes Medical Institute at the University of California, Los Angeles. This work was also supported by grants from the NIH (HL-66088 and HL-30568).
Footnotes
Nonstandard abbreviations used: CETP, cholesteryl ester transport protein; LXR, liver X receptor; LXRE, LXR-responsive element; MCP, monocyte chemoattractant protein; N-CoR, nuclear receptor corepressor; oxLDL, oxidized LDL; RXR, retinoid X receptor; SMRT, silencing mediator of retinoic acid and thyroid hormone receptor.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Apfel R, et al. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol. Cell. Biol. 1994;14:7025–7035. doi: 10.1128/mcb.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willy PJ, et al. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann JM, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 4.Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 5.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 6.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 7.Horlein AJ, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377:397–404. doi: 10.1038/377397a0. [DOI] [PubMed] [Google Scholar]
- 8.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- 9.Castrillo A, Tontonoz P. Nuclear receptors in macrophage biology: at the crossroads of lipid metabolism and inflammation. Annu. Rev. Cell Dev. Biol. 2004;20:455–480. doi: 10.1146/annurev.cellbio.20.012103.134432. [DOI] [PubMed] [Google Scholar]
- 10.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 11.Peet DJ, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 12.Chiang JY, Kimmel R, Stroup D. Regulation of cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription by the liver orphan receptor (LXRalpha) Gene. 2001;262:257–265. doi: 10.1016/s0378-1119(00)00518-7. [DOI] [PubMed] [Google Scholar]
- 13.Alberti S, et al. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRβ-deficient mice. J. Clin. Invest. 2001;107:565–573. doi: 10.1172/JCI9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ. Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 15.Repa JJ, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 16.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 17.Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 18.Repa JJ, et al. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J. Biol. Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 19.Berge KE, et al. Heritability of plasma noncholesterol sterols and relationship to DNA sequence polymorphism in ABCG5 and ABCG8. J. Lipid Res. 2002;43:486–494. [PubMed] [Google Scholar]
- 20.Venkateswaran A, et al. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. A transcriptional role for specific oxysterols. J. Biol. Chem. 2000;275:14700–14707. doi: 10.1074/jbc.275.19.14700. [DOI] [PubMed] [Google Scholar]
- 21.Sabol SL, Brewer HB, Jr, Santamarina-Fojo S. The human ABCG1 gene: identification of LXR response elements that modulate expression in macrophages and liver. J. Lipid Res. 2005;46:2151–2167. doi: 10.1194/jlr.M500080-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy MA, et al. Characterization of the human ABCG1 gene: liver X receptor activates an internal promoter that produces a novel transcript encoding an alternative form of the protein. J. Biol. Chem. 2001;276:39438–39447. doi: 10.1074/jbc.M105863200. [DOI] [PubMed] [Google Scholar]
- 23.Bodzioch M, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 24.Brooks-Wilson A, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 25.Rust S, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz K, Lawn RM, Wade DP. ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochem. Biophys. Res. Commun. 2000;274:794–802. doi: 10.1006/bbrc.2000.3243. [DOI] [PubMed] [Google Scholar]
- 27.Venkateswaran A, et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graf GA, et al. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J. Clin. Invest. 2002;110:659–669. doi:10.1172/JCI200216000. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu L, et al. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee MH, et al. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat. Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MH, Lu K, Patel SB. Genetic basis of sitosterolemia. Curr. Opin. Lipidol. 2001;12:141–149. doi: 10.1097/00041433-200104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz JR, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joseph SB, et al. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 2002;277:11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- 34.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 2002;109:1125–1131. doi:10.1172/JCI200215593. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Repa JJ, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol. Endocrinol. 2003;17:985–993. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- 37.Beyer TP, et al. Coadministration of a liver X receptor agonist and a peroxisome proliferator activator receptor-alpha agonist in mice: effects of nuclear receptor interplay on high-density lipoprotein and triglyceride metabolism in vivo. J. Pharmacol. Exp. Ther. 2004;309:861–868. doi: 10.1124/jpet.103.064535. [DOI] [PubMed] [Google Scholar]
- 38.Laffitte BA, et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5419–5424. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao G, et al. Antidiabetic action of a liver x receptor agonist mediated by inhibition of hepatic gluconeogenesis. J. Biol. Chem. 2003;278:1131–1136. doi: 10.1074/jbc.M210208200. [DOI] [PubMed] [Google Scholar]
- 40.Dalen KT, Ulven SM, Bamberg K, Gustafsson JA, Nebb HI. Expression of the insulin-responsive glucose transporter GLUT4 in adipocytes is dependent on liver X receptor alpha. J. Biol. Chem. 2003;278:48283–48291. doi: 10.1074/jbc.M302287200. [DOI] [PubMed] [Google Scholar]
- 41.Efanov AM, Sewing S, Bokvist K, Gromada J. Liver X receptor activation stimulates insulin secretion via modulation of glucose and lipid metabolism in pancreatic beta-cells. Diabetes. 2004;53(Suppl. 3):S75–S78. doi: 10.2337/diabetes.53.suppl_3.s75. [DOI] [PubMed] [Google Scholar]
- 42.Gerin I, et al. LXRbeta is required for adipocyte growth, glucose homeostasis, and beta cell function. J. Biol. Chem. 2005;280:23024–23031. doi: 10.1074/jbc.M412564200. [DOI] [PubMed] [Google Scholar]
- 43.Kalaany NY, et al. LXRs regulate the balance between fat storage and oxidation. Cell Metab. 2005;1:231–244. doi: 10.1016/j.cmet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Kalaany, N.Y., and Mangelsdorf, D.J. 2005. LXRs and FXR: the yin and yang of cholesterol and fat metabolism. Annu. Rev. Physiol. doi:10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed]
- 45.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 47.Shanahan CM, Carpenter KL, Cary NR. A potential role for sterol 27-hydroxylase in atherogenesis. Atherosclerosis. 2001;154:269–276. doi: 10.1016/s0021-9150(00)00473-1. [DOI] [PubMed] [Google Scholar]
- 48.Quinn CM, Jessup W, Wong J, Kritharides L, Brown AJ. Expression and regulation of sterol 27-hydroxylase (CYP27A1) in human macrophages: a role for RXR and PPARgamma ligands. Biochem. J. 2005;385:823–830. doi: 10.1042/BJ20041776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szanto A, et al. Transcriptional regulation of human CYP27 integrates retinoid, peroxisome proliferator-activated receptor, and liver X receptor signaling in macrophages. Mol. Cell. Biol. 2004;24:8154–8166. doi: 10.1128/MCB.24.18.8154-8166.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu X, et al. 27-Hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J. Biol. Chem. 2001;276:38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 51.Wong J, Quinn CM, Brown AJ. Statins inhibit synthesis of an oxysterol ligand for the liver x receptor in human macrophages with consequences for cholesterol flux. Arterioscler. Thromb. Vasc. Biol. 2004;24:2365–2371. doi: 10.1161/01.ATV.0000148707.93054.7d. [DOI] [PubMed] [Google Scholar]
- 52.Rowe AH, et al. Enhanced synthesis of the oxysterol 24(S),25-epoxycholesterol in macrophages by inhibitors of 2,3-oxidosqualene:lanosterol cyclase: a novel mechanism for the attenuation of foam cell formation. Circ. Res. 2003;93:717–725. doi: 10.1161/01.RES.0000097606.43659.F4. [DOI] [PubMed] [Google Scholar]
- 53.Aiello RJ, et al. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler. Thromb. Vasc. Biol. 2002;22:630–637. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- 54.van Eck M, et al. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6298–6303. doi: 10.1073/pnas.092327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klucken J, et al. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc. Natl. Acad. Sci. U. S. A. 2000;97:817–822. doi: 10.1073/pnas.97.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cserepes J, et al. Functional expression and characterization of the human ABCG1 and ABCG4 proteins: indications for heterodimerization. Biochem. Biophys. Res. Commun. 2004;320:860–867. doi: 10.1016/j.bbrc.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 58.Kennedy MA, et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Rigamonti E, et al. Liver X receptor activation controls intracellular cholesterol trafficking and esterification in human macrophages. Circ. Res. 2005;97:682–689. doi: 10.1161/01.RES.0000184678.43488.9f. [DOI] [PubMed] [Google Scholar]
- 60.Engel T, et al. The human ABCG4 gene is regulated by oxysterols and retinoids in monocyte-derived macrophages. Biochem. Biophys. Res. Commun. 2001;288:483–488. doi: 10.1006/bbrc.2001.5756. [DOI] [PubMed] [Google Scholar]
- 61.Laffitte BA, et al. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl. Acad. Sci. U. S. A. 2001;98:507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mak PA, et al. Regulated expression of the apolipoprotein E/C-I/C-IV/C-II gene cluster in murine and human macrophages. A critical role for nuclear liver X receptors alpha and beta. J. Biol. Chem. 2002;277:31900–31908. doi: 10.1074/jbc.M202993200. [DOI] [PubMed] [Google Scholar]
- 63.Hummasti S, et al. Liver X receptors are regulators of adipocyte gene expression but not differentiation: identification of apoD as a direct target. J. Lipid Res. 2004;45:616–625. doi: 10.1194/jlr.M300312-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Curtiss LK, Boisvert WA. Apolipoprotein E and atherosclerosis. Curr. Opin. Lipidol. 2000;11:243–251. doi: 10.1097/00041433-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Liang Y, et al. Liver X receptors (LXRs) regulate apolipoprotein AIV-implications of the antiatherosclerotic effect of LXR agonists. Mol. Endocrinol. 2004;18:2000–2010. doi: 10.1210/me.2003-0477. [DOI] [PubMed] [Google Scholar]
- 66.Jakel H, et al. The liver X receptor ligand T0901317 down-regulates APOA5 gene expression through activation of SREBP-1c. J. Biol. Chem. 2004;279:45462–45469. doi: 10.1074/jbc.M404744200. [DOI] [PubMed] [Google Scholar]
- 67.Pennacchio LA, et al. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 68.Chawla A, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 69.Laffitte BA, et al. Autoregulation of the human liver X receptor alpha promoter. Mol. Cell. Biol. 2001;21:7558–7568. doi: 10.1128/MCB.21.22.7558-7568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whitney KD, et al. Liver X receptor (LXR) regulation of the LXRalpha gene in human macrophages. J. Biol. Chem. 2001;276:43509–43515. doi: 10.1074/jbc.M106155200. [DOI] [PubMed] [Google Scholar]
- 71.Tangirala RK, et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schuster GU, et al. Accumulation of foam cells in liver X receptor-deficient mice. Circulation. 2002;106:1147–1153. doi: 10.1161/01.cir.0000026802.79202.96. [DOI] [PubMed] [Google Scholar]
- 73.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 74.Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J. Biol. Chem. 2003;278:10443–10449. doi: 10.1074/jbc.M213071200. [DOI] [PubMed] [Google Scholar]
- 75.Terasaka N, et al. Liver X receptor agonists inhibit tissue factor expression in macrophages. FEBS J. 2005;272:1546–1556. doi: 10.1111/j.1742-4658.2005.04599.x. [DOI] [PubMed] [Google Scholar]
- 76.Ogawa D, et al. Liver x receptor agonists inhibit cytokine-induced osteopontin expression in macrophages through interference with activator protein-1 signaling pathways. Circ. Res. 2005;96:e59–e67. doi: 10.1161/01.RES.0000163630.86796.17. [DOI] [PubMed] [Google Scholar]
- 77.Fowler AJ, et al. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J. Invest. Dermatol. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 78.Castrillo A, et al. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol. Cell. 2003;12:805–816. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 79.McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 80.Pascual G, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ogawa S, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joseph SB, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Levin N, et al. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler. Thromb. Vasc. Biol. 2005;25:135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- 84.Byrne GI, Kalayoglu MV. Chlamydia pneumoniae and atherosclerosis: links to the disease process. Am. Heart J. 1999;138:S488–S490. doi: 10.1016/s0002-8703(99)70282-6. [DOI] [PubMed] [Google Scholar]
- 85.Chiu B, Viira E, Tucker W, Fong IW. Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of the carotid artery. Circulation. 1997;96:2144–2148. doi: 10.1161/01.cir.96.7.2144. [DOI] [PubMed] [Google Scholar]
- 86.Rassu M, et al. Demonstration of Chlamydia pneumoniae in atherosclerotic arteries from various vascular regions. Atherosclerosis. 2001;158:73–79. doi: 10.1016/s0021-9150(01)00411-7. [DOI] [PubMed] [Google Scholar]
- 87.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 88.Joseph SB, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 89.Valledor AF, et al. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17813–17818. doi: 10.1073/pnas.0407749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arai S, et al. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 91.Gustafsson JA. Therapeutic potential of selective estrogen receptor modulators. Curr. Opin. Chem. Biol. 1998;2:508–511. doi: 10.1016/s1367-5931(98)80127-0. [DOI] [PubMed] [Google Scholar]
- 92.Wagner BL, et al. Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol. Cell. Biol. 2003;23:5780–5789. doi: 10.1128/MCB.23.16.5780-5789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quinet EM, et al. Gene-selective modulation by a synthetic oxysterol ligand of the liver X receptor. J. Lipid Res. 2004;45:1929–1942. doi: 10.1194/jlr.M400257-JLR200. [DOI] [PubMed] [Google Scholar]
- 94.Svensson S, et al. Crystal structure of the heterodimeric complex of LXRalpha and RXRbeta ligand-binding domains in a fully agonistic conformation. EMBO J. 2003;22:4625–4633. doi: 10.1093/emboj/cdg456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lund, E.G., et al. 2005. Different roles of liver X receptor alpha and beta in lipid metabolism: effects of an alpha-selective and a dual agonist in mice deficient in each subtype. Biochem. Pharmacol. doi:10.1016/j.bcp.2005.11.004. [DOI] [PubMed]
- 96.Groot PH, et al. Synthetic LXR agonists increase LDL in CETP species. J. Lipid Res. 2005;46:2182–2191. doi: 10.1194/jlr.M500116-JLR200. [DOI] [PubMed] [Google Scholar]
- 97.Luo Y, Tall AR. Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J. Clin. Invest. 2000;105:513–520. doi: 10.1172/JCI8573. [DOI] [PMC free article] [PubMed] [Google Scholar]