Abstract
The relative importance of the anterior cingulate cortex (ACC) for the detection and resolution of response conflicts versus its role in error monitoring remains under debate. One disputed issue is whether conflict detection and error monitoring can be viewed as unitary functions performed by the same region of the ACC, or whether these processes can be dissociated functionally and anatomically. We used a combination of electrophysiological and neuropsychological methods to assess these competing hypotheses. A neurological patient with a rare focal lesion of rostral-to-middorsal ACC was tested in an event-related potential study designed to track the time course of neural activity during conflicts and erroneous responses. Compared with controls, the error-related negativity component after incorrect responses was attenuated in the patient, accompanied by lower error-correction rates. Conversely, the stimulus-locked component on correct conflict trials, the N450, was enhanced, and behavioral performance was impaired. We hypothesize that intact regions of lateral prefrontal cortex were able to detect response conflict, but damage to the dorsal ACC impaired response inhibition, which may be due to disconnection from cingulate and supplementary motor areas. The results implicate rostral-dorsal ACC in error monitoring and suggest this function can be dissociated from conflict-detection processes.
The anterior cingulate cortex (ACC) has been proposed to be an integral part of the brain's anterior attention system (1). Although often treated as a homogeneous structure, converging evidence indicates that the ACC exhibits functional specialization, with subdivisions involved in visceromotor and skeletomotor control, vocalization, and pain as well as attention (2). The rostral/ventral division has been linked to emotion, and the more dorsal/caudal region has been associated with cognition and higher-order motor control (3, 4). The motor-control processes demonstrate a further degree of specificity, showing a somatotopic organization based on response modality (5–8).
Nevertheless, the higher cognitive functions of the ACC are still a matter of intense debate. Detecting conflict between competing response alternatives and monitoring for errors in performance have been proposed as two of its roles. Event-related functional MRI (fMRI) experiments have demonstrated enhanced activity in the rostral ACC during the commission of errors compared with correct trials (9, 10). Neuroimaging studies have also observed increased activity in the dorsal ACC during a variety of cognitive tasks that induce response conflict including divided attention (11), flanker interference (12, 13), and the Stroop color-word interference task (14–17). In the Stroop task, subjects name the printed color used to display color names or neutral stimuli (18). In the incongruent condition (e.g., “green” written in a blue font), the automatic tendency to read the word must be suppressed, which requires attentional control over behavior.
Controversy swirls over whether these processes can be viewed as unitary (15, 19) or distinct (20, 21) functions of the ACC. Models postulating that error monitoring can be viewed as separate from conflict monitoring are based on data obtained from electrophysiological recordings (event-related brain potentials or ERPs). An ERP component called the error-related negativity (ERN) is generated when subjects make errors in speeded reaction-time (RT) tasks (22, 23). It has been assumed to originate in the ACC (24, 25), although direct confirmation of this idea has not been provided yet. The ERN is an on-line index of performance monitoring that may be independent of response conflict, because it is observed in simple choice-reaction tasks without response competition (20). In contrast, the conflict-monitoring hypothesis suggests that the primary function of the ACC is to detect response conflict, whereas the dorsolateral prefrontal cortex (PFC) implements top-down control of behavior (26). An event-related fMRI study reported that dorsal ACC showed increases in activity during error trials as well as correct trials with higher levels of conflict (19). Error processing is subsumed under the rubric of conflict monitoring in this model (19), because errors are more likely to occur when competing response tendencies must be resolved.
However, the sluggishness of the hemodynamic signal does not permit fMRI to provide evidence about when the ACC is active relative to the stimulus and the response. Thus, ERP recordings may provide further insight into the temporal dynamics of ACC activity during error and conflict monitoring. Examining ERP components in neurological patients with well defined lesions allows one to draw inferences about the location of critical neural generators (27). The current case study presents a distinctive patient, R.N., who has a unilateral lesion restricted to the left dorsal ACC region implicated in neuroimaging studies of conflict monitoring and executive control, with some extension into the rostral ACC area implicated in error detection (Figs. 1 and 2). Given the pervasiveness of ACC activations in neuroimaging studies, it is crucial to determine the conditions in which the ACC is essential for cognitive performance. There have been few reports of focal ACC lesions, most of which tested patients who underwent cingulotomy (29–31), a psychosurgical procedure occasionally performed on individuals with psychiatric disorders or chronic pain. By contrast, the focal ACC damage in R.N. was due to stroke.
Fig 1.
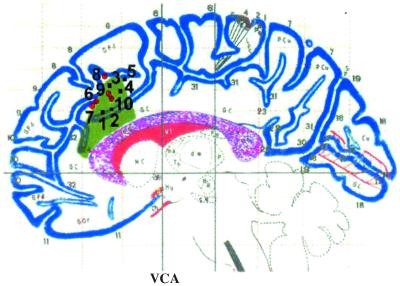
ACC lesion in patient R.N. overlaps with activation foci from neuroimaging studies of conflict detection. A sagittal view of the lesion is represented in green shading on the Talairach and Tournoux coordinate system (28). Peaks of activation from eight neuroimaging studies of response conflict are plotted inside or within close proximity of the region of damaged ACC. All activation foci are located in the left hemisphere or within 5 mm of the midline. Vocal or covert vocal responses in the Stroop task are depicted by black squares (1–5), and manual responses in other attentional tasks are represented by red circles (6–10). 1, incongruent − congruent blocks (16); 2, incongruent − neutral blocks (14); 3, high-conflict, incongruent − congruent (17); 4, incongruent − congruent blocks (16); 5, high-conflict, incongruent − congruent (15); 6, flanker incongruent − congruent (12); 7, flanker congruent/incongruent − incongruent/incongruent (12); 8, high-conflict, continuous-performance task (19); 9, divided attention (11); and 10, flanker incongruent − congruent (13). VCA, vertical anterior commissure.
Fig 2.
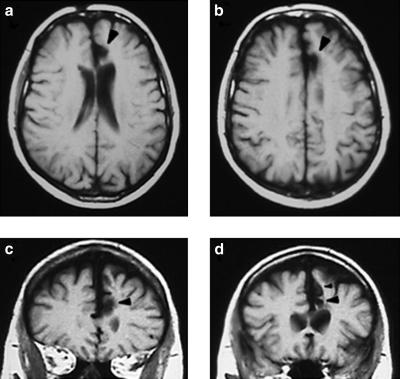
MRI scans showing R.N.'s lesion. (a and b) Horizontal sections illustrating the lesion in the left ACC (indicated by black arrowheads). (c and d) Coronal sections with ACC damage denoted by black arrowheads. In d, the larger arrowhead shows the damage in the cingulate sulcus, and the smaller arrowhead above it indicates the lesion in the paracingulate sulcus.
Here we report results from an ERP study designed to assess whether error-related and conflict-related activity within the ACC are separable phenomena or whether they have one and the same functional anatomical correlate. The experiments used a word-arrow version of the Stroop task in which the attended stimulus dimension (word or arrow) was cued on each trial. Two major questions were posed by the current experiments. First, we asked whether the ERN component that is time-locked to erroneous responses, and the ensuing compensatory behavior after committing an error, are intact in R.N. If error monitoring is a major function of the ACC, the ERN should be reduced in amplitude or eliminated entirely if its neural generator localized within the patient's lesion. Second, we examined whether the stimulus-locked ERP component related to the detection of competing response tendencies on incongruent trials is diminished in R.N., as would be predicted by the conflict-monitoring hypothesis (12, 15). If not, this would provide strong evidence that the ACC activations observed in multiple Stroop-like tasks do not reflect activity that is strictly time-locked to conflict-detection processes.
Experimental Procedures
Participants.
Patient R.N. (right-handed male, born in 1931, 14 yrs of education) has a focal left-hemisphere lesion extending from the rostral ACC (around the genu of the corpus callosum) to mid-ACC (Figs. 1 and 2). In the terminology of Paus et al. (32), the dorsal extent of R.N.'s lesion is in paralimbic ACC, which is above the cingulate sulcus and includes the paracingulate sulcus (area 32). The rostral (perigenual) extent of the damage is considered supracallosal (Z > 2) limbic ACC. The posterior motor areas (Y < 10), which are spared in R.N., are referred to as caudal ACC. The lesion presumably was due to occlusion of the pericallosal branch of the anterior cerebral artery. The date of the infarct is unknown, because neither R.N. nor his wife noted any behavioral changes suggesting the occurrence of a stroke.
Eight controls (mean age 67.0 yrs, 14.9 yrs of education) were tested for comparison to R.N. (age 69 yrs). All participants were free from significant medical complications, substance abuse, psychiatric disturbances, and dementia. All subjects signed informed consent statements approved by the Institutional Review Boards of the Veterans Affairs Northern California Health Care System and the University of California at Davis. The subjects were paid for their participation.
Stimuli and Behavioral Task.
A cued version of the word-arrow Stroop paradigm was used, in which the relevant dimension (either word or arrow) was signaled on a trial-by-trial basis. Stimulus displays consisted of a word and an arrow located above and below the center of the screen. The arrow pointed either to the left or right, and the word was either “LEFT” or “RIGHT.” The positions of the word and arrow were varied randomly from trial to trial. Each trial started with a cue word (“arrow” or “word”) presented in the center of the screen for 350 msec followed by the stimulus 1,500 msec later. The cue indicated which of the two stimuli was the relevant, to-be-attended dimension. When the cue was “arrow,” subjects were instructed to press the left or right button corresponding to the direction of the arrow, ignoring the word. When the cue was “word,” the instructions were to press the button indicated by the word, ignoring the arrow. The cue varied randomly from trial to trial. Stimulus displays (presented for 350 msec) could be congruent or incongruent (50% probability for each, randomly presented). Subjects were told to respond as fast as possible without making an excessive number of errors. They were encouraged also to correct themselves quickly after making an error. The interval from stimulus onset to the beginning of the next trial was 3,125 msec (stimulus onset asynchrony from onset of stimulus display to onset of next trial cue). Subjects were given a maximum time interval of 2,000 msec for their first response, and error corrections were allowed at any time before the end of the trial. There were a total of 12 blocks, each with 64 trials. In each block, 32 trials were “attend word” (16 congruent, 16 incongruent), and the other 32 were “attend arrow” (16 congruent, 16 incongruent).
ERP Recording and Analysis.
Electrophysiological signals were recorded by using an Electro-Cap with 48 tin electrodes placed according to the extended International 10–20 system. Forty-four scalp electroencephalographic channels, two electro-oculographic electrodes, and the right mastoid were referenced to the left mastoid. Signals were amplified (×50,000) and filtered (0.1–80 Hz) via an SA Instrumentation acquisition system. The electroencephalogram was digitized continuously at 16-bit resolution by using a 256-Hz sampling rate.
Twenty-nine channels of electroencephalogram were selected initially for further analysis. Data were re-referenced to the average of the mastoids off-line and digitally low-pass-filtered (20 Hz). Trials containing eye movement, excessive peak-to-peak deflections, electromyographic artifact, or amplifier blocking were rejected automatically from the averaged data. Trials with “correctable” blinks (i.e., uncontaminated by other artifacts) were corrected with an adaptive filtering algorithm developed by Dale (33) and included in the relevant average. ERP averages for each stimulus type and condition were computed for individual subjects; group averages were computed for the age-matched controls and compared with patient R.N. Stimulus- and response-locked averages were extracted during the analysis process. A digital filter of 2–10 Hz was also applied to the response-locked averages to isolate the ERN from temporally overlapping slow positive potentials (34). ERPs were quantified by computing peak amplitudes or mean amplitudes in defined latency windows relative to a 100-msec prestimulus or 200-msec preresponse baseline. Peak latency was referred to stimulus or response onset. The data were evaluated statistically with repeated-measures ANOVAs using Greenhouse–Geisser corrections for multiple comparisons when appropriate. ERP measures in controls were analyzed by attended stimulus type (arrow, word), congruity (congruent, incongruent), and electrode. Data from R.N. were compared with the range for his age-matched control group, and values falling outside the 95% confidence interval were considered significantly different from controls.
Results
Behavioral Performance.
All subjects exhibited slower RTs for incongruent stimuli than for congruent stimuli (Table 1). In controls, the main effect of congruity was highly significant [F(1,7) = 81.10, P < 0.0001]. The interaction between congruity and the attended stimulus dimension approached significance [F(1,7) = 5.01, P = 0.06] such that larger interference effects were observed in the attend-word condition compared with the attend-arrow condition, as has been observed for manual responses (35). The word/manual condition has a less automatic response mapping than the arrow/manual condition. When vocal responses are required (8, 35), however, the attend-arrow condition yields greater interference.
Table 1.
Mean RTs (msec), error rates (%), and the interference effect (%Int) for patient R.N. and his age-matched control group for the word-arrow Stroop task
|
|
Attend arrow | Attend word | ||||
|---|---|---|---|---|---|---|
| Congruent | Incongruent | %Int | Congruent | Incongruent | %Int | |
| Mean RTs | ||||||
| Controls | 467 ± 30 | 507 ± 38 | 8.1 ± 1.3 | 476 ± 25 | 542 ± 26 | 14 ± 1.6 |
| R.N. | 467 | 575 | 23.1 | 496 | 631 | 27.1 |
| Errors | ||||||
| Controls | 3.1 ± 1.0 | 8.6 ± 1.1 | 2.7 ± 0.8 | 14.6 ± 3.0 | ||
| R.N. | 0.5 | 17.7 | 4.2 | 13.1 | ||
Standard errors are shown for the control data.
Above the upper 99% confidence interval for controls.
The data from R.N. were compared with the 95% confidence intervals for controls, and values outside this range were considered significantly different. R.N.'s RTs to congruent stimuli were well matched to his control group, but his RTs for incongruent stimuli were slower than controls (significantly so for incongruent stimuli in the attend-word condition, P < 0.05). To compare directly the magnitude of the congruity effect in R.N. vs. controls, interference was expressed as a percentage [(incongruous RT − congruous RT)/congruous RT × 100]. Control subjects showed greater interference in the attend-word condition than in the attend-arrow condition [F(1,7) = 6.70, P < 0.05]. R.N. showed excessive interference effects for both the word and arrow conditions.
The accuracy data in controls (Table 1) revealed significantly higher error rates for incongruent stimuli [F(1,7) = 61.67, P < 0.0001] and a trend for worse performance in the attend-word condition [F(1,7) = 3.88, P < 0.09]. R.N. showed a higher error rate than controls for incongruent trials in the attend-arrow condition (P < 0.01); he was well outside the 99% confidence intervals for controls. Additionally, unlike controls, he was less accurate on incongruent trials when attending to arrows than to words.
After committing an error, control subjects typically engage in compensatory behavior in the form of both overt error correction and adjustments in strategy (slower RT on the trial after an error). As seen in Table 2, R.N. showed posterror slowing comparable with controls. Likewise, he did not show an elevated percentage of erroneous corrections (“corrections” of correct responses). For the overt error-correction measure, however, R.N. was impaired significantly (P < 0.05); he fell outside the 95% confidence intervals for controls.
Table 2.
Error compensation data for R.N. and age-matched controls
| Controls | R.N. | |
|---|---|---|
| Error correction, % | 88.0 (3.0) | 77.9 |
| Bad correction, % | 6.9 (1.9) | 8.6 |
| Post-error slowing, % | 11.4 (6.9) | 10.0 |
“Bad corrections” are correct responses followed by an incorrect correction, calculated as a percentage of all corrections. Standard errors are shown for controls.
Below the 95% confidence interval for controls.
ERPs.
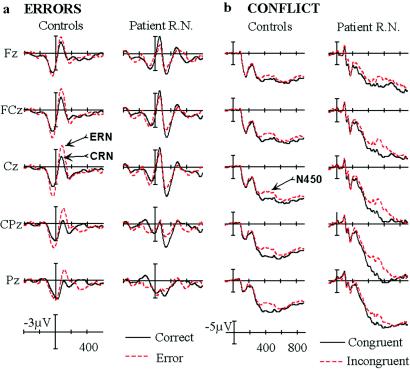
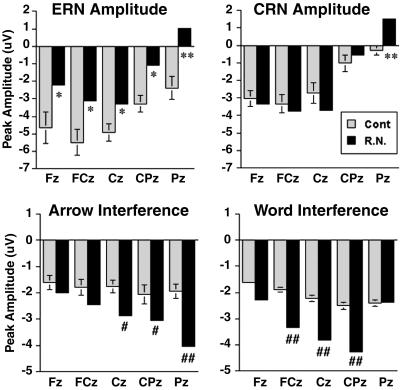
In the response-locked ERP recordings for error trials, control subjects generated an ERN component peaking ≈65 msec after incorrect responses (Fig. 3A). The ERN was largest at the frontal-central midline electrodes (FCz and Cz). As in previous studies of healthy older adults (25), a negative component was also observed on correct trials, which is called the correct-related negativity or CRN (36). ERN peak amplitude was measured at six midline electrodes (Fz, FCz, Cz, CPz, Pz, and POz) in the 20- to 125-msec postresponse window and entered into an ANOVA with the additional factor of accuracy. The ERN on error trials (−3.78 μV) was significantly greater than the negative component on correct trials (−1.75 μV) [F(1,7) = 16.46, P < 0.005]. Conversely, R.N. did not show a difference in amplitude between correct and error trials (error, −1.37 μV; correct, −1.38 μV). Although CRN amplitude appeared to be enhanced in R.N., this was not statistically significant (Fig. 4) in contrast to the reduction in ERN amplitude. Analysis of the ERN − CRN difference measure revealed that R.N. showed a significant reduction (P < 0.01), with a reversal of the effect (CRN > ERN) at the more anterior electrodes. On the other hand, neither ERN nor CRN latency were altered in R.N.
Fig 3.
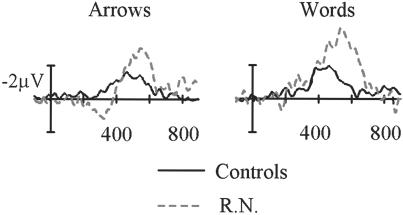
Dissociation of error-related and conflict-related ERPs in R.N. (a) The ERN and the CRN in the response-locked waves at midline electrode sites arranged from frontal (Fz) to parietal (Pz). Response onset occurs at the vertical bar (time = 0 msec), tic marks are 200 msec, and negative is plotted upward. (b) ERPs related to conflict (the N450 component) in the stimulus-locked waves at midline electrode sites, arranged from frontal to parietal. Stimulus onset occurs at the vertical bar (time = 0 msec).
Fig 4.
ERN amplitude is reduced, and the ERP interference effect is enhanced in R.N. The bar graphs illustrate the peak amplitudes of ERN, CRN, and the N450 conflict effect (incongruent − congruent). * and **, above the upper 95% and 99% confidence interval for controls (Cont), respectively; # and ##, below the 95% and 99% confidence interval for controls, respectively. Error bars are shown for the control data.
The effects of interference were evident in controls in the form of a negative component peaking at ≈450 msec in the stimulus-locked waves, which was largest at Cpz (Fig. 3B). The potentials elicited by correct incongruent stimuli were significantly more negative (i.e., less positive in amplitude) in the 350- to 500-msec poststimulus interval than the ERPs to correct congruent stimuli. To initially quantify this conflict-related N450 component, mean amplitude was measured at the midline sites in the 350- to 500-msec window and entered into an ANOVA with the additional factors of congruity and attended stimulus dimension. The N450 component on incongruent trials (10.69 μV) was less positive than on congruent trials (13.01 μV) [F(1,7) = 117.67, P < 0.0001]. R.N. appeared to show a delay in the latency and an enhancement in the amplitude of the N450 deflection on incongruent trials. These effects were quantified further by obtaining subtraction waveforms (incongruent − congruent, as shown in Fig. 5) and measuring peak latency and amplitude in the 350- to 550-msec (controls) and 400- to 625-msec (R.N.) windows. Peak latency for the N450 was delayed significantly in R.N. (524 vs. 449 msec), and this was true for both the word (506 vs. 441 msec) and arrow (543 vs. 457 msec) conditions (all Ps < 0.01). However, the onset of N450 appeared to be identical in controls and R.N. in the attend-word condition (≈350 msec), suggesting that R.N.'s brain detected the conflict at the same time as controls but engaged in prolonged evaluation and conflict-resolution processes. In the attend-arrow condition, R.N. showed an early reversal of the effect at ≈325 msec, which was close to the approximate N450 onset time for controls. Perhaps this pattern can be related to his poor performance in the arrow condition, which was the easier condition for controls. In addition, N450 amplitude showed a significant enhancement in R.N. compared with controls (Fig. 4).
Fig 5.
ERP effects of conflict detection are increased and prolonged in R.N. Subtraction waveforms (incongruent − congruent) for the N450 conflict effect are shown. Data from the central-parietal midline electrode (CPz) are illustrated here. Stimulus onset occurs at the vertical bar (time = 0 msec).
Discussion
A dissociation between error-detection and conflict-detection processes was observed in patient R.N., who has a lesion in the dorsal ACC region associated with conflict monitoring in fMRI studies (12, 15, 19). This patient showed deficits in the amplitude of the ERN component related to error detection and in the correction of erroneous responses. Conversely, the conflict-related N450 component on correct trials, recorded in response to incongruent stimuli, was enhanced in R.N. The current experiment aimed to address two major issues regarding the function of the human ACC, in particular the regions implicated in neuroimaging studies of attention and cognition. Although we acknowledge the limitations of generalizing from a single case study, the results do present a challenge for the conflict-monitoring hypothesis.
Error Monitoring.
The ACC has been implicated as a critical generator of the ERN, which is thought to reflect the activity of a neural system responsible for error monitoring (23). Although an ERN on incorrect trials was observed in R.N., the amplitude of this negative peak did not distinguish it from the CRN component on correct trials. One explanation for this finding is that the damaged area of the ACC plays a modulatory role in generating the ERN but is not the primary neural source of the component, because the lesion did not eliminate it entirely. Because of a lack of input from the ACC, the ERN generator no longer can distinguish between correct responses and errors. This view presupposes that the ERN and CRN represent activity from the exact same neural system, which may not be an accurate assumption.
The second interpretation relies on an alternate conception of ERN and CRN as related, but not identical, components (36, 37). In this view, the CRN is thought to signal the outcome of a general, response-related monitoring process, and the ERN reflects this generic monitoring process plus error-specific activity. ACC damage eliminated this error-specific activity but spared the more general response-monitoring process. Accumulating evidence points to a possible affective element in the scalp-recorded error potential, in particular, an association of the ERN with negative affective responses (38). This finding agrees with neuroimaging results demonstrating increased activity in rostral ACC during errors of commission in Go/NoGo tasks (9, 10). There was some overlap between these studies and R.N.'s lesion, but the activations also extended to areas rostral and ventral to R.N.'s lesion. These latter regions of the ACC are generally associated with affect, rather than cognition (3). If part of an affective monitoring system was compromised by rostral ACC damage, this could result in the lack of ERN activity on error trials.
In addition, R.N. corrected fewer of his erroneous responses than controls. However, this impairment cannot account for his attenuated ERN, because an analysis of only his corrected errors also revealed a diminished ERN. This is in agreement with prior demonstrations that ERN amplitude is not related directly to compensatory behavior: it is large for false-alarm “NoGo” errors that cannot be corrected, and it does not differ for corrected-vs.-uncorrected errors (20). Therefore, ERN also may reflect some sort of internal monitoring or affective process that occurs on error trials rather than only error detection itself. If R.N. showed a dampened affective response to errors, his motivation to self-correct could have been reduced. This explanation is supported by a recent study linking similar medial frontal activity to financial loss in a gambling task rather than errors (39). The fact that R.N. did not “overcorrect” his correct responses also supports this interpretation rather than a deficit in knowing when he committed an error.
Another form of compensatory behavior, posterror slowing, was spared in R.N. Posterror slowing may be a different phenomenon from overt error correction, perhaps reflecting the outcome of a less selective control process that affects response preparation (40). This type of control is independent of both ACC and lateral PFC; R.N.'s behavioral findings resemble those from patients with PFC lesions (25). The PFC patients did not show differential electrophysiological activity on error and correct trials, either. However, in that study, CRN amplitude was increased, but ERN amplitude was the same as controls (25), whereas the opposite was true in R.N. (and also in Parkinson's patients; ref. 37). Thus, the present data provide further confirmation of a performance-monitoring circuit that includes PFC, ACC, and the basal ganglia.
What sort of model of PFC–ACC interactions do these findings support? Which structure is signaling which? Before addressing these questions, it is important to consider the reciprocal nature of the connections between these structures (41), which may operate in different ways under conditions of response competition and error monitoring. Further, we suggest that rostral ACC is more specific to affective aspects of error processing, whereas dorsal ACC is engaged under difficult, attention-demanding task conditions that can include, but are not limited to, error detection and response conflict. Single-unit recording studies in monkeys have demonstrated that ACC neurons are heterogeneous physiologically, including cells specifically sensitive to the anticipation of a behavioral response, the motor response itself, error commission, and reward (42).
Several possible models for PFC–ACC communication have been put forward (25, 40). One prominent theory proposes that the ACC detects response conflict on both correct trials and errors and then signals the PFC to perform the control function of adjusting performance accordingly (19, 26). Another role of lateral PFC is to maintain representations of the stimulus-response mapping rules. If these representations are weakened by PFC damage, this would result in the perception of greater conflict by the ACC (40). This contention assumes that the ERN is only a reflection of conflict monitoring. We argue below that this assumption is invalid, because the conflict-related N450 component was intact in R.N.
Instead, the current results suggest a more complex arrangement, in which the rostral and dorsal ACC and the lateral PFC work with other regions (e.g., ventral ACC, orbitofrontal cortex, basal ganglia, and supplementary motor area) to mediate error-monitoring functions, which is in general agreement with Gehring and Knight (25). Models of sensorimotor integration (43) are informative for encompassing the motor-control and performance-monitoring aspects of medial frontal activity. Given the time course of the ERN, a representation of the actual response must be derived from a central rather than peripheral feedback system (21). An efference copy is sent to the monitoring system, where a mismatch between the intended response and the erroneous response is detected. One theory holds that the comparison between intended and actual responses is performed by the basal ganglia, and the output of this comparison process (terminating in dorsal ACC and/or cingulate motor areas) produces the ERN (21). Building on this work, Luu et al. (44) proposed that the ERN is comprised of signals from two distinct generators: one from rostral ACC that is specific to errors and another in caudal ACC/supplementary motor area that also is active during delayed feedback about task performance. Perhaps the more general performance-monitoring function is mediated by the latter regions, caudal and dorsal to R.N.'s lesion, which is why the CRN was spared. A related possibility is that the ERN reflects a phase alignment of ongoing frontal midline theta activity (34, 44), and damage to rostral ACC removed the phase-reset signal in R.N.
Conflict Monitoring.
The ERP data revealed that on correct trials, incongruent stimuli elicited a more negative-going potential in both controls and R.N. This conflict negativity resembles the N2c component to incompatible stimuli in a flanker task (45) and the N450 component in the color-word Stroop task (46, 47). This negative potential seems to reflect neural processing related to the detection of response conflict (48). The N450 was not only intact after focal lesion of the dorsal ACC but in fact was enhanced. Of critical importance is the electrophysiological dissociation between error and conflict; ERN amplitude was reduced in R.N., but the N450 was increased. These results are at odds with the contention that the exact same area of dorsal ACC mediates both conflict detection and error monitoring (19, 48). The present combination of ERP and lesion approaches provides a persuasive demonstration that conflict-detection processes, before the motor response, were not compromised by dorsal ACC damage.
Another important observation is that although the onset of the ERP effect to conflict was the same as in controls, its duration was prolonged in R.N. One possible explanation for the enhanced N450 is an impairment in engaging the control processes that reduce the effects of response conflict. This observation combined with his exaggerated behavioral interference effects suggest that the locus of the deficit is in the recruitment of executive control processes under difficult task conditions (49). This interpretation agrees with R.N.'s prior results in the color-word Stroop task, where he committed more errors on incongruent trials than controls (50). We hypothesize that intact regions of R.N.'s brain, perhaps in lateral PFC and the basal ganglia, are able to detect response conflict, but damage to the dorsal ACC renders him impaired in response inhibition, which may be due to disconnection from cingulate and supplementary motor areas. Our findings are consistent with the proposal that the PFC detects incompatible response options on incongruent trials and signals conflict-resolution processes, perhaps in caudal ACC and supplementary motor areas (6, 7). One explanation for the divergence between the present ERP results and prior fMRI studies is that the magnetic resonance signal reflects ACC activity that occurs after conflict-detection processes.
Conclusions
In conclusion, the ERP results in patient R.N. revealed an important distinction between two attentional control functions attributed to the ACC. The ERP signature of error monitoring was reduced in R.N., as was his ability (and/or motivation) to correct erroneous responses. In contrast, the ERP correlate of conflict detection was enhanced, strongly suggesting that dorsal ACC does not generate this component. Instead, we propose that lateral PFC detects competing response tendencies and communicates with dorsal ACC and caudal cingulate and supplementary motor areas to resolve the conflict. This hypothesis disagrees with the conflict-monitoring view (19, 26), in which dorsal ACC detects response conflict and signals lateral PFC to resolve the conflict. Single-unit recordings in monkeys (42) and humans (51), as well as a recent fMRI study (52), have also suggested that dorsal ACC consists of a functionally heterogeneous collection of cells that participate in a broad range of functions. Further, our data support the emerging notion that error monitoring and conflict monitoring are dissociated within the ACC (53, 54), with the more rostral, perigenual region related to the rapid evaluation of erroneous responses (44). These results provide further evidence for the existence of functional specialization in the human ACC, which has the anatomical connectivity to enable the integration of affect, motivational state, cognition, and motor control (4).
Acknowledgments
We thank Robert Knight and Robert Rafal for patient referrals and Jelena Jovanovic, Jonathan Kopelovich, Jary Larsen, Kim Miller, and Caitlin Roxby for their assistance. This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC03424 and James S. McDonnell Foundation Grant 98-47 CNS-QUA.05.
Abbreviations
ACC, anterior cingulate cortex
fMRI, functional MRI
ERP, event-related brain potential
ERN, error-related negativity
PFC, prefrontal cortex
RT, reaction time
CRN, correct-related negativity
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Posner M. I. & Petersen, S. E. (1990) Annu. Rev. Neurosci. 13, 25-42. [DOI] [PubMed] [Google Scholar]
- 2.Devinsky O., Morrell, M. J. & Vogt, B. A. (1995) Brain 118, 279-306. [DOI] [PubMed] [Google Scholar]
- 3.Bush G., Luu, P. & Posner, M. I. (2000) Trends Cogn. Sci. 4, 215-222. [DOI] [PubMed] [Google Scholar]
- 4.Paus T. (2001) Nat. Rev. Neurosci. 2, 417-424. [DOI] [PubMed] [Google Scholar]
- 5.Shima K., Aya, K., Mushiake, H., Inase, M., Aizawa, H. & Tanji, J. (1991) J. Neurophysiol. 65, 188-202. [DOI] [PubMed] [Google Scholar]
- 6.Paus T., Petrides, M., Evans, A. C. & Meyer, E. (1993) J. Neurophysiol. 70, 453-469. [DOI] [PubMed] [Google Scholar]
- 7.Picard N. & Strick, P. L. (1996) Cereb. Cortex 6, 342-353. [DOI] [PubMed] [Google Scholar]
- 8.Turken A. U. & Swick, D. (1999) Nat. Neurosci. 2, 920-924. [DOI] [PubMed] [Google Scholar]
- 9.Kiehl K. A., Liddle, P. F. & Hopfinger, J. B. (2000) Psychophysiology 37, 216-223. [PubMed] [Google Scholar]
- 10.Menon V., Adleman, N. E., White, C. D., Glover, G. H. & Reiss, A. L. (2001) Hum. Brain Mapp. 12, 131-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbetta M., Miezin, F. M., Dobmeyer, S., Shulman, G. L. & Petersen, S. E. (1991) J. Neurosci. 11, 2383-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botvinick M., Nystrom, L., Fissell, K., Carter, C. S. & Cohen, J. D. (1999) Nature 402, 179-181. [DOI] [PubMed] [Google Scholar]
- 13.Casey B. J., Thomas, K. M., Welsh, T. F., Badgaiyan, R. D., Eccard, C. H., Jennings, R. J. & Crone, E. A. (2000) Proc. Natl. Acad. Sci. USA 97, 8728-8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter C. S., Mintun, M. & Cohen, J. D. (1995) Neuroimage 2, 264-272. [DOI] [PubMed] [Google Scholar]
- 15.Carter C. S., Macdonald, A. M., Botvinick, M., Ross, L. L., Stenger, V. A., Noll, D. & Cohen, J. D. (2000) Proc. Natl. Acad. Sci. USA 97, 1944-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson B. S., Skudlarski, P., Gatenby, J. C., Zhang, H., Anderson, A. W. & Gore, J. C. (1999) Biol. Psychiatry 45, 1237-1258. [DOI] [PubMed] [Google Scholar]
- 17.Leung H.-C., Skudlarski, P., Gatenby, J. C., Peterson, B. S. & Gore, J. C. (2000) Cereb. Cortex 10, 552-560. [DOI] [PubMed] [Google Scholar]
- 18.MacLeod C. M. & MacDonald, P. A. (2000) Trends Cogn. Sci. 4, 383-391. [DOI] [PubMed] [Google Scholar]
- 19.Carter C. S., Braver, T. S., Barch, D. M., Botvinick, M. M., Noll, D. & Cohen, J. D. (1998) Science 280, 747-749. [DOI] [PubMed] [Google Scholar]
- 20.Falkenstein M., Hoormann, J., Christ, S. & Hohnsbein, J. (2000) Biol. Psychol. 51, 87-107. [DOI] [PubMed] [Google Scholar]
- 21.Coles M. G. H., Scheffers, M. K. & Holroyd, C. B. (2001) Biol. Psychol. 56, 173-189. [DOI] [PubMed] [Google Scholar]
- 22.Falkenstein M., Hohnsbein, J., Hoorman, J. & Blanke, L. (1990) in Psychophysiological Brain Research, eds. Brunia, C. H. M., Gaillard, A. W. K. & Kok, A. (Tilburg Univ. Press, Tilburg, The Netherlands), pp. 192–195.
- 23.Gehring W. J., Goss, B., Coles, M. G. H., Meyer, D. E. & Donchin, E. (1993) Psychol. Sci. 4, 385-390. [DOI] [PubMed] [Google Scholar]
- 24.Dehaene S., Posner, M. I. & Tucker, D. M. (1994) Psychol. Sci. 5, 303-305. [Google Scholar]
- 25.Gehring W. J. & Knight, R. T. (2000) Nat. Neurosci. 3, 516-520. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald A. W., Cohen, J. D., Stenger, V. A. & Carter, C. S. (2000) Science 288, 1835-1838. [DOI] [PubMed] [Google Scholar]
- 27.Swick D. & Knight, R. T. (1998) in The Attentive Brain, ed. Parasuraman, R. (MIT Press, Cambridge, MA), pp. 143–162.
- 28.Talairach J. & Tournoux, P., (1988) Co-Planar Stereotaxic Atlas of the Human Brain (Thieme, Stuttgart).
- 29.Janer K. W. & Pardo, J. V. (1991) J. Cognit. Neurosci. 3, 231-241. [DOI] [PubMed] [Google Scholar]
- 30.Cohen R. A., Kaplan, R. F., Zuffante, P., Moser, D. J., Jenkins, M. A., Salloway, S. & Wilkinson, H. (1999) J. Neuropsychiatry Clin. Neurosci. 11, 444-453. [DOI] [PubMed] [Google Scholar]
- 31.Ochsner K. N., Kosslyn, S. M., Cosgrove, G. R., Cassem, E. H., Price, B. H., Nierenberg, A. A. & Rauch, S. L. (2001) Neuropsychologia 39, 219-230. [DOI] [PubMed] [Google Scholar]
- 32.Paus T., Koski, L., Caramanos, Z. & Westbury, C. (1998) NeuroReport 9, R37-R47. [DOI] [PubMed] [Google Scholar]
- 33.Dale A. M., (1994) Ph.D. dissertation (Univ. of California, San Diego).
- 34.Luu P. & Tucker, D. M. (2001) Clin. Neurophysiol. 112, 1295-1469. [DOI] [PubMed] [Google Scholar]
- 35.Baldo J. V., Shimamura, A. P. & Prinzmetal, W. (1998) Percept. Psychophys. 60, 427-437. [DOI] [PubMed] [Google Scholar]
- 36.Mathalon D. H., Fedor, M., Faustman, W. O., Gray, M., Askari, N. & Ford, J. M. (2002) J. Abnorm. Psychol. 111, 22-41. [PubMed] [Google Scholar]
- 37.Falkenstein M., Hielscher, H., Dziobek, I., Schwarzenau, P., Hoormann, J., Sundermann, B. & Hohnsbein, J. (2001) NeuroReport 12, 157-161. [DOI] [PubMed] [Google Scholar]
- 38.Luu P., Collins, P. & Tucker, D. M. (2000) J. Exp. Psychol. Gen. 129, 43-60. [DOI] [PubMed] [Google Scholar]
- 39.Gehring W. J. & Willoughby, A. R. (2002) Science 295, 2279-2282. [DOI] [PubMed] [Google Scholar]
- 40.Cohen J. D., Botvinick, M. & Carter, C. S. (2000) Nat. Neurosci. 3, 421-423. [DOI] [PubMed] [Google Scholar]
- 41.Bates J. F. & Goldman-Rakic, P. S. (1993) J. Comp. Neurol. 336, 211-228. [DOI] [PubMed] [Google Scholar]
- 42.Niki H. & Watanabe, M. (1979) Brain Res. 171, 213-224. [DOI] [PubMed] [Google Scholar]
- 43.Nelson R. J. (1996) Curr. Opin. Neurobiol. 6, 801-810. [DOI] [PubMed] [Google Scholar]
- 44.Luu, P., Tucker, D. M., Derryberry, D. & Reed, M. (2002) Psychol. Sci., in press. [DOI] [PubMed]
- 45.Kopp B., Rist, F. & Mattler, U. (1996) Psychophysiology 33, 282-294. [DOI] [PubMed] [Google Scholar]
- 46.West R. & Alain, C. (2000) Brain Res. 873, 102-111. [DOI] [PubMed] [Google Scholar]
- 47.Liotti M., Woldorff, M. G., Perez, R., III & Mayberg, H. S. (2000) Neuropsychologia 38, 701-711. [DOI] [PubMed] [Google Scholar]
- 48.van Veen V. & Carter, C. S. (2002) J. Cognit. Neurosci. 14, 593-602. [DOI] [PubMed] [Google Scholar]
- 49.Posner M. I. & DiGirolamo, G. J. (1998) in The Attentive Brain, ed. Parasuraman, R. (MIT Press, Cambridge, MA), pp. 401–423.
- 50.Swick D. & Jovanovich, J. (2002) Neuropsychologia 40, 1240-1253. [DOI] [PubMed] [Google Scholar]
- 51.Davis K. D., Hutchison, W. D., Lozano, A. M., Tasker, R. R. & Dostrovsky, J. O. (2000) J. Neurophysiol. 83, 3575-3577. [DOI] [PubMed] [Google Scholar]
- 52.Bush G., Vogt, B. A., Holmes, J., Dale, A. M., Greve, D., Jenike, M. A. & Rosen, B. R. (2002) Proc. Natl. Acad. Sci. USA 99, 523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ullsperger M. & von Cramon, D. Y. (2001) Neuroimage 14, 1387-1401. [DOI] [PubMed] [Google Scholar]
- 54.Braver T. S., Barch, D. M., Gray, J. R., Molfese, D. L. & Snyder, A. (2001) Cereb. Cortex 11, 825-836. [DOI] [PubMed] [Google Scholar]