Abstract
PPARα is a nuclear receptor that regulates liver and skeletal muscle lipid metabolism as well as glucose homeostasis. Acting as a molecular sensor of endogenous fatty acids (FAs) and their derivatives, this ligand-activated transcription factor regulates the expression of genes encoding enzymes and transport proteins controlling lipid homeostasis, thereby stimulating FA oxidation and improving lipoprotein metabolism. PPARα also exerts pleiotropic antiinflammatory and antiproliferative effects and prevents the proatherogenic effects of cholesterol accumulation in macrophages by stimulating cholesterol efflux. Cellular and animal models of PPARα help explain the clinical actions of fibrates, synthetic PPARα agonists used to treat dyslipidemia and reduce cardiovascular disease and its complications in patients with the metabolic syndrome. Although these preclinical studies cannot predict all of the effects of PPARα in humans, recent findings have revealed potential adverse effects of PPARα action, underlining the need for further study. This Review will focus on the mechanisms of action of PPARα in metabolic diseases and their associated vascular pathologies.
Introduction
Nutrient metabolism and energy homeostasis are tightly regulated by endocrine, paracrine, and autocrine signals that control the expression and activity of key metabolic enzymes and transport proteins by transcriptional and posttranscriptional mechanisms. Lipid mediators play a critical role in metabolic control, and the PPARs (NR1Cs), a class of ligand-activated transcription factors, have emerged as master transcriptional regulators of lipid and carbohydrate metabolism. Saturated and unsaturated long-chain fatty acids (FAs) and their eicosanoid derivatives are natural activators of this subclass of nuclear receptors. Increased recognition of a role for PPARs in metabolic regulation came following the discovery that the hypolipidemic fibrates and the insulin sensitizers thiazolidinediones were synthetic ligands for PPARα (NR1C1; refs. 1, 2) and PPARγ (NR1C3; ref. 3), respectively. PPAR&Dgr; (NR1C3), also known as PPARβ, is the third PPAR isotype.
Accumulating evidence supports a link between the 3 PPARs and diabetes, obesity, dyslipidemia, and inflammation. PPARα controls liver and skeletal muscle lipid metabolism, and glucose homeostasis. PPARα influences intracellular lipid and carbohydrate metabolism through direct transcriptional control of genes involved in peroxisomal and mitochondrial β-oxidation pathways, FA uptake, and triglyceride (TG) catabolism. Moreover, preclinical data suggest a role for PPARα in body weight control, supporting the use of PPARα agonists to treat obesity (4). Mice deficient in PPARα exhibit a delayed response to inflammatory stimuli (5). Several clinical trials demonstrate the efficiency of fibrates at decreasing circulatory inflammatory markers and reducing the progression of coronary atherosclerotic lesions. The ability of PPARα to improve symptoms of the metabolic syndrome (visceral obesity, insulin resistance, atherogenic dyslipidemia, and inflammation) suggests that PPARα may be beneficial in the prevention or treatment of type 2 diabetes mellitus and associated complications.
Structure of PPARα
PPARα has a functional domain structure analogous to those of other nuclear receptor (NR) superfamily members. Like steroid receptors, PPARα interacts with hsp90 (6). The central DNA-binding domain (DBD) of PPARα is flanked by an N-terminal region called activating function–1 (AF-1) (7) that is activated by phosphorylation, as shown by insulin-stimulated AF-1 phosphorylation (S1). The DBD confers to PPARα the ability to bind to PPAR response elements (PPREs) in the promoter of target genes as an obligate heterodimer with retinoid X receptor (RXR) isotypes (8). PPREs typically are organized as direct repeats of the core sequence AGGTCA separated by 1 or 2 nucleotides (DR1 and DR2), flanked upstream by A/T–rich sequences (S2). While PPRE geometry ensures specificity for PPAR/RXR heterodimers, DR1 and DR2 PPREs are also recognized by RXR homodimers or retinoic acid receptor (RAR)/RXR heterodimers, suggesting cross-talk with RARs and RXRs that may influence metabolic control (9). The C-terminus of PPARα, whose 3D structure has been solved (10, 11), contains the ligand-regulated E domain or AF-2 or ligand-binding domain (LBD), which harbors a large T-shaped ligand-binding pocket (1,300 Å3) to accommodate various natural and synthetic ligands.
Transcriptional activation by PPARα
The transactivation process by NRs relies on 5 major steps: ligand binding; stable binding of liganded NR to DNA; corepressor dismissal and coactivator recruitment; activation of transcription; and dissociation of the transcriptional complex, followed by either shut-down or reinitiation of transcription. Crystallographic studies suggest that ligand binding to PPARα induces a global stabilization of the receptor conformation (11), without major structural reorganization, unlike the prototypical retinoic acid receptor LBD that undergoes major structural transitions upon agonist binding (S3). This suggests that the major contribution of the PPARα ligand is the stabilization of a predefined structure able to engage protein-protein interactions with coactivators (agonist-bound PPARα) or corepressors (antagonist-bound PPARα) (11). This feature is common to PPARα, PPAR&Dgr;, and PPARγ (S4, S5), but a recent refinement of the structure of the LBD of PPAR&Dgr; showed that this polypeptide is able to trap endogenous bacterial FAs prior to crystallization (S6). Therefore, the possibility arises that PPARα, and other isotypes, might shift from an inactive to an active conformation, similarly to other NRs. However, it is not known whether PPARα is, in a biological context, constitutively bound to endogenous FAs.
Coactivator and corepressor complexes possess distinct enzymatic activities (such as acetylase, deacetylase, methylase, demethylase, and kinase activities) targeting chromatin, components of the basal transcriptional machinery, and other coactivators and corepressors. The orchestrated recruitment and dismissal of coactivators and corepressors leads to chromatin decompaction and preinitiation complex assembly on promoters. The transcriptional response is also strongly influenced by the chemical structure of the ligand, the nature of the PPRE (12), the structure of the promoter, and the expression of coactivators and corepressors in a given cell type. The direct interaction of coactivators and corepressors with PPARα requires 1 or more cores of a degenerated LXXLL motif on the coregulator protein, and several proteins have features of a bona fide coactivator or corepressor for PPARα (S7–S13). However, none has been proven essential for PPARα-induced transcription, including the prototypical SRC1 molecule (13), reflecting a likely functional redundancy between coactivators, or the lack of appropriate models to study such mechanisms. This general mechanism for transcriptional activation by PPARα is likely similar for other PPARα target genes.
Gene repression by PPARα
PPARα also interferes negatively with other nuclear signaling pathways such as the AP1 (14) and NF-κB pathways. Indeed, PPARα inhibits genes induced by NF-κB, such as VCAM-1, COX-2, and IL-6 (15, 16), providing a molecular basis for the antiinflammatory effects of PPARα ligands in vivo. PPARα upregulates expression of the NF-κB repressor IκBα (17) by increasing occupancy of the NF-κB binding site present in the IκBα promoter, thereby potentiating a negative feedback loop. This occurs independently of PPARα binding to DNA and thus could involve direct protein-protein interaction of PPARα with the NF-κB complex (18). A similar mechanism has been described for the fibrate-mediated inhibition of IL-1–induced expression of C-reactive protein (CRP) (19). Interference of PPARα with the CAATT/enhancer binding protein (C/EBP) signaling pathway is the molecular basis for the inhibition of IL-6–induced fibrinogen-α and -β and of serum amyloid A expression (20). PPARα also decreases the expression of IL-6 receptor components as well as that of C/EBPs (21).
The biological and therapeutic activities of PPARα are therefore the result of the combination of both transactivating and transrepressive properties of this receptor. In addition, posttranslational modifications are important regulatory controls. SUMOylation and acetylation regulate transrepressive and transactivating activities of some NRs, and phosphorylation may inhibit transrepression by PPARα. PKC inhibition increases repression of the fibrinogen-β gene by PPARα by modulating the phosphorylation state of the PPARα D domain (22). The ability of NRs to regulate transcription is also a function of promoter architecture, ligand structure, cell type, and physiological and pathological conditions. This raises the possibility of designing ligands with dissociated transactivating and transrepressive activities, enabling specific targeting of gene subsets. However, despite extensive knowledge of PPARα molecular biology, the design of such ligands remains purely empirical.
Mechanisms controlling PPARα activity
There are several levels at which PPARα activity can be controlled. These include the regulation of its expression, the nature of the ligand, the levels of coactivators and corepressors, and posttranslational modifications of PPARα and the associated coactivators and corepressors. Temporal expression of PPARα in rats is controlled by the circadian clock (23), through the positive control of PPARα expression by glucocorticoids (24, 25) and the clock gene Bmal1 (26). PPARα expression is induced during fasting in Sv129 mice (27, 28), and influenced by hormonal signals such as leptin, growth hormone, and insulin (24, 29, 30). Synthetic PPARα ligands such as Wy14,643, GW7647, or fibrates increase the half-life of the PPARα polypeptide by preventing its ubiquitination and its subsequent degradation via the proteasome (31).
Human PPARα promoter activity is induced by PPARα itself and by the nuclear receptor HNF4, a major regulator of gluconeogenesis (32). Glucose decreases PPARα expression in the pancreas, leading to diminished fatty acid oxidation (FAO) and TG accumulation, a supposed cause for pancreatic lipotoxicity (33).
Extracellularly regulated signaling pathways impact on PPARα through phosphorylation. The role of PKC in specifying PPARα transcriptional activities has been described above, and a phosphorylation-dependent increase in PPARα activity by the stress-activated p38 protein kinase has been shown to favor PPARα-mediated transactivation (34).
Endogenous PPARα ligands
The quest for “the” endogenous PPARα ligand is still ongoing. Early reports identified mono- and polyunsaturated FA as well as eicosanoids as natural PPARα ligands (2, 35). Long-chain fatty acyl-CoAs and saturated FAs also bind and activate PPARα with EC50 values in the high-micromolar range (36, 37). Recently, Chakravarthy and colleagues reported that liver-specific inactivation of the fatty acid synthase (FAS) gene in mice resulted in a phenotype identical to that of fasting PPARα-deficient mice (38). Defects in FAO, cholesterol metabolism, and gluconeogenesis could be corrected by PPARα agonist treatment in FAS-deficient mice, suggesting that de novo synthesized end products of FAS, including palmitate, regulate PPARα activity. The data also suggest that FAs released from adipocytes are inactive with respect to PPARα and thus exert physiologically distinct activities.
PPARα and intracellular lipid metabolism
PPARα is highly expressed in tissues displaying a high catabolic rate of FAs, such as the liver, skeletal muscles (mostly in slow-twitch, oxidative type I fibers [S14]), brown fat, heart, kidneys, and cells of atherosclerotic lesions (endothelial cells, smooth muscle cells, monocytes/macrophages). In rodents, PPARα activation leads to peroxisome proliferation and hepatocarcinoma, a property intrinsic to mouse PPARα and, fortunately, not observed in humans (39). Targeted disruption of the PPARα gene in mice revealed its role in mitochondrial and peroxisomal FA β-oxidation (FAO), FA uptake, and lipoprotein assembly and transport (27, 28, 40–46). While the phenotype of PPARα-deficient mice fed ad libitum is mild, fasting or inhibition of mitochondrial FA import severely impairs FA uptake and FAO, leading to sex-specific liver steatosis and cardiac lipid accumulation, hypoglycemia, and hypothermia (28, 43).
PPAR α in hepatic lipid metabolism.
Short-term adjustment of mitochondrial FA β-oxidation occurs through regulation of carnitine palmitoyl transferase 1 (CPT-1), which controls FA import into the mitochondria. CPT-1 expression is regulated by PPARα in liver and myocytes (44), as well as that of major enzymes of the β-oxidation pathway (acyl-CoA synthetase, very-long- and medium-chain acyl-CoA dehydrogenases, 3-ketoacyl-CoA thiolase). Partial oxidation of very-long-chain and long-chain FAs, as well as of other lipid derivatives such as eicosanoids or branched FAs, occurs in peroxisomes to provide substrates for mitochondrial oxidation. The expression of key enzymes catalyzing the degradation of straight-chain FAs (acyl-CoA oxidase, L-bifunctional protein, thiolase) in peroxisomes is regulated by PPARα. The mitochondrial HMG-CoA synthase, which converts acetyl-CoA units into ketone bodies during fasting or diabetes, is also upregulated by PPARα (45). Thus PPARα acts in liver and other organs to reduce intracellular FA concentrations, likely contributing to decreased VLDL particle production and plasma TG levels in patients treated with an agonist. PPARα’s role in energy homeostasis is thus clearly demonstrated in animal models, but unclear at present in humans. Moreover, the lower expression of PPARα in human compared with rodent liver (47), as well the dominant-negative splice variant of PPARα in human liver (48), suggests a more modest role of PPARα in humans.
PPAR α and cellular FA uptake.
PPARα also modulates FA cellular uptake. Fatty acid translocase, or CD36, is a glycoprotein regulating FA uptake in multiple cell types, including hepatocytes, adipocytes, and monocytes, as well as cells in muscle and intestine. PPARα activation upregulates CD36 expression in liver and intestine, but not in skeletal muscle (49). Similarly, expression of the fatty acid transport protein, an integral membrane protein involved in FA uptake, is upregulated by PPARα activation in hepatocytes (46).
PPAR α in cardiac lipid metabolism.
The hearts of PPARα-deficient mice express very low levels of mitochondrial FAO enzymes, relying almost exclusively on glucose oxidation for energy, similarly to fetal hearts (42, 50, 51). A PPARα-dependent transcriptional network is activated in the heart during the transition from fetus to newborn, creating a metabolic switch from glucose to FAO (S15). Moreover, the metabolic rate of FA is increased in wild-type cardiomyocytes upon treatment with a synthetic PPARα agonist (52), and cardiac overexpression of PPARα leads to upregulation of FAO enzymes, and to downregulation of enzymes controlling glucose uptake and oxidation (51). The cardiac hypertrophy and dysfunction in these PPARα-overexpressing mice thus resemble the cardiac phenotype of diabetic mice, and cardiac as well as age-related liver insulin resistance is observed in these mice (53). Thus PPARα plays a regulatory role in controlling cardiac metabolic switches. A decreased expression of PPARα in the nondiabetic, hypertrophic heart alters FAO in cardiomyocytes and may contribute to cardiac dysfunction (54), whereas a decreased PPARα expression in the diabetic heart may be a mechanism to protect the heart from further oxidative stress–induced damage due to excessive FAO (55).
Extrapolating these findings in mice to human pathology is difficult. The NR field has provided ample evidence that ligand-mediated activation of a receptor can trigger biological responses distinct from those that result from receptor overexpression (see ref. 51, for example). Similarly, the outcomes of gene inactivation studies are not always predictable. Moreover, the metabolic response (such as TG lowering) to agonist-induced systemic PPARα activation may alter cardiac metabolism. Nevertheless, data in genetic models suggest that chronic activation of PPARα can lead to ventricular dysfunction, and recent evidence shows that treating cardiomyopathic mice with fenofibrate worsened heart function (56). However, an important unresolved issue is the contribution of peroxisome proliferation to the cardiac phenotype, which occurs in mice but not humans (39). Thus, although there is no evidence that such events occur in humans treated with weak PPARα agonists (i.e., fibrates), monitoring of cardiac function in diabetic patients appears to be indicated for potent PPARα agonists.
Lipoprotein metabolism
PPAR α and TG and LDL metabolism.
The therapeutic benefit of fibrates is due in part to reduced VLDL production and enhanced catabolism of TG-rich particles, which indirectly decreases small dense LDL (sdLDL) particles, enhancing the formation of HDL particles and hepatic elimination of excess cholesterol. Fibrates have a marked effect on VLDL and chylomicron TG hydrolysis mediated by PPARα, which upregulates lipoprotein lipase (LPL) transcription in liver and muscle (57). LPL is a triacylglycerol hydrolase in the capillary endothelium of peripheral tissues, where its inactivation leads to severe hypertriglyceridemia and decreased HDL formation (S16). Another control of TG catabolism is regulation of the ratio of lipolytic versus antilipolytic apolipoprotein content in TG-rich particles. ApoC-III is an inhibitor of both LPL activity and remnant clearance, and apoC-III–overexpressing mice are severely hypertriglyceridemic (S17). ApoC-III synthesis is lowered by PPARα agonists in murine and human hepatocytes, both in vivo and in vitro (42, 58), thereby favoring VLDL lipolysis and generation of large LDL particles that are more efficiently cleared via the LDL receptor. Interestingly, expression of the recently identified apoA-V, a potent activator of lipolysis, is upregulated by PPARα agonists (59).
PPAR α and HDL metabolism.
HDLs are protective against atherosclerotic vascular disease and are the main vehicle of reverse cholesterol transport (RCT). The interaction of HDL or apoA-I with scavenger receptor BI (SR-BI) and ABC transporter A1 (ABCA1), G1, or G4 triggers cholesterol efflux from peripheral tissues, and HDL particles direct cholesterol for hepatic excretion into the bile (60). Macrophage cholesterol efflux is of paramount importance for atherosclerosis, although it represents only a small fraction of the whole-body RCT. PPARα agonists induce ABCA1 and SR-BI expression in macrophages (61, 62), thereby enhancing the first steps of macrophage RCT. Expression of the major human HDL apolipoprotein genes apoA-I and apoA-II is activated in response to fibrate treatment in vitro (63, 64) and in humans (65, 66) via direct transcriptional control by PPARα. Devoid of any functional PPRE in its promoter, the murine apoA-I gene is negatively regulated by PPARα agonists through an indirect pathway implicating the PPARα-dependent induction of the orphan NR Rev-erbα, a negative regulator of transcription (67). This is an example of the major differences between rodent and human metabolic control by PPARα (see below). Thus PPARα activation, by virtue of its effects on the transcriptional activities of genes involved in lipoprotein metabolism, elicits a global normolipidemic response, by reducing TG-rich particle production, increasing their lipolysis, and promoting HDL metabolism and RCT. These collective effects should enhance transport of cholesterol from peripheral tissues to the liver.
Glucose metabolism
It is well established that excess fat intake promotes insulin resistance, resulting in increased gluconeogenesis and hepatic glucose production. In addition, hepatic and peripheral tissue lipotoxicity is a major causative factor for the development of type 2 diabetes mellitus. TGs provide the gluconeogenesis pathway with the essential substrate, glycerol, in addition to acetyl-CoA, reducing equivalents and ATP.
The mild PPARα-deficient phenotype becomes pronounced upon exposure to thermic, metabolic, or inflammatory stress (28, 43). The severe hypoglycemia observed specifically in PPARα-deficient mice upon fasting, characterized by a 50% drop in blood glucose concentration after 24 hours of fasting, suggested a role for PPARα in glucose homeostasis (28). Several mechanisms may account for this fasting hypoglycemia, including normal glucose-6-phosphate production in liver accompanied by the shift from glucose to glycogen production (68). Other authors attribute the fasting hypoglycemia to decreased production of lactate and hepatic glucose (69). Fasting induces the conversion of glycerol into glucose through the induction of several hepatic enzymes such as glycerol-3-phosphate dehydrogenase (GPDH) and glycerol kinase. The expression of these enzymes, and of the glycerol transporters aquaporins 3 and 9, is PPARα-dependent (70). GPDH deficiency in mice and humans leads to hypoglycemia, underlining the important role of glycerol as a substrate for glucose synthesis (S18, S19).
The mammalian ortholog of TRB3, another PPARα-target gene, is an important link between glucose and lipid metabolism. TRB3 is an inhibitor of Akt/protein kinase B, a positive regulator of cellular responses to insulin (S20). Upregulation of TRB3 expression through direct transcriptional control by PPARα may impact negatively on liver insulin signaling, and in turn perturb glucose homeostasis (71). Moreover, glucocorticoid-induced diabetes is PPARα-dependent (72), and, accordingly, PPARα-deficient mice are protected from high-fat diet–induced insulin resistance (73, 74). Thus, PPARα is a key player in hepatic glucose homeostasis.
The response to fasting is also dependent on the pancreas, and PPARα-deficient mice inefficiently suppress insulin secretion upon fasting, resulting in relative hyperinsulinemia (75). However, treatment of obese mice with PPARα agonists improves insulin sensitivity and decreases blood glucose and insulin levels (76). A similar treatment of severely insulin-resistant lipoatrophic mice decreases blood glucose but does not normalize insulin levels (77). In another model of lipoatrophy, PPARα treatment improved both blood glucose and insulin levels (78). In prediabetic monkeys, lipoatrophic rats, or high-fat-fed rodents, PPARα activation by a high-affinity agonist reversed insulin resistance, likely because of increased FA clearance from insulin-sensitive organs (76, 77, 79, 80). Interestingly, PPARα activation in pancreatic islet β cells also increases pancreatic FAO and potentiates glucose-induced insulin secretion, suggesting that PPARα activation protects pancreatic islets from lipotoxicity (81). This raises the exciting yet untested hypothesis that PPARα activation may prevent progression from a prediabetic, insulin-resistant state to type 2 diabetes.
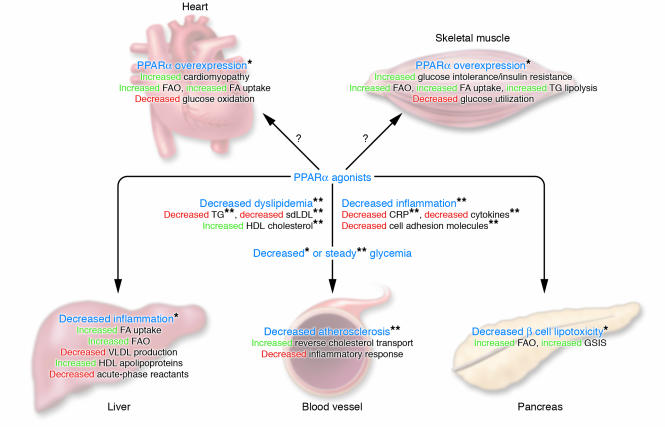
While it is clear that PPARα plays an obligatory role in liver and heart FAO, the importance of PPARα in skeletal muscle FAO is obliterated by a compensatory role of the ubiquitous PPAR&Dgr; (82). However, overexpression of PPARα in skeletal muscle in vivo impacts on peripheral glucose homeostasis. The increased FAO in PPARα-overexpressing muscles is accompanied by a decreased insulin-stimulated glucose uptake. Mice are resistant to diet-induced obesity but exhibit glucose intolerance, revealing a link between muscle PPARα-driven FAO and insulin resistance (83). This suggests that PPARα activation in skeletal muscles shifts substrate utilization from glucose to FA, a conclusion supported by loss-of-function experiments (73, 83). However, only a few clinical trials report an improvement of glucose homeostasis after fibrate treatment (84–87). Moreover, the recent Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study did not reveal any effect of fenofibrate on glucose parameters in diabetic patients (88); this suggests that effects on glucose homeostasis may be species specific. The multiple metabolic actions of PPARα are summarized in Figure 1.
Figure 1.
Metabolic actions of PPARα and potential pathophysiological consequences. The main effects of PPARα overexpression or of PPARα ligands in mice (denoted by a single asterisk) and in humans (denoted by a double asterisk) are shown. GSIS, glucose-stimulated insulin secretion.
PPARα and atherosclerosis
The identification of PPARα expression in cell types of the atherosclerotic lesion has led to a thorough investigation of its modulatory role in this process. The gradual process of atherosclerotic lesion formation involves multiple cell types. Initiation occurs when large arteries are exposed to atherogenic stimuli, such as bacterial products, dyslipidemia, proinflammatory cytokines, or vasoconstrictor hormones such as angiotensin II. At this early stage, VCAM-1, activated through an NF-κB–dependent pathway, is believed to play a role in monocyte recruitment to nascent atherosclerotic lesions. Transmigration of monocytes and mast cells into the arterial intima, attracted by chemokines such as monocyte chemoattractant protein-1 (MCP-1) and IL-8, perturbs intercellular communications and promotes monocyte differentiation into macrophages and mast cell degranulation. Mast cells produce granule remnants rich in heparin proteoglycans, which interact with apoB-100, enhancing LDL retention. In this inflammatory milieu, SMCs migrate and proliferate, releasing MMPs that disrupt the ECM, exposing proteoglycans. Enhanced binding of lipoproteins to these proteoglycans favors their oxidation and glycation, perpetuating the inflammatory cycle. Macrophages, expressing scavenger receptors of the B class (CD36) and the A class (SR-A), internalize modified and oxidized LDL to form foam cells, which produce additional cytokines and growth factors. Following modified LDL uptake, cholesteryl esters (CEs) are continuously hydrolyzed by a CE hydrolase and re-esterified by acyl-CoA:cholesterol acyltransferase (ACAT). Eventually calcification occurs, particularly in patients on renal dialysis. In addition, apoptosis and necrosis of foam cells increase tissue factor, which can initiate thrombus formation. Formation of a necrotic core in the atherosclerotic plaque eventually progresses to plaque erosion and rupture, leading to clinical manifestations such as unstable angina, stroke, and myocardial infarction. However, when cholesterol acceptors are present in the extracellular fluid, RCT is initiated, and cholesterol flows out of cells, through the ABC transporters ABCA1 and ABCG1/G4, which may reverse the atherosclerotic lesion.
Clinical trials as well as in vitro data provide compelling evidence that PPARα acts as an antiatherogenic factor by interfering at multiple stages of the atherosclerosis process (Figure 2). Results from animal models have yielded conflicting results, which may be due to inherent differences in the models, and the species differences found in rodent and human metabolisms (74, 89–92). In the absence of inflammatory stimuli, PPARα may promote proatherogenic responses. The expression level of MCP-1 and IL-8 in endothelial cells is upregulated upon PPARα activation (93). In addition, PPARα ligands exert ROS-generating effects in unactivated macrophages (94). By contrast, fibrates increase Cu-Zn superoxide dismutase and decrease NADPH oxidase in endothelial cells, potentially decreasing LDL oxidation (95). This suggests that PPARα actions are distinct from inflammatory status, as demonstrated by MCP-1 upregulation in early atherosclerotic lesions and MCP-1 downregulation in late lesions during PPARα activation (90). Antiatherogenic effects can be attributed to PPARα-dependent repression of CRP-induced MCP-1 expression (96), inhibition of the expression of ET-1 (14), inhibition of IL-1–induced IL-6 release (15), and inhibition of LPS-induced VCAM-1 expression (16, 97, 98). Similarly, high IFN-γ serum levels are observed in atherosclerotic patients, and IFN-γ release by activated T lymphocytes is blunted by PPARα activators (99).
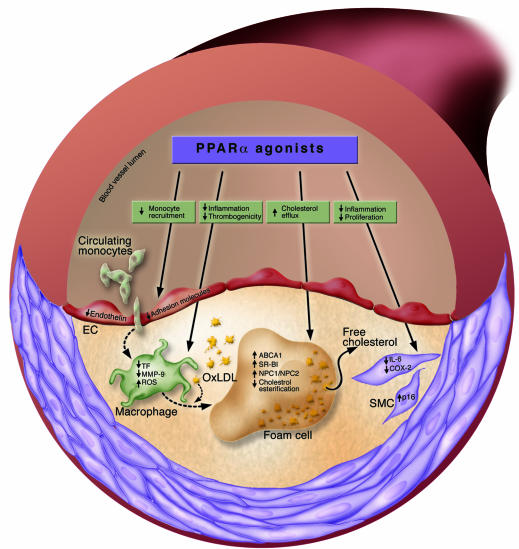
Figure 2.
PPARα and atherosclerosis. The effects of PPARα agonists in atherosclerosis are depicted for the most prominent cell type present in atherosclerotic lesions. NPC1 and 2, Niemann-Pick type C proteins 1 and 2; OxLDL, oxidized LDL; SR-BI, scavenger receptor BI; TF, tissue factor.
Furthermore, PPARα has a critical role in controlling the cholesterol cycle in macrophages. The expression of ABCA1 is stimulated by PPARα in foam cells in a liver X receptor–dependent manner, promoting apoA-I–mediated cholesterol efflux (62). The expression of Niemann-Pick type C proteins 1 and 2, transporters of cholesterol from lysosomes to the plasma membrane, is also regulated by PPARα, which promotes cholesterol availability for efflux (100). SR-BI, which plays a role in both the uptake of HDL-CE by the liver and cholesterol efflux from macrophages, is upregulated by PPARα ligands in macrophages (61), favoring cholesterol removal. Another parameter controlling macrophage cholesterol uptake is the availability and activity of released LPL. PPARα reduces LPL secretion and decreases macrophage uptake of glycated LDL (101). FAO is also induced in macrophages, as in liver. CPT-1 expression is upregulated by PPARα ligands, decreasing the FA pool available for cholesterol esterification (102).
Some of the later steps of atherosclerosis are also regulated by PPARα. Activation of SMC proliferation is a key event in atherosclerosis development and its complications. Upon vascular injury, SMCs migrate from the media into the neointimal layer of the vascular wall, where they proliferate and synthesize proteoglycans, leading to intimal hyperplasia. SMC proliferation is also one of the primary mechanisms underlying restenosis, an occlusive complication of corrective angioplasty procedures. PPARα inhibits SMC proliferation by blocking G1/S cell cycle transition, through the induction of the cyclin-dependent kinase inhibitor p16. This results in SMC growth inhibition and reduced neointima formation in a mouse model of carotid artery injury (103). The migration of SMCs requires the degradation of the extracellular matrix by MMPs. Among them, MMP-9 contributes significantly to SMC migration, and its expression is reduced by PPARα (104). Furthermore, by inhibiting the expression of tissue factor, a major procoagulant, PPARα may block atherothrombosis (105, 106).
PPARα modulates hepatic inflammation
Fibrates decrease the level of CRP, a major acute-phase protein stimulated by IL-1 and IL-6 and a risk factor for cardiovascular disease. Synthesized in the liver, PPARα ligands suppress CRP expression through an indirect transcriptional mechanism (19). The expression of fibrinogen-α and -β and of serum amyloid A is repressed in a similar fashion (107). Thus PPARα acts as an antiatherogenic factor by modulating local and systemic inflammatory responses, as well as lipid homeostasis in cell types that constitute the atherosclerotic plaque.
Animal models of PPARα action in atherosclerosis
Despite a wealth of evidence documenting antiatherogenic properties of PPARα ligands in vitro, mouse models have yielded contradictory results, which are furthermore difficult to extrapolate to human disease. First, mice are notoriously resistant to atherosclerosis, and only an aggressive diet rich in fat, cholesterol, and cholate and/or genetic manipulation, such as knockout of apoE or LDL receptor genes or knock-in of human apoE2, yields models that mimic some features of human dyslipidemia and atherosclerosis. Here again, the literature points to inconsistencies between effects of PPARα agonists and the phenotype of PPARα-deficient mice. In the apoE–/– background, PPARα deficiency was shown to protect mice from atherosclerosis, hinting at a proatherogenic role of PPARα (74). However, apoE–/– mice fed a Western diet developed atherosclerotic lesions that regressed moderately upon fenofibrate treatment, an effect accentuated in the apoE–/– strain expressing a human apoA-I transgene (89). In LDL receptor–deficient mice, another model of hypercholesterolemia, GW7647, a highly active PPARα agonist, strongly decreases lesion formation (90). Similarly, in the apoE2 knock-in mouse, a model of mixed dyslipidemia, fenofibrates also lower lesion size (92). While these few examples highlight the inequities of knockout studies and agonist treatment, they also allude to the distinct pharmacokinetic properties of PPARα ligands. Fibrates are known to act preferentially in the liver and are low-affinity ligands for PPARα, whereas high-affinity ligands such as Wy14,643 and GW7647 are suspected of acting more efficiently in peripheral tissues. These discrepancies may also arise from differences in mouse and human lipid metabolism. In mice, HDL is the main transporter of cholesterol, whereas LDL is the principal carrier in humans. In addition, interspecies variation of the mode of regulation of metabolic genes may be considerable, as mentioned for the apoA-I gene. It is also worth noting that PPARα has species-specific functional (39) and ligand-binding properties (108–110), so caution must be used in extrapolating data from the murine to the human situation.
Animal models are also used to identify PPARα-regulated pathologies other than atherosclerosis. For example, pretreatment of rats or mice with PPARα agonists protects the heart from reperfusion injury induced by coronary occlusion (111, 112). Cerebral ischemia is also a major cause of stroke, and preventive treatment by fibrates reduces the susceptibility of mice to stroke and decreases cerebral infarct size (113, 114). These studies point to a potential preventive application of PPARα ligands in such pathologies and, interestingly, expand the biological roles of PPARα to other organs.
Clinical consequences of PPARα activation
The actions of fibrates in humans have been tested in several clinical studies. Treatment with fibrates, such as fenofibrate, improves endothelial dysfunction in patients with type 2 diabetes (115). Evidence that PPARα signaling is critical in the progression of atherosclerotic lesion formation in humans is provided by coronary angiography in both nondiabetic and diabetic patients. A decreased atherosclerosis progression was observed with gemfibrozil in the Lopid Coronary Angiography Trial, with bezafibrate in the Bezafibrate Coronary Atherosclerosis Intervention Trial, and with fenofibrate in the Diabetes Atherosclerosis Intervention Study (116–118). More importantly, the influence of fibrate treatment on cardiovascular morbidity and mortality was studied in primary (Helsinki Heart Study; FIELD) and secondary (Bezafibrate Infarction Prevention; Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial; FIELD) prevention studies (88, 119–121). In the Helsinki Heart Study, cardiovascular disease risk reduction upon gemfibrozil treatment was most pronounced in overweight patients with metabolic syndrome or diabetes and atherogenic dyslipidemia (122, 123). In the Bezafibrate Infarction Prevention trial, reduction in coronary events with bezafibrate was observed only in patients with serum TG concentrations greater than 200 mg/dl (119), whereas the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial showed the most significant benefits of gemfibrozil in diabetics or in nondiabetics with high insulin levels (124). Altogether, these observations indicate that fibrates are particularly useful to treat the cardiovascular risk in insulin-resistant prediabetic individuals, and in diabetic patients with dyslipidemia. The most recent results come from the FIELD study (88), a combined primary and secondary prevention study testing the effects of fenofibrate on coronary heart disease in 9,795 type 2 diabetes patients who had no indication for lipid-lowering therapy. The primary endpoints, death from coronary heart disease or nonfatal myocardial infarction, were decreased, although not significantly, by 11% (mean follow-up, 5 years). However, during the course of the study, there was a gradual increase in statin use, which was greater in the placebo group than in the fenofibrate group. Since statins can decrease cardiovascular risk in type 2 diabetic patients (125), the actual benefit of fenofibrate thus may be underestimated because of the higher use of statins in the placebo arm. After correction for the statin effect, it was estimated that fenofibrate treatment resulted in a 19% reduction of relative risk of the primary endpoints. The benefits of fenofibrate were mainly due to reductions in nonfatal myocardial infarction and coronary revascularization. Moreover, fenofibrate treatment reduced microvascular complications (such as progression to microalbuminuria and intervention for retinopathy); this was not explained by changes in blood glucose control. Finally, the FIELD trial does not suggest that there are safety issues associated with fenofibrate-statin combination therapy. The major unanswered question is whether fenofibrate treatment confers additional benefit when given on top of a statin. This issue will be addressed in the ongoing Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, the results of which are expected in early 2010 (S21).
Conclusion
The use of the PPARα agonists, fibrates, as hypolipidemic agents for several decades has demonstrated their safety and efficacy for lipid lowering, an important parameter in the prevention of cardiovascular diseases. Moreover, increasing evidence attributing antiinflammatory activities to PPARα emerges, documented largely in in vitro and animal studies. Prediabetic metabolic syndrome patients with atherogenic dyslipidemia (inflammation, low HDL, high TG, and sdLDL) are highly susceptible to cardiovascular morbidity and respond extremely well to fibrate treatment. In type 2 diabetics, fibrate treatment was recently demonstrated to reduce nonfatal myocardial infarction and coronary revascularization, suggesting beneficial effects of these drugs in these patients. Whether activation of PPARα has detrimental effects in the hearts of diabetic patients, as observed after PPARα overexpression in mouse cardiomyocytes, is unclear. However, at present, there is no indication that fibrate treatment would increase chronic heart insufficiency in humans; this points to possibly distinct responses of humans versus mice to chronic stimulation of cardiac metabolism by PPARα activators. This view is strengthened by the fact that PPARα activators exert species-specific activities and may induce peroxisome proliferation in mouse hearts, which could increase oxidative stress. Additionally, these species-specific responses are illustrated by the observation that fibrate treatment does not perturb glucose homeostasis in humans, although a negative effect could have been predicted from mouse data showing that PPARα overexpression in skeletal muscle provokes insulin resistance. PPARα activators appear to be particularly indicated to treat dyslipidemia of the metabolic syndrome and type 2 diabetes, although adverse effects on cardiac and skeletal muscle should be monitored in the development of novel, more potent PPARα activators. Promising future developments undoubtedly lie in the field of selective PPARα modulators (SPPARMs).
Footnotes
Nonstandard abbreviations used: ABCA1, ABC transporter A1; AF, activating function; CE, cholesteryl ester; CPT-1, carnitine palmitoyl transferase 1; CRP, C-reactive protein; FA, fatty acid; FAO, FA oxidation; FIELD, Fenofibrate Intervention and Event Lowering in Diabetes; LBD, ligand-binding domain; LPL, lipoprotein lipase; MCP-1, monocyte chemoattractant protein-1; NR, nuclear receptor; PPRE, PPAR response element; RCT, reverse cholesterol transport; RXR, retinoid X receptor; sdLDL, small dense LDL; SR, scavenger receptor; TG, triglyceride.
Conflict of interest: The authors have declared that no conflict of interest exists.
Note: References S1-S21 are available online with this article; doi:10.1172/JCI27989DS1.
References
- 1.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 2.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehmann JM, et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPAR γ) J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 4.Fu J, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPARα. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 5.Devchand PR, et al. The PPARα-leukotriene B-4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 6.Sumanasekera WK, et al. Heat shock protein-90 (hsp90) acts as a repressor of peroxisome proliferator-activated receptor α and PPARγ activity. Biochemistry. 2003;42:10726–10735. doi: 10.1021/bi0347353. [DOI] [PubMed] [Google Scholar]
- 7.Hi R, Osada S, Yumoto N, Osumi T. Characterization of the amino-terminal activation domain of peroxisome proliferator-activated receptor α. Importance of α-helical structure in the transactivating function. J. Biol. Chem. 1999;274:35152–35158. doi: 10.1074/jbc.274.49.35152. [DOI] [PubMed] [Google Scholar]
- 8.Wan YJY, et al. Peroxisome proliferator-activated receptor α-mediated pathways are altered in hepatocyte-specific retinoid X receptor α-deficient mice. J. Biol. Chem. 2000;275:28285–28290. doi: 10.1074/jbc.M000934200. [DOI] [PubMed] [Google Scholar]
- 9.Ijpenberg A, et al. In vivo activation of PPAR target genes by RXR homodimers. EMBO J. 2004;23:2083–2091. doi: 10.1038/sj.emboj.7600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronet P, et al. Structure of the PPARα and PPARγ ligand binding domain in complex with AZ 242; ligand selectivity and agonist activation in the PPAR family. Structure. 2001;9:699–706. doi: 10.1016/s0969-2126(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 11.Xu HE, et al. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPAR α. Nature. 2002;415:813–817. doi: 10.1038/415813a. [DOI] [PubMed] [Google Scholar]
- 12.Duez H, et al. Regulation of human ApoA-I by gemfibrozil and fenofibrate through selective peroxisome proliferator-activated receptor α modulation. Arterioscler. Thromb. Vasc. Biol. 2005;25:585–591. doi: 10.1161/01.ATV.0000154140.73570.00. [DOI] [PubMed] [Google Scholar]
- 13.Qi C, et al. Mouse steroid receptor coactivator-1 is not essential for peroxisome proliferator-activated receptor α-regulated gene expression. Proc. Natl. Acad. Sci. U. S. A. 1999;96:1585–1590. doi: 10.1073/pnas.96.4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delerive P, et al. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ. Res. 1999;85:394–402. doi: 10.1161/01.res.85.5.394. [DOI] [PubMed] [Google Scholar]
- 15.Staels B, et al. Activation of human aortic smooth-muscle cells is inhibited by PPARα but not by PPARγ activators. Nature. 1998;393:790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- 16.Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. PPARα activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999;99:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delerive P, Gervois P, Fruchart JC, Staels B. Induction of Ikappa-Bα expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-α activators. J. Biol. Chem. 2000;275:36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 18.Delerive P, et al. DNA binding-independent induction of IkappaBα gene transcription by PPARα. Mol. Endocrinol. 2002;16:1029–1039. doi: 10.1210/mend.16.5.0826. [DOI] [PubMed] [Google Scholar]
- 19.Kleemann R, et al. Fibrates down-regulate IL-1-stimulated C-reactive protein gene expression in hepatocytes by reducing nuclear p50-NFkappa B-C/EBP-β complex formation. Blood. 2003;101:545–551. doi: 10.1182/blood-2002-06-1762. [DOI] [PubMed] [Google Scholar]
- 20.Gervois P, et al. Negative regulation of human fibrinogen gene expression by peroxisome proliferator-activated receptor α agonists via inhibition of CCAAT Box/Enhancer-binding protein β. J. Biol. Chem. 2001;276:33471–33477. doi: 10.1074/jbc.M102839200. [DOI] [PubMed] [Google Scholar]
- 21.Gervois P, et al. Global suppression of IL-6-induced acute phase response gene expression after chronic in vivo treatment with the peroxisome proliferator-activated receptor-α activator fenofibrate. J. Biol. Chem. 2004;279:16154–16160. doi: 10.1074/jbc.M400346200. [DOI] [PubMed] [Google Scholar]
- 22.Blanquart C, et al. The protein kinase C signaling pathway regulates a molecular switch between transactivation and transrepression activity of the peroxisome proliferator-activated receptor α. Mol. Endocrinol. 2004;18:1906–1918. doi: 10.1210/me.2003-0327. [DOI] [PubMed] [Google Scholar]
- 23.Lemberger T, et al. Expression of the peroxisome proliferator-activated receptor α gene is stimulated by stress and follows a diurnal rhythm. J. Biol. Chem. 1996;271:1764–1769. doi: 10.1074/jbc.271.3.1764. [DOI] [PubMed] [Google Scholar]
- 24.Steineger HH, et al. Dexamethasone and insulin demonstrate marked and opposite regulation of the steady-state mRNA level of the peroxisomal proliferator-activated receptor (PPAR) in hepatic cells. Hormonal modulation of fatty-acid-induced transcription. Eur. J. Biochem. 1994;225:967–974. doi: 10.1111/j.1432-1033.1994.0967b.x. [DOI] [PubMed] [Google Scholar]
- 25.Lemberger T, et al. Regulation of the peroxisome proliferator-activated receptor α gene by glucocorticoids. J. Biol. Chem. 1994;269:24527–24530. [PubMed] [Google Scholar]
- 26.Oishi K, Shirai H, Ishida N. CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor α (PPARα) in mice. Biochem. J. 2005;386:575–581. doi: 10.1042/BJ20041150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor α (PPARα) in the cellular fasting response: the PPARα-null mouse as a model of fatty acid oxidation disorders. Proc. Natl. Acad. Sci. U. S. A. 1999;96:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kersten S, et al. Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J. Clin. Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou YT, et al. Role of peroxisome proliferator-activated receptor α in disease of pancreatic β cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:8898–8903. doi: 10.1073/pnas.95.15.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jalouli M, et al. Sex difference in hepatic peroxisome proliferator-activated receptor α expression: influence of pituitary and gonadal hormones. Endocrinology. 2003;144:101–109. doi: 10.1210/en.2002-220630. [DOI] [PubMed] [Google Scholar]
- 31.Blanquart C, Barbier O, Fruchart JC, Staels B, Glineur C. Peroxisome proliferator-activated receptor α (PPARα) turnover by the ubiquitin-proteasome system controls the ligand-induced expression level of its target genes. J. Biol. Chem. 2002;277:37254–37259. doi: 10.1074/jbc.M110598200. [DOI] [PubMed] [Google Scholar]
- 32.Pineda Torra I, Jamshidi Y, Flavell DM, Fruchart JC, Staels B. Characterization of the human PPARα promoter: identification of a functional nuclear receptor response element. Mol. Endocrinol. 2002;16:1013–1028. doi: 10.1210/mend.16.5.0833. [DOI] [PubMed] [Google Scholar]
- 33.Roduit R, et al. Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-α gene in the pancreatic β-cell. J. Biol. Chem. 2000;275:35799–35806. doi: 10.1074/jbc.M006001200. [DOI] [PubMed] [Google Scholar]
- 34.Barger PM, Browning AC, Garner AN, Kelly DP. p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor α. A potential role in the cardiac metabolic stress response. J. Biol. Chem. 2001;276:44495–44501. doi: 10.1074/jbc.M105945200. [DOI] [PubMed] [Google Scholar]
- 35.Kliewer SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hostetler HA, Petrescu AD, Kier AB, Schroeder F. Peroxisome proliferator-activated receptor α interacts with high affinity and is conformationally responsive to endogenous ligands. J. Biol. Chem. 2005;280:18667–18682. doi: 10.1074/jbc.M412062200. [DOI] [PubMed] [Google Scholar]
- 37.Xu HE, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol. Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 38.Chakravarthy MV, et al. “New” hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Cheung C, et al. Diminished hepatocellular proliferation in mice humanized for the nuclear receptor peroxisome proliferator-activated receptor α. Cancer Res. 2004;64:3849–3854. doi: 10.1158/0008-5472.CAN-04-0322. [DOI] [PubMed] [Google Scholar]
- 40.Aoyama T, et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα) J. Biol. Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 41.Lee SS, et al. Targeted disruption of the α isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters JM, et al. Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor α-deficient mice. J. Biol. Chem. 1997;272:27307–27312. doi: 10.1074/jbc.272.43.27307. [DOI] [PubMed] [Google Scholar]
- 43.Djouadi F, et al. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor α-deficient mice. J. Clin. Invest. 1998;102:1083–1091. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandt JM, Djouadi F, Kelly DP. Fatty acids activate transcription of the muscle carnitine palmitoyltransferase I gene in cardiac myocytes via the peroxisome proliferator-activated receptor α. J. Biol. Chem. 1998;273:23786–23792. doi: 10.1074/jbc.273.37.23786. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez JC, Gil-Gomez G, Hegardt FG, Haro D. Peroxisome proliferator-activated receptor mediates induction of the mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene by fatty acids. J. Biol. Chem. 1994;269:18767–18772. [PubMed] [Google Scholar]
- 46.Martin G, Schoonjans K, Lefebvre AM, Staels B, Auwerx J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. J. Biol. Chem. 1997;272:28210–28217. doi: 10.1074/jbc.272.45.28210. [DOI] [PubMed] [Google Scholar]
- 47.Palmer CN, Hsu MH, Griffin KJ, Raucy JL, Johnson EF. Peroxisome proliferator activated receptor-α expression in human liver. Mol. Pharmacol. 1998;53:14–22. [PubMed] [Google Scholar]
- 48.Gervois P, et al. A truncated human peroxisome proliferator-activated receptor α splice variant with dominant negative activity. Mol. Endocrinol. 1999;13:1535–1549. doi: 10.1210/mend.13.9.0341. [DOI] [PubMed] [Google Scholar]
- 49.Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor α and γ activators in a tissue- and inducer-specific manner. J. Biol. Chem. 1998;273:16710–16714. doi: 10.1074/jbc.273.27.16710. [DOI] [PubMed] [Google Scholar]
- 50.Lee SS, et al. Targeted disruption of the α isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finck BN, et al. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J. Clin. Invest. 2002;109:121–130. doi:10.1172/JCI200214080. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilde AJ, et al. Peroxisome proliferator-activated receptor (PPAR) α and PPARβ/δ, but not PPARγ, modulate the expression of genes involved in cardiac lipid metabolism. Circ. Res. 2003;92:518–524. doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- 53.Park SY, et al. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-α causes insulin resistance in heart and liver. Diabetes. 2005;54:2514–2524. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 54.Barger PM, Brandt JM, Leone TC, Weinheimer CJ, Kelly DP. Deactivation of peroxisome proliferator-activated receptor-α during cardiac hypertrophic growth. J. Clin. Invest. 2000;105:1723–1730. doi: 10.1172/JCI9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finck BN, et al. A critical role for PPARα-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc. Natl. Acad. Sci. U. S. A. 2003;100:1226–1231. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vikramadithyan RK, et al. Peroxisome proliferator-activated receptor agonists modulate heart function in transgenic mice with lipotoxic cardiomyopathy. J. Pharmacol. Exp. Ther. 2005;313:586–593. doi: 10.1124/jpet.104.080259. [DOI] [PubMed] [Google Scholar]
- 57.Schoonjans K, et al. PPAR α and PPAR γ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 58.Staels B, et al. Fibrates downregulate apolipoprotein C-III expression independent of induction of peroxisomal acyl coenzyme A oxidase. A potential mechanism for the hypolipidemic action of fibrates. J. Clin. Invest. 1995;95:705–712. doi: 10.1172/JCI117717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vu-Dac N, et al. Apolipoprotein A5, a crucial determinant of plasma TG levels, is highly responsive to peroxisome proliferator-activated receptor α activators. J. Biol. Chem. 2003;278:17982–17985. doi: 10.1074/jbc.M212191200. [DOI] [PubMed] [Google Scholar]
- 60.Eriksson M, Carlson LA, Miettinen TA, Angelin B. Stimulation of fecal steroid excretion after infusion of recombinant proapolipoprotein A-I. Potential reverse cholesterol transport in humans. Circulation. 1999;100:594–598. doi: 10.1161/01.cir.100.6.594. [DOI] [PubMed] [Google Scholar]
- 61.Chinetti G, et al. CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors. Circulation. 2000;101:2411–2417. doi: 10.1161/01.cir.101.20.2411. [DOI] [PubMed] [Google Scholar]
- 62.Chinetti G, et al. PPAR-α and PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 63.Hennuyer N, et al. Beneficial effects of fibrates on apolipoprotein A-I metabolism occur independently of any peroxisome proliferative response. Circulation. 1999;99:2445–2451. doi: 10.1161/01.cir.99.18.2445. [DOI] [PubMed] [Google Scholar]
- 64.Vu-Dac N, et al. Negative regulation of the human apolipoprotein A-I promoter by fibrates can be attenuated by the interaction of the peroxisome proliferator-activated receptor with its response element. J. Biol. Chem. 1994;269:31012–31018. [PubMed] [Google Scholar]
- 65.Watts GF, et al. Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes. 2003;52:803–811. doi: 10.2337/diabetes.52.3.803. [DOI] [PubMed] [Google Scholar]
- 66.Vu-Dac N, et al. Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. J. Clin. Invest. 1995;96:741–750. doi: 10.1172/JCI118118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vu-Dac N, et al. The nuclear receptors peroxisome proliferator-activated receptor α and Rev-erbα mediate the species-specific regulation of apolipoprotein A-I expression by fibrates. J. Biol. Chem. 1998;273:25713–25720. doi: 10.1074/jbc.273.40.25713. [DOI] [PubMed] [Google Scholar]
- 68.Bandsma RHJ, et al. Hepatic de-novo synthesis of glucose 6-phosphate is not affected in peroxisome proliferator-activated receptor α-deficient mice but is preferentially directed toward hepatic glycogen stores after a short term fast. J. Biol. Chem. 2004;279:8930–8937. doi: 10.1074/jbc.M310067200. [DOI] [PubMed] [Google Scholar]
- 69.Xu J, et al. Peroxisome proliferator-activated receptor α (PPARα) influences substrate utilization for hepatic glucose production. J. Biol. Chem. 2002;277:50237–50244. doi: 10.1074/jbc.M201208200. [DOI] [PubMed] [Google Scholar]
- 70.Patsouris D, et al. PPARα governs glycerol metabolism. J. Clin. Invest. 2004;114:94–103. doi:10.1172/JCI200420468. doi: 10.1172/JCI20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koo SH, et al. PGC-1 promotes insulin resistance in liver through PPAR-α-dependent induction of TRB-3. Nat. Med. 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 72.Bernal-Mizrachi C, et al. Dexamethasone induction of hypertension and diabetes is PPAR-α dependent in LDL receptor-null mice. Nat. Med. 2003;9:1069–1075. doi: 10.1038/nm898. [DOI] [PubMed] [Google Scholar]
- 73.Guerre-Millo M, et al. PPARα-null mice are protected from high-fat diet-induced insulin resistance. Diabetes. 2001;50:2809–2814. doi: 10.2337/diabetes.50.12.2809. [DOI] [PubMed] [Google Scholar]
- 74.Tordjman K, et al. PPARα deficiency reduces insulin resistance and atherosclerosis in ApoE-null mice. J. Clin. Invest. 2001;107:1025–1034. doi: 10.1172/JCI11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gremlich S, et al. Pancreatic islet adaptation to fasting is dependent on peroxisome proliferator-activated receptor α transcriptional up-regulation of fatty acid oxidation. Endocrinology. 2005;146:375–382. doi: 10.1210/en.2004-0667. [DOI] [PubMed] [Google Scholar]
- 76.Guerre-Millo M, et al. Peroxisome proliferator-activated receptor α activators improve insulin sensitivity and reduce adiposity. J. Biol. Chem. 2000;275:16638–16642. doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- 77.Chou CJ, et al. WY14,643, a peroxisome proliferator-activated receptor α (PPARα) agonist, improves hepatic and muscle steatosis and reverses insulin resistance in lipoatrophic A-ZIP/F-1 mice. J. Biol. Chem. 2002;277:24484–24489. doi: 10.1074/jbc.M202449200. [DOI] [PubMed] [Google Scholar]
- 78.Kim H, et al. Peroxisome proliferator-activated receptor-α agonist treatment in a transgenic model of type 2 diabetes reverses the lipotoxic state and improves glucose homeostasis. Diabetes. 2003;52:1770–1778. doi: 10.2337/diabetes.52.7.1770. [DOI] [PubMed] [Google Scholar]
- 79.Schafer SA, Hansen BC, Volkl A, Fahimi HD, Pill J. Biochemical and morphological effects of K-111, a peroxisome proliferator-activated receptor (PPAR)-α activator, in non-human primates. Biochem. Pharmacol. 2004;68:239–251. doi: 10.1016/j.bcp.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 80.Ye JM, et al. Peroxisome proliferator–activated receptor (PPAR)-α activation lowers muscle lipids and improves insulin sensitivity in high fat–fed rats: comparison with PPAR-γ activation. Diabetes. 2001;50:411–417. doi: 10.2337/diabetes.50.2.411. [DOI] [PubMed] [Google Scholar]
- 81.Ravnskjaer K, et al. Peroxisome proliferator-activated receptor α (PPARα) potentiates, whereas PPARγ attenuates, glucose-stimulated insulin secretion in pancreatic β-cells. Endocrinology. 2005;146:3266–3276. doi: 10.1210/en.2004-1430. [DOI] [PubMed] [Google Scholar]
- 82.Muoio DM, et al. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) α knock-out mice. Evidence for compensatory regulation by PPAR δ. J. Biol. Chem. 2002;277:26089–26097. doi: 10.1074/jbc.M203997200. [DOI] [PubMed] [Google Scholar]
- 83.Finck BN, et al. A potential link between muscle peroxisome proliferator-activated receptor-α signaling and obesity-related diabetes. Cell Metab. 2005;1:133–144. doi: 10.1016/j.cmet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 84.Steiner G. Altering TG concentrations changes insulin-glucose relationships in hypertriglyceridemic patients. Double-blind study with gemfibrozil with implications for atherosclerosis. Diabetes Care. 1991;14:1077–1081. doi: 10.2337/diacare.14.11.1077. [DOI] [PubMed] [Google Scholar]
- 85.Ferrari C, et al. Effects of short-term clofibrate administration on glucose tolerance and insulin secretion in patients with chemical diabetes or hypertriglyceridemia. Metabolism. 1977;26:129–139. doi: 10.1016/0026-0495(77)90048-8. [DOI] [PubMed] [Google Scholar]
- 86.Murakami K, et al. Clofibrate enhances the affinity of insulin receptors in non-insulin dependent diabetes mellitus. Br. J. Clin. Pharmacol. 1984;17:89–91. doi: 10.1111/j.1365-2125.1984.tb05005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kobayashi M, et al. Improvement of glucose tolerance in NIDDM by clofibrate. Randomized double-blind study. Diabetes Care. 1988;11:495–499. doi: 10.2337/diacare.11.6.495. [DOI] [PubMed] [Google Scholar]
- 88.Keech A, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 89.Duez H, et al. Reduction of atherosclerosis by the peroxisome proliferator-activated receptor α agonist fenofibrate in mice. J. Biol. Chem. 2002;277:48051–48057. doi: 10.1074/jbc.M206966200. [DOI] [PubMed] [Google Scholar]
- 90.Li AC, et al. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARα, β/δ, and γ. J. Clin. Invest. 2004;114:1564–1576. doi:10.1172/JCI200418730. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fu T, Mukhopadhyay D, Davidson NO, Borensztajn J. The peroxisome proliferator-activated receptor α (PPARα) agonist ciprofibrate inhibits apolipoprotein B mRNA editing in low density lipoprotein receptor-deficient mice: effects on plasma lipoproteins and the development of atherosclerotic lesions. J. Biol. Chem. 2004;279:28662–28669. doi: 10.1074/jbc.M403271200. [DOI] [PubMed] [Google Scholar]
- 92.Hennuyer N, et al. PPARα, but not PPARγ, activators decrease macrophage-laden atherosclerotic lesions in a nondiabetic mouse model of mixed dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2005;25:1897–1902. doi: 10.1161/01.ATV.0000175756.56818.ee. [DOI] [PubMed] [Google Scholar]
- 93.Lee H, et al. Role for peroxisome proliferator-activated receptor α in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ. Res. 2000;87:516–521. doi: 10.1161/01.res.87.6.516. [DOI] [PubMed] [Google Scholar]
- 94.Teissier E, et al. Peroxisome proliferator-activated receptor α induces NADPH oxidase activity in macrophages, leading to the generation of LDL with PPAR-α activation properties. Circ. Res. 2004;95:1174–1182. doi: 10.1161/01.RES.0000150594.95988.45. [DOI] [PubMed] [Google Scholar]
- 95.Inoue I, et al. The ligands/activators for peroxisome proliferator-activated receptor α (PPARα) and PPARγ increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism. 2001;50:3–11. doi: 10.1053/meta.2001.19415. [DOI] [PubMed] [Google Scholar]
- 96.Pasceri V, Cheng JS, Willerson JT, Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103:2531–2534. doi: 10.1161/01.cir.103.21.2531. [DOI] [PubMed] [Google Scholar]
- 97.Jackson SM, et al. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler. Thromb. Vasc. Biol. 1999;19:2094–2104. doi: 10.1161/01.atv.19.9.2094. [DOI] [PubMed] [Google Scholar]
- 98.Rival Y, et al. PPARα and PPAR&Dgr; activators inhibit cytokine-induced nuclear translocation of NF-kappaB and expression of VCAM-1 in EAhy926 endothelial cells. Eur. J. Pharmacol. 2002;435:143–151. doi: 10.1016/s0014-2999(01)01589-8. [DOI] [PubMed] [Google Scholar]
- 99.Marx N, et al. PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ. Res. 2002;90:703–710. doi: 10.1161/01.res.0000014225.20727.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chinetti-Gbaguidi G, et al. Peroxisome proliferator-activated receptor α controls cellular cholesterol trafficking in macrophages. J. Lipid Res. 2005;46:2717–2725. doi: 10.1194/jlr.M500326-JLR200. [DOI] [PubMed] [Google Scholar]
- 101.Gbaguidi FG, et al. Peroxisome proliferator-activated receptor (PPAR) agonists decrease lipoprotein lipase secretion and glycated LDL uptake by human macrophages. FEBS Lett. 2002;512:85–90. doi: 10.1016/s0014-5793(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 102.Chinetti G, Lestavel S, Fruchart JC, Clavey V, Staels B. Peroxisome proliferator-activated receptor α reduces cholesterol esterification in macrophages. Circ. Res. 2003;92:212–217. doi: 10.1161/01.res.0000053386.46813.e9. [DOI] [PubMed] [Google Scholar]
- 103.Gizard F, et al. PPARα inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16. J. Clin. Invest. 2005;115:3228–3238. doi:10.1172/JCI22756. doi: 10.1172/JCI22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shu H, et al. Activation of PPARα or γ reduces secretion of matrix metalloproteinase 9 but not interleukin 8 from human monocytic THP-1 cells. Biochem. Biophys. Res. Commun. 2000;267:345–349. doi: 10.1006/bbrc.1999.1968. [DOI] [PubMed] [Google Scholar]
- 105.Marx N, et al. PPARα activators inhibit tissue factor expression and activity in human monocytes. Circulation. 2001;103:213–219. doi: 10.1161/01.cir.103.2.213. [DOI] [PubMed] [Google Scholar]
- 106.Neve BP, et al. PPARα agonists inhibit tissue factor expression in human monocytes and macrophages. Circulation. 2001;103:207–212. doi: 10.1161/01.cir.103.2.207. [DOI] [PubMed] [Google Scholar]
- 107.Gervois P, et al. Negative regulation of human fibrinogen gene expression by peroxisome proliferator-activated receptor α agonists via inhibition of CCAAT box/enhancer-binding protein β. J. Biol. Chem. 2001;276:33471–33477. doi: 10.1074/jbc.M102839200. [DOI] [PubMed] [Google Scholar]
- 108.Nagasawa M, et al. Pharmacological characterization of a human-specific peroxisome proliferator-activated receptor α (PPARα) agonist in dogs. Biochem. Pharmacol. 2004;67:2057–2069. doi: 10.1016/j.bcp.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 109.Mukherjee R, Jow L, Noonan D, McDonnell DP. Human and rat peroxisome proliferator activated receptors (PPARs) demonstrate similar tissue distribution but different responsiveness to PPAR activators. J. Steroid Biochem. Mol. Biol. 1994;51:157–166. doi: 10.1016/0960-0760(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 110.Keller H, Devchand PR, Perroud M, Wahli W. PPARα structure-function relationships derived from species-specific differences in responsiveness to hypolipidemic agents. Biol. Chem. 1997;378:651–655. doi: 10.1515/bchm.1997.378.7.651. [DOI] [PubMed] [Google Scholar]
- 111.Yue T-L, et al. Activation of peroxisome proliferator-activated receptor α protects the heart from ischemia/reperfusion injury. Circulation. 2003;108:2393–2399. doi: 10.1161/01.CIR.0000093187.42015.6C. [DOI] [PubMed] [Google Scholar]
- 112.Wayman NS, et al. Ligands of the peroxisome proliferator-activated receptors (PPARγ and PPARα) reduce myocardial infarct size. FASEB J. 2002;16:1027–1040. doi: 10.1096/fj.01-0793com. [DOI] [PubMed] [Google Scholar]
- 113.Deplanque D, et al. Peroxisome proliferator-activated receptor α activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J. Neurosci. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Inoue H, et al. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor α in mice. Neurosci. Lett. 2003;352:203–206. doi: 10.1016/j.neulet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 115.Playford DA, Watts GF, Best JD, Burke V. Effect of fenofibrate on brachial artery flow-mediated dilatation in type 2 diabetes mellitus. Am. J. Cardiol. 2002;90:1254–1257. doi: 10.1016/s0002-9149(02)02847-3. [DOI] [PubMed] [Google Scholar]
- 116.Frick MH, et al. Prevention of the angiographic progression of coronary and vein-graft atherosclerosis by gemfibrozil after coronary bypass surgery in men with low levels of HDL cholesterol. Lopid Coronary Angiography Trial (LOCAT) Study Group. Circulation. 1997;96:2137–2143. doi: 10.1161/01.cir.96.7.2137. [DOI] [PubMed] [Google Scholar]
- 117.Ruotolo G, et al. Treatment effects on serum lipoprotein lipids, apolipoproteins and low density lipoprotein particle size and relationships of lipoprotein variables to progression of coronary artery disease in the Bezafibrate Coronary Atherosclerosis Intervention Trial (BECAIT) J. Am. Coll. Cardiol. 1998;32:1648–1656. doi: 10.1016/s0735-1097(98)00442-2. [DOI] [PubMed] [Google Scholar]
- 118.[Anonymous]. Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001;357:905–910. [PubMed] [Google Scholar]
- 119.[Anonymous]. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000;102:21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 120.Frick MH, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N. Engl. J. Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 121.Rubins HB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N. Engl. J. Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 122.Manninen V, et al. Joint effects of serum TG and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation. 1992;85:37–45. doi: 10.1161/01.cir.85.1.37. [DOI] [PubMed] [Google Scholar]
- 123.Tenkanen L, Manttari M, Manninen V. Some coronary risk factors related to the insulin resistance syndrome and treatment with gemfibrozil. Experience from the Helsinki Heart Study. Circulation. 1995;92:1779–1785. doi: 10.1161/01.cir.92.7.1779. [DOI] [PubMed] [Google Scholar]
- 124.Robins SJ, et al. Insulin resistance and cardiovascular events with low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT) Diabetes Care. 2003;26:1513–1517. doi: 10.2337/diacare.26.5.1513. [DOI] [PubMed] [Google Scholar]
- 125.Colhoun HM, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomized placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]