Abstract
Tissue damage resulting from chemical, mechanical, and biological injury, or from interrupted blood flow and reperfusion, is often life threatening. The subsequent tissue response involves an intricate series of events including inflammation, oxidative stress, immune cell recruitment, and cell survival, proliferation, migration, and differentiation. In addition, fibrotic repair characterized by myofibroblast transdifferentiation and the deposition of ECM proteins is activated. Failure to initiate, maintain, or stop this repair program has dramatic consequences, such as cell death and associated tissue necrosis or carcinogenesis. In this sense, inflammation and oxidative stress, which are beneficial defense processes, can become harmful if they do not resolve in time. This repair program is largely based on rapid and specific changes in gene expression controlled by transcription factors that sense injury. PPARs are such factors and are activated by lipid mediators produced after wounding. Here we highlight advances in our understanding of PPAR action during tissue repair and discuss the potential for these nuclear receptors as therapeutic targets for tissue injury.
An overview of tissue injury
The clinical significance of tissue injury and the need for therapeutic agents to treat organ damage have called for an improved understanding of the causes of tissue injury and subsequent healing. The complex nature of these processes creates a challenge in identifying the specific cell types and biochemical pathways involved. Moreover, tissue protection and regeneration require tight control of cell survival and death, cell growth and differentiation, and ECM remodeling and breakdown. Chemical, biological, and mechanical stress is deleterious to epithelial tissue, and even whole organs are vulnerable to damage. For example, the liver, which metabolizes nutrients and drugs absorbed from the digestive tract, is particularly susceptible to injury, since all blood leaving the intestines and stomach must pass through it before reaching the rest of the body. Organ damage also occurs in response to an inadequate supply of oxygen (hypoxia), usually caused by blood vessel constriction or obstruction (ischemia). Under normal physiological conditions, oxygenation levels and sensitivity to hypoxia differ among the various organs. Since short periods of ischemia and reperfusion (ischemia/reperfusion, or I/R) cause extensive damage, the goal of the survival response is to maintain tissue viability. As a result, the hypoxia response requires optimal revascularization for efficient recovery.
Inflammation is a major component of early healing, and its control is essential for efficient repair. The inflammatory cytokines and eicosanoids produced during the first hours after injury recruit neutrophils and macrophages to the wound. These cells amplify the early response through their production of additional inflammatory mediators. Some of these factors promote cell proliferation and migration and are thus directly involved in wound closure. Others increase pain, delay wound healing, and promote neovascularization (angiogenesis). A full understanding of these responses will help therapeutic interventions through the identification of molecular targets. Among such targets are transcription factors that control various pathways of cellular repair. In particular, PPARs have recently received attention for their protective and healing attributes. The PPAR agonists can be synthetic molecules, such as those used to treat hypertriglyceridemia (fibrates) and insulin resistance (thiazolidinediones), or natural ligands, such as fatty acids (FAs) and their derivatives (eicosanoids). Recent work has unveiled a variety of natural lipid-derived molecules that activate PPARs, but little is known about their action in vivo. Here we summarize the role of PPARs in repair of multistratified and single-cell-layer epithelia, and of injured organs.
PPAR involvement in healing stratified epithelia: skin wound healing as a model
The epidermis is renewed continuously, and its integrity depends on a tightly regulated balance among cell proliferation, differentiation, and apoptosis. During its maturation, the epidermis evolves from a single layer of epithelial cells to a fully stratified and differentiated epithelium. The outermost layer of the epidermis, the stratum corneum, is the end product of keratinocyte differentiation and consists of a layer of cross-linked proteins and lipids, which functions as a barrier to transepidermal water loss and as a defense against physical damage, microbes, and xenobiotics. Several studies have examined the role of PPARs in this process.
No gross defect is seen in epidermal maturation in mice deficient in either PPARα or PPARδ (also called PPARβ). Similarly, PPARγ-null mice born after placental rescue (to prevent lethal placenta maturation defects) show no alterations in epidermal maturation. These results suggest that epidermal maturation is PPAR independent. However, PPARs can stimulate keratinocyte differentiation (1–3). All 3 PPARs are undetectable in adult mouse interfollicular epidermis, but PPARα and PPARγ are found upon proliferative stimuli, such as at wound edges after an injury (1). PPARα expression is only transiently increased after injury. Interestingly, the expression of PPARα is upregulated by antiinflammatory glucocorticoids, which increase during injury (4, 5). The inhibitory effect of PPARα on NF-κB action may create a negative feedback loop, which would explain the transient PPARα expression that allows control of early inflammation (5).
Following tissue damage, injured cells release proinflammatory cytokines. These stimulate PPARδ expression via stress-associated protein kinase/JNK–mediated activation of the activator protein-1 (AP-1) transcription complex (6). PPARδ, which is activated by ligands whose production is also triggered by proinflammatory cytokines, then coordinates transcriptional upregulation of integrin-linked kinase and 3-phosphoinositide–dependent kinase and repression of the phosphatase and tensin homologue 10 (PTEN). As a consequence, the activity of protein kinase Bα (PKBα, also known as Akt-1) is increased and apoptosis cascades are repressed (7). The resulting increased resistance to cell death helps to maintain a sufficient number of viable wound keratinocytes for re-epithelialization (Figure 1). Interestingly, PPARδ-deficient keratinocytes exhibit a migration defect and reduced expression of the gelatinolytic enzyme MMP-9. Not surprisingly, PPARδ-null mice exhibit delayed wound closure after an injury (1).
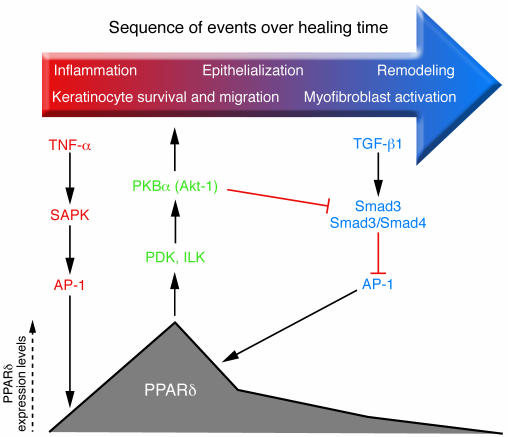
Figure 1.
Dynamic control of PPARδ expression after skin injury. Left: TNF-α released by injured epidermal keratinocytes activates stress-associated protein kinase (SAPK) and induces AP-1 binding to the PPARδ promoter and transcription of PPARδ target genes. TNF-α also triggers production of endogenous PPARδ ligands, which activate PPARδ in keratinocytes and macrophages. Center: PPARδ activation helps maintain a sufficient number of keratinocytes for re-epithelialization by improving apoptosis resistance through expression of integrin-linked kinase (ILK) and 3-phosphoinositide–dependent kinase (PDK), as well as via activation of the PKB/Akt-1 survival pathway. Right: The initial inflammatory signals that stimulate PPARδ are countered by TGF-β1/Smad3–mediated suppression of PPARδ in the late re-epithelialization/remodeling stage. This suppression occurs via Smad3/4 complex–mediated abrogation of AP-1 activity. In addition, TGF-β1 released by dermal wound fibroblasts increases macrophage numbers and stimulates ECM production for wound remodeling. The diverse cell types and feedback signals regulating would repair are discussed in detail in the text.
A balanced interplay among keratinocytes, dermal fibroblasts, and macrophages is critical for efficient wound healing. This process involves secretion of TGF-β1 by dermal wound fibroblasts, leading to the recruitment of neutrophils and macrophages. These inflammatory cells secrete TNF-α and thereby sustain PPARδ expression and ligand production in wound keratinocytes. After levels of PPARδ peak, its promoter activity and expression levels fall for the remainder of the healing process because of TGF-β1/Smad3–mediated reductions in AP-1 DNA binding (1, 8) (Figure 1). Additional mechanisms that repress PPARδ promoter activity in mouse keratinocytes involve CCAAT/enhancer-binding protein-α (C/EBPα) and C/EBPβ binding to a specific C/EBP response element, and histone deacetylation via histone deacetylase-1 (9). These regulatory controls suggest that expression of PPARδ and C/EBPs in interfollicular and hair follicle keratinocytes is mutually exclusive.
Role of PPARs in different organs after I/R injury
PPARs in kidney I/R.
The kidney is vulnerable to damage by toxins, infection, immune reactions, and ischemia. Acute renal failure (ARF) affects about 5% of hospitalized patients and carries a high mortality. Damage to renal tubules alters epithelial cells and is accompanied by the shedding of cells into the tubule lumen and the back-leakage of glomerular filtrate, further increasing epithelial apoptosis and necrosis. Surviving cells participate in regeneration of the epithelium and restoration of renal function. The prognosis for ARF is complicated by secondary injuries induced by free radicals formed during I/R, although inadequate renal cortical-medullary reperfusion may be more deleterious (10). Today, there is no treatment for this devastating clinical syndrome.
A role for PPARs in reducing renal injury and dysfunction is established in animal models. PPARα-null mice subjected to I/R injury by arterial ligation show enhanced cortical necrosis and impaired renal function (11). Conversely, induction of FA oxidation enzymes by PPARα is thought to preserve kidney structure and function during renal I/R injury (11). In humans, nephrotoxicity is a common side effect of treatment with the antitumor agent cisplastin (12). In mice, PPARα ligands attenuate cisplatin-induced ARF by preventing the inhibition of FA oxidation, reducing apoptosis and necrosis in the proximal tubule (13), and repressing inflammation via inhibition of NF-κB binding activity, which attenuates neutrophil infiltration and cytokine/chemokine release (14).
Consistent with their defect in skin healing, PPARδ-deficient mice exhibit greater kidney injury and dysfunction than wild-type counterparts after renal I/R. Conversely, wild-type mice pretreated with a PPARδ ligand are protected from I/R damage, with a reduction in medullary necrosis, apoptosis, and inflammation. Cell culture studies show that PPARδ ligands activate the PKB/Akt pathway, as they do in keratinocytes, and increase the spread of tubular epithelial cells. In vivo, these events may accelerate healing by suppressing tubular epithelial shedding and anoikis (15).
The PPARγ agonists rosiglitazone and pioglitazone have protective effects against not only I/R, but also various kidney injuries including diabetic nephropathy, hypertensive nephropathy, experimental glomerulonephritis, and cyclosporine-induced renal injury (reviewed in refs. 16, 17). This protection reflects both improved glucose metabolism and insulin resistance as well as the antiinflammatory, antifibrotic, and antiapoptotic effects of PPARγ ligands (18). The mechanisms underlying these beneficial properties are similar for synthetic agonists and the natural cyclopentenone prostaglandin 15d-PGJ2. The pathways involve inhibition of NF-κB activation, together with reduced expression and/or activity of AP-1, TGF-β1, monocyte chemoattractant protein-1 (MCP-1), ICAM-1, iNOS, fibronectin, and collagen I. The outcome of these signaling changes includes attenuated infiltration of polymorphonuclear cells into renal tissues, reducing oxidative stress and inflammation (19–23).
PPARs in lung I/R and fibrosis.
Patients with end-stage pulmonary diseases are often treated with lung transplantation. Although improvements in techniques such as the preservation of vascular endothelium have significantly improved survival, I/R lung injury still occurs in over 20% of patients and remains the main cause of death during the first month after transplantation (24). Rodent models show that PPAR ligands, such as rosiglitazone and pioglitazone, can significantly attenuate I/R–induced lung injuries (17). Furthermore, treatment with the PPARγ agonist pioglitazone before ischemia reduces I/R–induced lung damage in rats. The mechanism involves inhibition of proinflammatory cytokines (TNF-α, cytokine-induced neutrophil chemoattractant 1) and polymorphonuclear cell infiltration into the lung interstitium, resulting in reduced pulmonary edema (25). Similarly, in a murine I/R model, pretreatment with the PPARγ agonist troglitazone prevents induction of the zinc finger transcription factor early growth response gene-1 (Egr-1), a master switch for the inflammatory response in ischemic vessels. Thus, PPARγ neutralizes the potential for harm caused by Egr-1 target genes such as IL-1β, MCP-1, and macrophage inflammatory protein-2. As a consequence of this protection, leukostasis is decreased, while oxygenation and overall survival are increased (26).
The term pulmonary fibrosis covers several life-threatening diseases for which no effective therapy exists. All have a similar pathology characterized by an immune response closely resembling a Th2-type phenotype, with proliferation and accumulation of myofibroblasts and excessive deposition of ECM proteins in the lung parenchyma. The clinical features are shortness of breath, evident diffuse pulmonary infiltrates, and varying degrees of inflammation and fibrosis (27). In humans, bleomycin treatment for cancer chemotherapy induces interstitial lung fibrosis (28). In human pulmonary fibroblast cultures, PPARγ agonists interrupt the profibrotic effects of TGF-β (29). Similarly, mice subjected to intratracheal administration of bleomycin develop lung fibrosis, which is significantly reduced by PPARγ agonists. As expected, this beneficial effect is attenuated by the PPARγ antagonist bisphenol A diglycidyl ether (BADGE), suggesting that PPARγ activity is required for protection (30).
PPARs in digestive tract I/R.
Acute mesenteric ischemia, abdominal aortic aneurysm, hemorrhagic, traumatic, or septic shock, small bowel transplantation, and severe burns cause intestinal I/R, a severe condition characterized by endothelial cell swelling, capillary plugging, and mucosal barrier dysfunction (31). In rodent models of intestinal I/R injury, PPARγ activation downregulates TNF-α and ICAM-1 (probably via inhibition of NF-κB), and pretreatment with a PPARγ agonist before ischemia significantly reduces neutrophil infiltration (32, 33). These protective effects are attenuated by PPARγ antagonists or reduction of PPARγ levels in mutant PPARγ heterozygous animals (32, 34). Similarly, activation of PPARα attenuates I/R injury by reducing ICAM-1 expression, peroxynitrite activity, and the production of proinflammatory cytokines (35). Additionally, enteral nutrition is beneficial when administered soon after severe gut I/R insults. For instance, the solute glutamine maintains small bowel function depending on cellular energetics and epithelial cell functions after I/R injury in rats. This effect is associated with PPARγ induction and, logically, abrogated by a PPARγ antagonist (36).
Evidence is also accumulating for a beneficial role of PPARγ agonists in healing gastric mucosal damage associated with I/R in animal models (37–40). Activation of PPARγ reduces gastric lesions and attenuates levels of lipid peroxidation, ICAM-1, TNF-α, COX-2, iNOS, and apoptosis after gastric I/R injury. As a result, PPARγ alleviates oxidative injury and inflammation (here again the mechanism likely involves inhibition of NF-κB). The protective and healing effects of all 3 PPAR isotypes on kidney, lung, and digestive tract epithelia after injury are summarized in Figure 2.
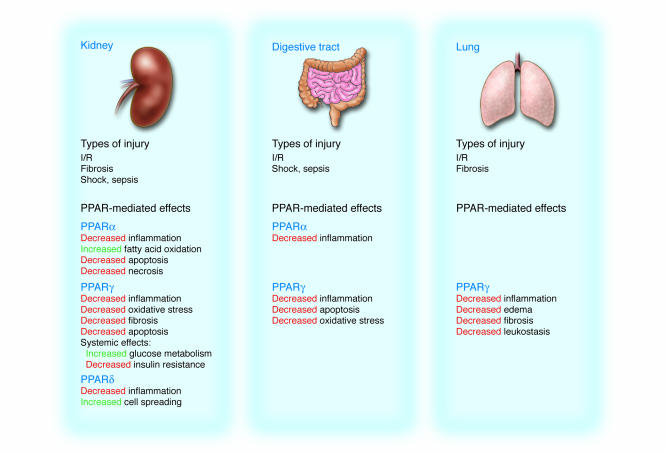
Figure 2.
Epithelial repair pathways controlled by PPARs during kidney, digestive tract, and lung injury. Common to all tissue injury is a rapid increase in inflammation. PPAR activation, mediated by the binding of natural and synthetic ligands, restricts inflammation to prevent extensive tissue necrosis and chronic oxidative damage.
PPARs in liver injury (cirrhosis and fibrosis).
Chronic liver disease remains an important cause of mortality and morbidity. Recurring or chronic injury and inflammation trigger tissue remodeling pathways that can lead to severe fibrosis and end-stage cirrhosis. Unfortunately, no effective treatment exists for these injuries except liver transplantation (41). The causes of liver fibrosis and cirrhosis include genetic abnormalities, toxic, alcoholic, and autoimmune-mediated damage, nonalcoholic steato-hepatitis associated with the metabolic syndrome, and viral hepatitis forms B and C. Liver fibrosis involves proliferation of myofibroblasts derived from hepatic stellate cells (HSCs, also called Ito cells or lipocytes). In the damaged areas, the transition of normally quiescent HSCs to proliferative myofibroblasts, through the action of cytokines and oxidative stress, increases ECM deposition. In the fibrotic and cirrhotic liver, matrix degradation by MMPs occurs but is restricted by tissue inhibitors of metalloproteinases (TIMPs). Encouraging results from experimental models suggest that fibrosis could be attenuated by enhancing apoptosis of stellate cells or blocking their transdifferentiation, as well as by manipulating the TIMP-MMP balance to facilitate matrix degradation and improve liver architecture (42).
PPARγ agonists suppress the growth and fibrotic activity of HSCs by the downregulation of proteins such as α1(I) collagen, fibronectin, α-SMAs, and MCP-1, which is consistent with reduced PPARγ levels in transdifferentiated HSCs (43–46). Experimental overexpression of PPARγ in myofibroblasts reverses their phenotype to quiescent cells, restores their ability to store retinyl esters, and represses activation markers such as α1(I) procollagen and TGF-β1 by suppressing AP-1 and nuclear factor-1 activities (47, 48). Interestingly, an analogy has been proposed between preadipocyte-adipocyte differentiation and HSC transdifferentiation. The high level of expression of adipogenic transcription factors in quiescent HSCs rapidly declines during their transdifferentiation to myofibroblastic HSCs. Similarly, treating these cells with an adipocyte differentiation “cocktail” or ectopic expression of SREBP-1c or PPARγ reverts them to quiescent HSCs (49). The COX-2 inhibitor SC-236 attenuates liver inflammation and fibrosis through PPARγ activation and downregulation of α-SMA expression and MMP-2 and -9 activities, as well as by the induction of Kupffer cells and HSC apoptosis (50). In addition, ligands of the farnesoid X receptor stimulate expression of PPARγ in HSCs and maintain its enhanced levels after injury, thereby promoting the antifibrotic action of PPARγ agonists (51). In addition to their action on HSCs, PPARγ ligands also reduce ductal proliferation and fibrosis after bile duct ligation in rats, showing that PPARγ attenuates fibrosis through not only direct action on matrix-producing cells, but also modulation of the epithelial-mesenchymal interactions in chronic obstructive cholestasis (52).
The function of PPARδ in fibrogenesis is less well studied, but PPARδ expression is strongly induced after HSC activation. In a model of carbon tetrachloride–induced acute liver damage, PPARδ activation induces HSC proliferation during early fibrogenesis and enhances expression of fibrotic markers (53). Thus, PPARγ and PPARδ appear to have antagonistic effects that require further investigation using PPARγ- and PPARδ-deficient mice. The possibility of manipulating the balance of PPARγ and PPARδ pharmacologically signifies a promising development for the attenuation of liver fibrosis. Future studies should improve our understanding of pathways regulating HSC survival, death, and clearance, leading to potential therapies to induce HSC apoptosis (41).
PPARα ligands also have antifibrotic effects in the rat thioacetemide model of liver cirrhosis, probably via their antioxidant action associated with enhanced catalase expression and activity (54). Interestingly, in a mouse model of I/R, PPARα regulates hepatic neutrophil accumulation and reduces iNOS expression after hepatocellular injury. This finding is important because activation of PPARα in hepatocytes protects against oxidant injury, indicating that parenchymal cells might impact the inflammatory response (55). Finally, the function of PPARα in liver regeneration after partial hepatectomy remains unclear. PPARα is not necessary for compensatory hyperplasia induced by partial hepatectomy, yet PPARα-dependent regulation of genes associated with cell cycle progression, cytokine signaling, and metabolic changes appears to be involved (56–59).
PPARs in ischemic brain injury and neurodegenerative disease.
Brain injury resulting from insufficient blood (oxygen) supply can be transient (from syncope or ischemic attack) or permanent (from infarct or irreversible stroke). The latter is a major cause of disability and death in developed countries, and because of limited therapeutic strategies there is increasing interest in prophylactic pharmacological treatment (60). It was first observed that the fibrate gemfibrozil reduces stroke incidence in men with low HDL cholesterol and low LDL cholesterol who suffer from coronary heart disease (61). In mice this outcome is associated with improved endothelial relaxation, reduced brain oxidative stress, and decreased VCAM-1 and ICAM-1 expression and is thus independent of lipid metabolism (62). Similarly, the PPARα and PPARγ agonist resveratrol, a polyphenol found in grapes, protects the murine brain from stroke, in a PPARα-dependent manner (63). Thus, PPARα agonists may prevent or reduce the severity of ischemic stroke in humans. In rat hippocampal neurons, the PPARα agonist Wy-14,463 induces peroxisomal proliferation that attenuates β-amyloid peptide–dependent neurotoxicity and decreases intraneuronal oxidative stress (64). In addition, PPARγ ligands have neuroprotective effects in experimental models of ischemic injury, Alzheimer disease, multiple sclerosis, and autoimmune encephalomyelitis. The benefits result from suppressing inflammation (65–68). In addition, in a mouse model of amyotrophic lateral sclerosis for which neuroinflammation may contribute to motor neuron death, the PPARγ ligand pioglitazone improves muscle strength and body weight, delays disease onset, and increases lifespan (69).
PPARs in cardiac I/R.
Myocardial I/R is a clinically relevant problem associated with reestablishment of blood flow by coronary bypass surgery, thrombolysis, and angioplasty, and with the need to minimize myocardial damage after heart infarct. Heart tissue normally uses FAs as the major energy source. However, hypoxia or pressure overload in the heart results in a substrate switch from FAs to glucose, caused by downregulation of PPARα (70). It is thought that partial inhibition of FA oxidation improves the functional recovery of the heart during reperfusion (71). In support of this idea, experimental overexpression of PPARα in the heart impairs cardiac recovery after ischemia (72). Thus, pharmacological treatments that stimulate glucose oxidation and repress FA oxidation appear to be beneficial for cardiac recovery (73). Along this line of thought, it has been proposed that downregulation of PPARγ coactivator-1 and PPARα may shift myocytes toward a more glycolytic metabolism (74). However, beneficial effects of PPARα agonists on I/R damage have been reported as well (75–77). Experimentally, this contradiction could be resolved by determination of whether PPARα agonists improve myocardial function via metabolic and antiinflammatory actions, and whether cardiac overexpression of PPARα has deleterious effects on the heart when circulating FA levels are high. Nevertheless, these observations suggest that cardiac PPARα antagonism could be a therapy for treating I/R damage (72).
In healthy, diabetic, or obese animals (76, 78–82), PPARγ agonists reduce myocardial infarct size. These effects are associated with increased glucose uptake and improved insulin sensitivity. In addition, PPARγ agonists reduce postischemic myocardial apoptosis (83). However, the role of PPARγ in heart failure is debated, particularly with regard to patients with type 2 diabetes mellitus. The Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) study concluded that pioglitazone may improve cardiovascular outcome (84), while a retrospective cohort study suggested that thiazolidinediones (TZDs) may increase the risk of heart failure. Since type 2 diabetes patients are at increased risk of heart failure, those with underlying myocardial disease may be especially vulnerable to the effects of TZDs (85). Exacerbation of heart failure is documented in animal studies. TZDs are associated with increased post–myocardial infarction mortality in rats (86), and increased susceptibility to ventricular fibrillation during myocardial I/R in pigs (87). Finally, PPARα or PPARγ stimulation prevents or attenuates cardiac fibrosis by reducing endothelin-I, collagen type I, and MMP-1 production, and by improving myocardial inflammation in anoxia/reoxygenation and pressure-overloaded hearts (88–91).
These experimental studies suggest that PPARs exert beneficial effects in reducing infarct size, myocardial reperfusion injury, and hypertrophic signaling and inflammatory responses. However, clinical applications have revealed some undesirable side effects, suggesting that TZDs should be used with caution in diabetic patients predisposed to heart failure (92). Obviously, there are uncertainties that require additional research (93). The protective effects of PPARs on liver, brain, and heart injury are summarized in Figure 3.
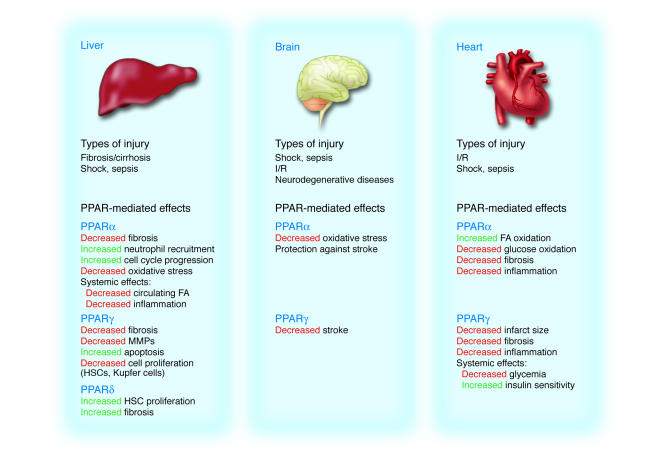
Figure 3.
Role of PPARs in repair of liver, brain, and heart damage. Various systemic states such as shock or sepsis can lead to organ injury and failure. These injuries, as well as tissue-specific insults such as cirrhosis, fibrosis, and I/R injury, can be partially alleviated or prevented through the actions of PPARs.
PPARs in shock and sepsis
Sepsis and shock are intrinsically complex, causing failure of multiple organs, including the gastrointestinal tract, kidneys, pancreas, heart, and brain. Since these conditions are a major cause of death in intensive care units, they are high-priority targets for new therapies. Mortality levels increase with the number of failed organs, reaching over 80% when dysfunction occurs in 4 or more organs (94). Preclinical studies have investigated the protective effects of activated PPARs against multiple-organ failure resulting from septicemic and hemorrhagic shocks. Several studies provide evidence for an amelioration through pharmacological treatment with 15d-PGJ2 of endotoxic shock induced in rodents by bacterial products such as LPS, and wall fragments of Gram-positive and Gram-negative bacteria (reviewed in refs. 95, 96). The 15d-PGJ2 likely counteracts the inflammatory response by activating PPARγ, repressing NF-κB, and enhancing the heat shock response (97). Evidence for direct involvement of PPARγ in organ protection is provided by a reduction of the beneficial action of 15d-PGJ2 in the presence of the PPARγ antagonist GW9662 (95). Similarly, pretreatment with the PPARα agonist fenofibrate protects the endothelia in rabbit E. coli endotoxin–induced shock (98). Severe hemorrhage and subsequent resuscitation also causes multiorgan injury. A study in rats suggested that treatment with 15d-PGJ2 before hemorrhagic shock attenuates liver injury and kidney dysfunction, an effect that is reduced by GW9662 (99). Although none of these sepsis or shock models is ideal, they can improve our knowledge of disease mechanisms and help us identify patient populations that would most benefit from therapeutic trials (100).
PPARs in cancer
Wound healing after injury is a high-priority survival response. In this situation, epithelial cells change their intercellular contacts, modify their matrix, proliferate, and migrate over the wound. In addition, new blood vessels form rapidly. Interestingly, each of these healing behaviors is similarly involved in tumorigenesis and metastasis. As mentioned earlier, epithelia are highly susceptible to, and are efficient healers of, injury, which correlates with the observation that 95% of all cancer deaths are from epithelial tumors. This suggests that the repair mechanisms activated in response to injury may promote cancer if uncontrolled. Indeed, some tumors, especially those prone to metastasis, activate wound-healing genes (101). Although the PPARs may be involved in tumor-associated pathways, their regulation of wound-healing genes within specific tumor types remains largely unexplored (102).
Tumor development involves changes in noncancerous cells and tissues of the transformed mass, including activation of stromal cells, inflammation, and angiogenesis. As a result, these changes are popular targets for cancer therapy design. For example, as angiogenesis is necessary for wound healing and tissue repair, inhibition of angiogenesis represents a promising feature of anticancer therapy. Indeed, PPARγ agonists have received much attention in this field (reviewed in ref. 103). Surprisingly, although they upregulate VEGF in cultured cells, PPARγ agonists such as 15d-PGJ2, pioglitazone, rosiglitazone, ciglitazone, and BRL49653 are potent angiogenesis inhibitors. These data are obtained from in vitro and in vivo models (104–108). Several direct and indirect actions of PPARγ ligands are reported in these studies, such as decreased VEGF-C and angiogenic chemokine production by tumor cells, inhibition of urokinase plasminogen activator, reduction of VEGF receptors 1 (Flt-1) and 2 (fetal liver kinase-1/KDR), increased plasminogen activator inhibitor type 1, and inhibition of tube formation (reviewed in ref. 103). In addition, PPARγ agonists downregulate leptin gene expression and block leptin-induced endothelial cell migration by inhibiting Akt and iNOS (109, 110). Unfortunately, PPARγ involvement is not yet validated by gene deletion or antagonists. Finally, PPARγ upregulates expression of several fibrinogen/angiopoietin–related proteins (111–113). Although the function of these proteins in angiogenesis is unclear, they share structural homologies with angiopoietins, a family of proteins with roles in vascular development. Much less is known about the effects of PPARα and PPARδ. The PPARα agonist fenofibrate inhibits capillary tube formation in vitro and angiogenesis in vivo. The mechanism involves disorganization of the actin cytoskeleton with decreased bFGF-induced Akt activity and COX-2 gene expression (114). Fenofibrate also inhibits constrictive remodeling after angioplasty through repression of inflammation and neovascularization (115). Like PPARγ, PPARα controls expression of a protein known as fasting-induced adipose factor/angiopoietin-like protein (FIAF) (111). A study of 35 individuals found that microvessel density among PPARδ-immunoreactive squamous cell carcinomas (SCCs) was higher than that of nonreactive SCCs (116), consistent with an association between VEGF and PPARδ in head and neck SCCs (117). PPARδ may also be involved in vascular development via modulation of the angiogenesis-associated PKBα/Akt-1 pathway (7, 118). Together, these results suggest that the PPARα and PPARγ agonists already used in clinics may be harnessed for angiogenic diseases.
Conclusions
PPARs are major regulators of lipid, glucose, and amino acid metabolism. Here we have presented some of their less well known functions in tissue protection and repair. A majority of the studies reviewed herein are descriptive, and even the use of specific ligands does not necessarily distinguish between PPAR isotypes or between PPAR-dependent and -independent mechanisms. However, collectively, the studies have improved our understanding of the role of PPARs in healing. Their actions are simultaneously systemic and cellular. Systemic effects are antiinflammatory, antioxidant, and metabolic, such as the normalization of circulating lipids and insulin resistance. During the early postinjury inflammatory phase, lipoxygenases and cyclooxygenases stimulate production of PPAR ligands. Indeed, perhaps the most striking action of PPARs is the control of inflammation, an event first observed 10 years ago (119). Inhibition of the NF-κB pathway appears to be central to this process.
Although protective against infections, the inflammatory milieu is a hostile environment for resident host cells at the injury site. This insult is abrogated by the antiapoptotic role of PPARδ. These PPARδ effects are best described in skin repair, where PKBα/Akt-1 activity is central. Fibroblasts in inflamed wound areas further support healing. These cells transdifferentiate to activated myofibroblasts with elevated α-SMA expression and contractility to constrict the exposed wound surface. They secrete growth factors, inflammatory cytokines, and ECM components that provide a scaffold for migration of epithelial cells (120). Chronic injury, repetitive injury-repair cycles, or failure to shut off the healing signals leads to fibrosis, during which there are antagonistic actions of PPARγ and PPARδ. PPARγ sustains fibroblast quiescence and promotes the reversal of myofibroblasts to quiescent cells, while PPARδ expression is high in differentiated myofibroblasts (although its functions are unclear). After skin injury, the interaction of PPAR and TGF-β1 pathways is indispensable for spatiotemporal control of the repair program, suggesting a role for PPARs in fibrosis. Studies to determine how PPAR pathways communicate with TGF-β1, angiotensin II, leptin, and endothelin pathways may inspire novel therapeutic strategies for tissue fibrosis (121–124).
A recurrent observation in wound-healing studies is the protective effect of PPAR ligands. Today, the precise nature and function of natural lipid activators of PPARs in repair are unclear. This knowledge should assist the development of truly selective drugs to augment or antagonize PPAR action according to the desired cellular response. In terms of PPAR biology and pharmacology, the acute conditions described herein are quite distinct from the chronic metabolic disorders for which PPAR agonist use is already established. A collective effort is required to translate promising basic-science data on PPAR-mediated tissue repair into clinical application for acute injuries. As discussed above, short-term preconditioning strategies with PPAR agonists can be protective in animal models. Thus, PPARs appear to have potential as new strategies for injury prevention or preconditioning, and as targets for syndromes with high morbidity and mortality rates. Finally, tissue repair activates ECM remodeling, cell proliferation, migration, and angiogenesis pathways essential for not only normal tissue development but also cancer invasion and metastasis (101, 125). Identifying the distinct signals that trigger or block gene expression during the wound response will improve our understanding of tissue repair, fibrosis, organogenesis, and oncogenesis.
Acknowledgments
The authors acknowledge grant support from the Swiss National Science Foundation, the European Union EUMORPHIA Research Program, and the Etat de Vaud. The authors thank Jérôme Feige for critical reading of the manuscript and Nathalie Constantin for help in manuscript preparation.
Footnotes
Nonstandard abbreviations used: AP-1, activator protein-1; ARF, acute renal failure; C/EBP, CCAAT/enhancer-binding protein; FA, fatty acid; HSC, hepatic stellate cell; I/R, ischemia/reperfusion; MCP-1, monocyte chemoattractant protein-1; PKB, protein kinase B; SCC, squamous cell carcinoma; TZD, thiazolidinedione.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Michalik L, et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)alpha and PPARbeta mutant mice. J. Cell Biol. 2001;154:799–814. doi: 10.1083/jcb.200011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barak Y, et al. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc. Natl. Acad. Sci. U. S. A. 2002;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao-Qiang M, et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J. Invest. Dermatol. 2004;123:305–312. doi: 10.1111/j.0022-202X.2004.23235.x. [DOI] [PubMed] [Google Scholar]
- 4.Lemberger T, et al. Regulation of the peroxisome proliferator-activated receptor alpha gene by glucocorticoids. J. Biol. Chem. 1994;269:24527–24530. [PubMed] [Google Scholar]
- 5.Tan NS, Michalik L, Di-Poi N, Desvergne B, Wahli W. Critical roles of the nuclear receptor PPARbeta (peroxisome-proliferator-activated receptor beta) in skin wound healing. Biochem. Soc. Trans. 2004;32:97–102. doi: 10.1042/bst0320097. [DOI] [PubMed] [Google Scholar]
- 6.Tan NS, et al. Critical roles of PPAR beta/delta in keratinocyte response to inflammation. Genes Dev. 2001;15:3263–3277. doi: 10.1101/gad.207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di-Poi N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol. Cell. 2002;10:721–733. doi: 10.1016/s1097-2765(02)00646-9. [DOI] [PubMed] [Google Scholar]
- 8.Tan NS, et al. Essential role of Smad3 in the inhibition of inflammation-induced PPARbeta/delta expression. EMBO J. 2004;23:4211–4221. doi: 10.1038/sj.emboj.7600437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di-Poi N, Desvergne B, Michalik L, Wahli W. Transcriptional repression of peroxisome proliferator-activated receptor beta/delta in murine keratinocytes by CCAAT/enhancer-binding proteins. J. Biol. Chem. 2005;280:38700–38710. doi: 10.1074/jbc.M507782200. [DOI] [PubMed] [Google Scholar]
- 10.Molitoris BA, Sutton TA. Endothelial injury and dysfunction: role in the extension phase of acute renal failure. Kidney Int. 2004;66:496–499. doi: 10.1111/j.1523-1755.2004.761_5.x. [DOI] [PubMed] [Google Scholar]
- 11.Portilla D, et al. Etomoxir-induced PPARalpha-modulated enzymes protect during acute renal failure. Am. J. Physiol. Renal Physiol. 2000;278:F667–F675. doi: 10.1152/ajprenal.2000.278.4.F667. [DOI] [PubMed] [Google Scholar]
- 12.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin. Nephrol. 2003;23:460–464. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Gokden N, Okusa MD, Bhatt R, Portilla D. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am. J. Physiol. Renal Physiol. 2005;289:F469–F480. doi: 10.1152/ajprenal.00038.2005. [DOI] [PubMed] [Google Scholar]
- 14.Li S, et al. PPAR-alpha ligand ameliorates acute renal failure by reducing cisplatin-induced increased expression of renal endonuclease G. Am. J. Physiol. Renal Physiol. 2004;287:F990–F998. doi: 10.1152/ajprenal.00206.2004. [DOI] [PubMed] [Google Scholar]
- 15.Letavernier E, et al. Peroxisome proliferator-activated receptor beta/delta exerts a strong protection from ischemic acute renal failure. J. Am. Soc. Nephrol. 2005;16:2395–2402. doi: 10.1681/ASN.2004090802. [DOI] [PubMed] [Google Scholar]
- 16.Chung BH, et al. Protective effect of peroxisome proliferator activated receptor gamma agonists on diabetic and non-diabetic renal diseases. Nephrology (Carlton). 2005;10(Suppl. 2):S40–S43. doi: 10.1111/j.1440-1797.2005.00456.x. [DOI] [PubMed] [Google Scholar]
- 17.Cuzzocrea S. Peroxisome proliferator-activated receptors gamma ligands and ischemia and reperfusion injury. Vascul. Pharmacol. 2004;41:187–195. doi: 10.1016/j.vph.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Guan Y. Peroxisome proliferator-activated receptor family and its relationship to renal complications of the metabolic syndrome. J. Am. Soc. Nephrol. 2004;15:2801–2815. doi: 10.1097/01.ASN.0000139067.83419.46. [DOI] [PubMed] [Google Scholar]
- 19.Panchapakesan U, Sumual S, Pollock CA, Chen X. PPARgamma agonists exert antifibrotic effects in renal tubular cells exposed to high glucose. Am. J. Physiol. Renal Physiol. 2005;289:F1153–F1158. doi: 10.1152/ajprenal.00097.2005. [DOI] [PubMed] [Google Scholar]
- 20.Zafiriou S, Stanners SR, Polhill TS, Poronnik P, Pollock CA. Pioglitazone increases renal tubular cell albumin uptake but limits proinflammatory and fibrotic responses. Kidney Int. 2004;65:1647–1653. doi: 10.1111/j.1523-1755.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- 21.Sivarajah A, et al. Agonists of peroxisome-proliferator activated receptor-gamma reduce renal ischemia/reperfusion injury. Am. J. Nephrol. 2003;23:267–276. doi: 10.1159/000072088. [DOI] [PubMed] [Google Scholar]
- 22.Zheng F, et al. Upregulation of type I collagen by TGF-beta in mesangial cells is blocked by PPARgamma activation. Am. J. Physiol. Renal Physiol. 2002;282:F639–F648. doi: 10.1152/ajprenal.00189.2001. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee PK, et al. The cyclopentenone prostaglandin 15-deoxy-delta(12, 14)-prostaglandin J2 ameliorates ischemic acute renal failure. Cardiovasc. Res. 2004;61:630–643. doi: 10.1016/j.cardiores.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 24.King RC, et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann. Thorac. Surg. 2000;69:1681–1685. doi: 10.1016/s0003-4975(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 25.Ito K, et al. Protective effects of preischemic treatment with pioglitazone, a peroxisome proliferator-activated receptor-gamma ligand, on lung ischemia-reperfusion injury in rats. Eur. J. Cardiothorac. Surg. 2004;25:530–536. doi: 10.1016/j.ejcts.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Okada M, Yan SF, Pinsky DJ. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) activation suppresses ischemic induction of Egr-1 and its inflammatory gene targets. FASEB J. 2002;16:1861–1868. doi: 10.1096/fj.02-0503com. [DOI] [PubMed] [Google Scholar]
- 27.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 28.Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001;120:617–624. doi: 10.1378/chest.120.2.617. [DOI] [PubMed] [Google Scholar]
- 29.Burgess HA, et al. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L1146–L1153. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- 30.Genovese T, et al. Effect of rosiglitazone and 15-deoxy-delta12,14-prostaglandin J2 on bleomycin-induced lung injury. Eur. Respir. J. 2005;25:225–234. doi: 10.1183/09031936.05.00049704. [DOI] [PubMed] [Google Scholar]
- 31.Cappell MS. Intestinal (mesenteric) vasculopathy. II. Ischemic colitis and chronic mesenteric ischemia [review] Gastroenterol. Clin. North Am. 1998;27:827–860, vi. doi: 10.1016/s0889-8553(05)70034-0. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima A, et al. Endogenous PPAR gamma mediates anti-inflammatory activity in murine ischemia-reperfusion injury. Gastroenterology. 2001;120:460–469. doi: 10.1053/gast.2001.21191. [DOI] [PubMed] [Google Scholar]
- 33.Naito Y, et al. Suppression of intestinal ischemia-reperfusion injury by a specific peroxisome proliferator-activated receptor-gamma ligand, pioglitazone, in rats. Redox Rep. 2002;7:294–299. doi: 10.1179/135100002125000983. [DOI] [PubMed] [Google Scholar]
- 34.Cuzzocrea S, et al. Rosiglitazone and 15-deoxy-delta12,14-prostaglandin J2, ligands of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), reduce ischaemia/reperfusion injury of the gut. Br. J. Pharmacol. 2003;140:366–376. doi: 10.1038/sj.bjp.0705419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuzzocrea S, et al. WY 14643, a potent exogenous PPAR-alpha ligand, reduces intestinal injury associated with splanchnic artery occlusion shock. Shock. 2004;22:340–346. doi: 10.1097/01.shk.0000136704.26372.2d. [DOI] [PubMed] [Google Scholar]
- 36.Sato, N., et al. 2005. Differential induction of PPAR gamma by luminal glutamine and iNOS by luminal arginine in the rodent post ischemic small bowel. Am. J. Physiol. Gastrointest. Liver Physiol. doi:10.1152/ajpgi.00248.2005. [DOI] [PubMed]
- 37.Ichikawa H, et al. A specific peroxisome proliferator-activated receptor-gamma (PPAR-gamma) ligand, pioglitazone, ameliorates gastric mucosal damage induced by ischemia and reperfusion in rats. Redox Rep. 2002;7:343–346. doi: 10.1179/135100002125000956. [DOI] [PubMed] [Google Scholar]
- 38.Konturek PC, et al. Pioglitazone, a specific ligand of the peroxisome proliferator-activated receptor gamma reduces gastric mucosal injury induced by ischaemia/reperfusion in rat. Scand. J. Gastroenterol. 2003;38:468–476. [PubMed] [Google Scholar]
- 39.Wada K, et al. Protective effect of endogenous PPARgamma against acute gastric mucosal lesions associated with ischemia-reperfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G452–G458. doi: 10.1152/ajpgi.00523.2003. [DOI] [PubMed] [Google Scholar]
- 40.Villegas I, Martin AR, Toma W, de la Lastra CA. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, protects against gastric ischemia-reperfusion damage in rats: role of oxygen free radicals generation. Eur. J. Pharmacol. 2004;505:195–203. doi: 10.1016/j.ejphar.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 41.Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10:927–939. doi: 10.1007/s10495-005-1055-4. [DOI] [PubMed] [Google Scholar]
- 42.Iredale JP. Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ. 2003;327:143–147. doi: 10.1136/bmj.327.7407.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G20–G30. doi: 10.1152/ajpgi.00474.2002. [DOI] [PubMed] [Google Scholar]
- 44.Miyahara T, et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J. Biol. Chem. 2000;275:35715–35722. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 45.Marra F, et al. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466–478. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- 46.Galli A, et al. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924–1940. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- 47.Hazra S, et al. Peroxisome proliferator-activated receptor gamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J. Biol. Chem. 2004;279:11392–11401. doi: 10.1074/jbc.M310284200. [DOI] [PubMed] [Google Scholar]
- 48.Yavrom S, et al. Peroxisome proliferator-activated receptor gamma suppresses proximal alpha 1(I) collagen promoter via inhibition of p300-facilitated NF-I binding to DNA in hepatic stellate cells. J. Biol. Chem. 2005;280:40650–40659. doi: 10.1074/jbc.M510094200. [DOI] [PubMed] [Google Scholar]
- 49.She H, Xiong S, Hazra S, Tsukamoto H. Adipogenic transcriptional regulation of hepatic stellate cells. J. Biol. Chem. 2005;280:4959–4967. doi: 10.1074/jbc.M410078200. [DOI] [PubMed] [Google Scholar]
- 50.Planaguma A, et al. The selective cyclooxygenase-2 inhibitor SC-236 reduces liver fibrosis by mechanisms involving non-parenchymal cell apoptosis and PPARgamma activation. FASEB J. 2005;19:1120–1122. doi: 10.1096/fj.04-2753fje. [DOI] [PubMed] [Google Scholar]
- 51.Fiorucci S, et al. Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor gamma contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J. Pharmacol. Exp. Ther. 2005;315:58–68. doi: 10.1124/jpet.105.085597. [DOI] [PubMed] [Google Scholar]
- 52.Marra F, et al. Thiazolidinedione treatment inhibits bile duct proliferation and fibrosis in a rat model of chronic cholestasis. World J. Gastroenterol. 2005;11:4931–4938. doi: 10.3748/wjg.v11.i32.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hellemans K, et al. Peroxisome proliferator-activated receptor-beta signaling contributes to enhanced proliferation of hepatic stellate cells. Gastroenterology. 2003;124:184–201. doi: 10.1053/gast.2003.50015. [DOI] [PubMed] [Google Scholar]
- 54.Toyama T, et al. PPARalpha ligands activate antioxidant enzymes and suppress hepatic fibrosis in rats. Biochem. Biophys. Res. Commun. 2004;324:697–704. doi: 10.1016/j.bbrc.2004.09.110. [DOI] [PubMed] [Google Scholar]
- 55.Okaya T, Lentsch AB. Peroxisome proliferator-activated receptor-alpha regulates postischemic liver injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G606–G612. doi: 10.1152/ajpgi.00191.2003. [DOI] [PubMed] [Google Scholar]
- 56.Rao MS, Peters JM, Gonzalez FJ, Reddy JK. Hepatic regeneration in peroxisome proliferator-activated receptor alpha-null mice after partial hepatectomy. Hepatol. Res. 2002;22:52–57. doi: 10.1016/s1386-6346(01)00119-x. [DOI] [PubMed] [Google Scholar]
- 57.Wheeler MD, et al. Impaired Ras membrane association and activation in PPARalpha knockout mice after partial hepatectomy. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G302–G312. doi: 10.1152/ajpgi.00175.2002. [DOI] [PubMed] [Google Scholar]
- 58.Anderson SP, et al. Delayed liver regeneration in peroxisome proliferator-activated receptor-alpha-null mice. Hepatology. 2002;36:544–554. doi: 10.1053/jhep.2002.35276. [DOI] [PubMed] [Google Scholar]
- 59.Skrtic S, et al. Decreased expression of peroxisome proliferator-activated receptor alpha and liver fatty acid binding protein after partial hepatectomy of rats and mice. Liver Int. 2005;25:33–40. doi: 10.1111/j.1478-3231.2004.0998.x. [DOI] [PubMed] [Google Scholar]
- 60.Jonas S. Prophylactic pharmacologic neuroprotection against focal cerebral ischemia. Ann. N. Y. Acad. Sci. 1995;765:21–25; discussion 26–27. doi: 10.1111/j.1749-6632.1995.tb16555.x. [DOI] [PubMed] [Google Scholar]
- 61.Bloomfield Rubins H, et al. Reduction in stroke with gemfibrozil in men with coronary heart disease and low HDL cholesterol: The Veterans Affairs HDL Intervention Trial (VA-HIT) Circulation. 2001;103:2828–2833. doi: 10.1161/01.cir.103.23.2828. [DOI] [PubMed] [Google Scholar]
- 62.Deplanque D, et al. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J. Neurosci. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inoue H, et al. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci. Lett. 2003;352:203–206. doi: 10.1016/j.neulet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Santos MJ, et al. Peroxisomal proliferation protects from beta-amyloid neurodegeneration. J. Biol. Chem. 2005;280:41057–41068. doi: 10.1074/jbc.M505160200. [DOI] [PubMed] [Google Scholar]
- 65.Shimazu T, et al. A peroxisome proliferator-activated receptor-gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005;36:353–359. doi: 10.1161/01.STR.0000152271.21943.a2. [DOI] [PubMed] [Google Scholar]
- 66.Sundararajan S, et al. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J. The intracerebral application of the PPARgamma-ligand pioglitazone confers neuroprotection against focal ischaemia in the rat brain. Eur J. Neurosci. 2005;22:278–282. doi: 10.1111/j.1460-9568.2005.04200.x. [DOI] [PubMed] [Google Scholar]
- 68.Kielian T, Drew PD. Effects of peroxisome proliferator-activated receptor-gamma agonists on central nervous system inflammation. J. Neurosci. Res. 2003;71:315–325. doi: 10.1002/jnr.10501. [DOI] [PubMed] [Google Scholar]
- 69.Schutz B, et al. The oral antidiabetic pioglitazone protects from neurodegeneration and amyotrophic lateral sclerosis-like symptoms in superoxide dismutase-G93A transgenic mice. J. Neurosci. 2005;25:7805–7812. doi: 10.1523/JNEUROSCI.2038-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc. Med. 2000;10:238–245. doi: 10.1016/s1050-1738(00)00077-3. [DOI] [PubMed] [Google Scholar]
- 71.Fragasso G, et al. Short- and long-term beneficial effects of trimetazidine in patients with diabetes and ischemic cardiomyopathy. Am. Heart J. 2003;146:E18. doi: 10.1016/S0002-8703(03)00415-0. [DOI] [PubMed] [Google Scholar]
- 72.Sambandam N, et al. Chronic activation of PPARalpha is detrimental to cardiac recovery following ischemia. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H87–H95. doi: 10.1152/ajpheart.00285.2005. [DOI] [PubMed] [Google Scholar]
- 73.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ. Res. 2000;86:580–588. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- 74.Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J. Biol. Chem. 2002;277:22528–22533. doi: 10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- 75.Yue TL, et al. Activation of peroxisome proliferator-activated receptor-alpha protects the heart from ischemia/reperfusion injury. Circulation. 2003;108:2393–2399. doi: 10.1161/01.CIR.0000093187.42015.6C. [DOI] [PubMed] [Google Scholar]
- 76.Wayman NS, et al. Ligands of the peroxisome proliferator-activated receptors (PPAR-gamma and PPAR-alpha) reduce myocardial infarct size. FASEB J. 2002;16:1027–1040. doi: 10.1096/fj.01-0793com. [DOI] [PubMed] [Google Scholar]
- 77.Tabernero A, et al. Activation of the peroxisome proliferator-activated receptor alpha protects against myocardial ischaemic injury and improves endothelial vasodilatation. BMC Pharmacol. 2002;2:10. doi: 10.1186/1471-2210-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee TM, Chou TF. Troglitazone administration limits infarct size by reduced phosphorylation of canine myocardial connexin43 proteins. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1650–H1659. doi: 10.1152/ajpheart.00407.2002. [DOI] [PubMed] [Google Scholar]
- 79.Shiomi T, et al. Pioglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;106:3126–3132. doi: 10.1161/01.cir.0000039346.31538.2c. [DOI] [PubMed] [Google Scholar]
- 80.Yue T-L, et al. In vivo myocardial protection from ischemia/reperfusion injury by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2001;104:2588–2594. doi: 10.1161/hc4601.099403. [DOI] [PubMed] [Google Scholar]
- 81.Khandoudi N, et al. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma, inhibits the Jun NH(2)-terminal kinase/activating protein 1 pathway and protects the heart from ischemia/reperfusion injury. Diabetes. 2002;51:1507–1514. doi: 10.2337/diabetes.51.5.1507. [DOI] [PubMed] [Google Scholar]
- 82.Sidell RJ, et al. Thiazolidinedione treatment normalizes insulin resistance and ischemic injury in the zucker fatty rat heart. Diabetes. 2002;51:1110–1117. doi: 10.2337/diabetes.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 83.Liu HR, et al. Anti-apoptotic effects of rosiglitazone in hypercholesterolemic rabbits subjected to myocardial ischemia and reperfusion. Cardiovasc. Res. 2004;62:135–144. doi: 10.1016/j.cardiores.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 84.Dormandy JA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 85.Delea TE, Edelsberg JS, Hagiwara M, Oster G, Phillips LS. Use of thiazolidinediones and risk of heart failure in people with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2003;26:2983–2989. doi: 10.2337/diacare.26.11.2983. [DOI] [PubMed] [Google Scholar]
- 86.Lygate CA, et al. The PPARgamma-activator rosiglitazone does not alter remodeling but increases mortality in rats post-myocardial infarction. Cardiovasc. Res. 2003;58:632–637. doi: 10.1016/s0008-6363(03)00289-x. [DOI] [PubMed] [Google Scholar]
- 87.Xu Y, et al. Deleterious effects of acute treatment with a peroxisome proliferator-activated receptor-gamma activator in myocardial ischemia and reperfusion in pigs. Diabetes. 2003;52:1187–1194. doi: 10.2337/diabetes.52.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iglarz M, et al. Peroxisome proliferator-activated receptor-alpha and receptor-gamma activators prevent cardiac fibrosis in mineralocorticoid-dependent hypertension. Hypertension. 2003;42:737–743. doi: 10.1161/01.HYP.0000083511.91817.B1. [DOI] [PubMed] [Google Scholar]
- 89.Ogata T, et al. Myocardial fibrosis and diastolic dysfunction in deoxycorticosterone acetate-salt hypertensive rats is ameliorated by the peroxisome proliferator-activated receptor-alpha activator fenofibrate, partly by suppressing inflammatory responses associated with the nuclear factor-kappa-B pathway. J. Am. Coll. Cardiol. 2004;43:1481–1488. doi: 10.1016/j.jacc.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 90.Diep QN, et al. PPAR alpha activator fenofibrate inhibits myocardial inflammation and fibrosis in angiotensin II-infused rats. J. Mol. Cell. Cardiol. 2004;36:295–304. doi: 10.1016/j.yjmcc.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 91.Chen K, Li D, Zhang X, Hermonat PL, Mehta JL. Anoxia-reoxygenation stimulates collagen type-I and MMP-1 expression in cardiac fibroblasts: modulation by the PPAR-gamma ligand pioglitazone. J. Cardiovasc. Pharmacol. 2004;44:682–687. doi: 10.1097/00005344-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 92.Nikolaidis LA, Levine TB. Peroxisome proliferator activator receptors (PPAR), insulin resistance, and cardiomyopathy: friends or foes for the diabetic patient with heart failure? Cardiol. Rev. 2004;12:158–170. doi: 10.1097/01.crd.0000102419.52594.90. [DOI] [PubMed] [Google Scholar]
- 93.Rizza R, Henry R, Kahn R. Commentary on the results and clinical implications of the PROactive study. Diabetes Care. 2005;28:2965–2967. doi: 10.2337/diacare.28.12.2965. [DOI] [PubMed] [Google Scholar]
- 94.Baue AE, Durham R, Faist E. Systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), multiple organ failure (MOF): are we winning the battle? Shock. 1998;10:79–89. doi: 10.1097/00024382-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 95.Abdelrahman M, Sivarajah A, Thiemermann C. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc. Res. 2005;65:772–781. doi: 10.1016/j.cardiores.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 96.Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation. Shock. 2005;23:393–399. doi: 10.1097/01.shk.0000160521.91363.88. [DOI] [PubMed] [Google Scholar]
- 97.Kaplan JM, et al. 15-Deoxy-Delta(12, 14)-prostaglandin J(2) (15D-PGJ(2)), a peroxisome proliferator activated receptor gamma ligand, reduces tissue leukosequestration and mortality in endotoxic shock. Shock. 2005;24:59–65. doi: 10.1097/01.shk.0000167108.88376.f2. [DOI] [PubMed] [Google Scholar]
- 98.Wiel E, et al. Pretreatment with peroxysome proliferator-activated receptor alpha agonist fenofibrate protects endothelium in rabbit Escherichia coli endotoxin-induced shock. Intensive Care Med. 2005;31:1269–1279. doi: 10.1007/s00134-005-2730-1. [DOI] [PubMed] [Google Scholar]
- 99.Abdelrahman M, Collin M, Thiemermann C. The peroxisome proliferator-activated receptor-gamma ligand 15-deoxyDelta12,14 prostaglandin J2 reduces the organ injury in hemorrhagic shock. Shock. 2004;22:555–561. doi: 10.1097/01.shk.0000144132.13900.24. [DOI] [PubMed] [Google Scholar]
- 100.Marshall JC, et al. Preclinical models of shock and sepsis: what can they tell us? Shock. 2005;24(Suppl. 1):1–6. doi: 10.1097/01.shk.0000191383.34066.4b. [DOI] [PubMed] [Google Scholar]
- 101.Chang HY, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3738–3743. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat. Rev. Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 103.Margeli A, Kouraklis G, Theocharis S. Peroxisome proliferator activated receptor-gamma (PPAR-gamma) ligands and angiogenesis. Angiogenesis. 2003;6:165–169. doi: 10.1023/B:AGEN.0000021377.13669.c0. [DOI] [PubMed] [Google Scholar]
- 104.Keshamouni VG, et al. PPAR-gamma activation inhibits angiogenesis by blocking ELR+CXC chemokine production in non-small cell lung cancer. Neoplasia. 2005;7:294–301. doi: 10.1593/neo.04601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sarayba MA, et al. Inhibition of corneal neovascularization by a peroxisome proliferator-activated receptor-gamma ligand. Exp. Eye Res. 2005;80:435–442. doi: 10.1016/j.exer.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 106.Panigrahy D, et al. PPARγ ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J. Clin. Invest. 2002;110:923–932. doi:10.1172/JCI200215634. doi: 10.1172/JCI15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murata T, et al. Peroxisome proliferator-activated receptor-gamma ligands inhibit choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2000;41:2309–2317. [PubMed] [Google Scholar]
- 108.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J. Biol. Chem. 1999;274:9116–9121. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 109.Goetze S, et al. Leptin induces endothelial cell migration through Akt, which is inhibited by PPARgamma-ligands. Hypertension. 2002;40:748–754. doi: 10.1161/01.hyp.0000035522.63647.d3. [DOI] [PubMed] [Google Scholar]
- 110.Rieusset J, Auwerx J, Vidal H. Regulation of gene expression by activation of the peroxisome proliferator-activated receptor gamma with rosiglitazone (BRL 49653) in human adipocytes. Biochem. Biophys. Res. Commun. 1999;265:265–271. doi: 10.1006/bbrc.1999.1657. [DOI] [PubMed] [Google Scholar]
- 111.Kersten S, et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 2000;275:28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 112.Yoon JC, et al. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell. Biol. 2000;20:5343–5349. doi: 10.1128/mcb.20.14.5343-5349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Belanger AJ, et al. Hypoxia up-regulates expression of peroxisome proliferator-activated receptor gamma angiopoietin-related gene (PGAR) in cardiomyocytes: role of hypoxia inducible factor 1alpha. J. Mol. Cell. Cardiol. 2002;34:765–774. doi: 10.1006/jmcc.2002.2021. [DOI] [PubMed] [Google Scholar]
- 114.Varet J, et al. Fenofibrate inhibits angiogenesis in vitro and in vivo. Cell. Mol. Life Sci. 2003;60:810–819. doi: 10.1007/s00018-003-2322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kasai, T., Miyauchi, K., Yokoyama, T., Aihara, K., and Daida, H. 2005. Efficacy of peroxisome proliferative activated receptor (PPAR)-alpha ligands, fenofibrate, on intimal hyperplasia and constrictive remodeling after coronary angioplasty in porcine models. Atherosclerosis. doi:10.1016/j.atherosclerosis.2005.10.047. [DOI] [PubMed]
- 116.Nijsten T, Geluyckens E, Colpaert C, Lambert J. Peroxisome proliferator-activated receptors in squamous cell carcinoma and its precursors. J. Cutan. Pathol. 2005;32:340–347. doi: 10.1111/j.0303-6987.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 117.Jaeckel EC, et al. Correlation of expression of cyclooxygenase-2, vascular endothelial growth factor, and peroxisome proliferator-activated receptor delta with head and neck squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 2001;127:1253–1259. doi: 10.1001/archotol.127.10.1253. [DOI] [PubMed] [Google Scholar]
- 118.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ. Res. 2002;90:1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 119.Devchand PR, et al. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 120.Powell DW, Adegboyega PA, Di Mari JF, Mifflin RC. Epithelial cells and their neighbors. I. Role of intestinal myofibroblasts in development, repair, and cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G2–G7. doi: 10.1152/ajpgi.00075.2005. [DOI] [PubMed] [Google Scholar]
- 121.Hetzel M, Bachem M, Anders D, Trischler G, Faehling M. Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung. 2005;183:225–237. doi: 10.1007/s00408-004-2534-z. [DOI] [PubMed] [Google Scholar]
- 122.Bataller R, Brenner DA. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi:10.1172/JCI200524282. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chatziantoniou C, Dussaule JC. Insights into the mechanisms of renal fibrosis: is it possible to achieve regression? Am. J. Physiol. Renal Physiol. 2005;289:F227–F234. doi: 10.1152/ajprenal.00453.2004. [DOI] [PubMed] [Google Scholar]
- 124.Pershadsingh, H.A. 2005. Treating the metabolic syndrome using angiotensin receptor antagonists that selectively modulate peroxisome proliferator-activated receptor-γ. Int. J. Biochem. Cell Biol. doi:10.1016/j.biocel.2005.08.006. [DOI] [PubMed]
- 125.Yang ZZ, et al. Dosage-dependent effects of Akt1/protein kinase Balpha (PKBalpha) and Akt3/PKBgamma on thymus, skin, and cardiovascular and nervous system development in mice. Mol. Cell. Biol. 2005;25:10407–10418. doi: 10.1128/MCB.25.23.10407-10418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]